Ultrasound or Sectional Imaging Techniques as Screening Tools for Hepatocellular Carcinoma: Fall Forward or Move Forward?
Abstract
1. Topic Overview—Why Do We Need HCC Surveillance?
2. Surveillance Techniques and Ongoing Strategies for HCC
3. Ultrasound Aspects of HCC Discovered during Screening
4. Performance of US as Screening Tool
5. Factors Affecting US Performance
6. How to Improve US Screening?
6.1. An Adequate Ultrasound Examination and Potential Targets for Improvement
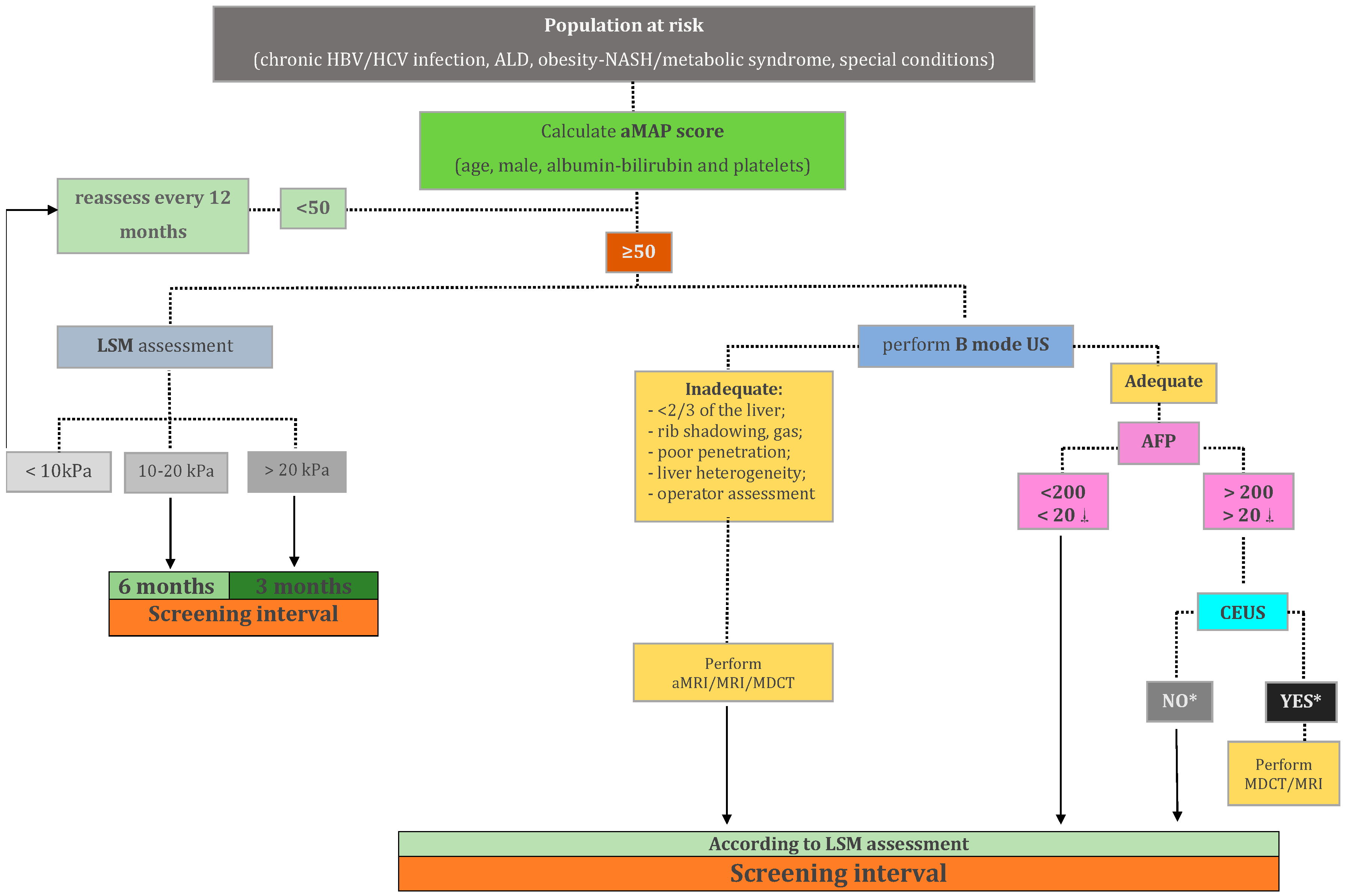
6.2. Defining Classes of Risk and Developing Imaging Strategies According to the Risk
6.3. Sectional Imaging as an Alternative to Ultrasound in the Screening for HCC
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M.; Coggeshall, M.; et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef]
- Altekruse, S.F.; McGlynn, K.A.; Reichman, M.E. Hepatocellular Carcinoma Incidence, Mortality, and Survival Trends in the United States From 1975 to 2005. J. Clin. Oncol. 2009, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Bagante, F.; Sahara, K.; Moris, D.; Hyer, J.M.; Wu, L.; Ratti, F.; Marques, H.P.; Soubrane, O.; Paredes, A.Z.; et al. Prognosis After Resection of Barcelona Clinic Liver Cancer (BCLC) Stage 0, A, and B Hepatocellular Carcinoma: A Comprehensive Assessment of the Current BCLC Classification. Ann. Surg. Oncol. 2019, 26, 3693–3700. [Google Scholar] [CrossRef]
- Bruix, J.; Raoul, J.-L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; SanGiovanni, A.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J. Hepatol. 2012, 57, 821–829. [Google Scholar] [CrossRef]
- Singal, A.; Volk, M.L.; Waljee, A.; Salgia, R.; Higgins, P.D.R.; Rogers, M.A.M.; Marrero, J.A. Meta-analysis: Surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment. Pharmacol. Ther. 2009, 30, 37–47. [Google Scholar] [CrossRef]
- Deng, L.X.; Mehta, N. Does Hepatocellular Carcinoma Surveillance Increase Survival in At-Risk Populations? Patient Selection, Biomarkers, and Barriers. Dig. Dis. Sci. 2020, 65, 3456–3462. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Yang, B.-H.; Tang, Z.-Y. Randomized controlled trial of screening for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2004, 130, 417–422. [Google Scholar] [CrossRef]
- Singal, A.G.; Pillai, A.; Tiro, J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLoS Med. 2014, 11, e1001624. [Google Scholar] [CrossRef]
- Kansagara, D.; Papak, J.; Pasha, A.S.; O’Neil, M.; Freeman, M.; Relevo, R.; Quiñones, A.; Motu’Apuaka, M.; Jou, J.H. Screening for Hepatocellular Carcinoma in Chronic Liver Disease. Ann. Intern. Med. 2014, 161, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Poustchi, H.; Farrell, G.C.; Strasser, S.I.; Lee, A.U.; McCaughan, G.W.; George, J. Feasibility of conducting a randomized control trial for liver cancer screening: Is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology 2011, 54, 1998–2004. [Google Scholar] [CrossRef]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.-C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Taddei, T.H.; Serper, M.; Mehta, R.; Dieperink, E.; Aytaman, A.; Baytarian, M.; Fox, R.; Hunt, K.; Pedrosa, M.; et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology 2017, 65, 864–874. [Google Scholar] [CrossRef]
- Davila, J.A.; Henderson, L.; Kramer, J.R.; Kanwal, F.; Richardson, P.A.; Duan, Z.; El-Serag, H.B. Utilization of Surveillance for Hepatocellular Carcinoma Among Hepatitis C Virus–Infected Veterans in the United States. Ann. Intern. Med. 2011, 154, 85–93. [Google Scholar] [CrossRef]
- Singal, A.G.; Li, X.; Tiro, J.; Kandunoori, P.; Adams-Huet, B.; Nehra, M.S.; Yopp, A. Racial, Social, and Clinical Determinants of Hepatocellular Carcinoma Surveillance. Am. J. Med. 2015, 128, 90.e1–90.e7. [Google Scholar] [CrossRef]
- Singal, A.G.; Yopp, A.C.; Gupta, S.; Skinner, C.S.; Halm, E.A.; Okolo, E.; Nehra, M.; Lee, W.M.; Marrero, J.A.; Tiro, J.A. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev. Res. 2012, 5, 1124–1130. [Google Scholar] [CrossRef]
- Singal, A.G.; Conjeevaram, H.S.; Volk, M.L.; Fu, S.; Fontana, R.J.; Askari, F.; Su, G.L.; Lok, A.S.; Marrero, J.A. Effectiveness of Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Prorok, P.C.; Marcus, P.M. Cancer Screening Trials: Nuts and Bolts. Semin. Oncol. 2010, 37, 216–223. [Google Scholar] [CrossRef][Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Bolondi, L. Screening for hepatocellular carcinoma in cirrhosis. J. Hepatol. 2003, 39, 1076–1084. [Google Scholar] [CrossRef]
- Wong, G.L.; Chan, H.L.; Tse, Y.-K.; Chan, H.-Y.; Tse, C.-H.; Lo, A.O.; Wong, V.W. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology 2013, 59, 986–995. [Google Scholar] [CrossRef]
- Tayob, N.; Lok, A.S.F.; Do, K.-A.; Feng, Z. Improved Detection of Hepatocellular Carcinoma by Using a Longitudinal Alpha-Fetoprotein Screening Algorithm. Clin. Gastroenterol. Hepatol. 2016, 14, 469–475.e2. [Google Scholar] [CrossRef] [PubMed]
- Biselli, M.; Conti, F.; Gramenzi, A.; Frigerio, M.; Cucchetti, A.; Fatti, G.; D’Angelo, M.; Dall’Agata, M.; Giannini, E.G.; Farinati, F.; et al. A new approach to the use of α-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. Br. J. Cancer 2015, 112, 69–76. [Google Scholar] [CrossRef]
- Chang, T.-S.; Wu, Y.-C.; Tung, S.-Y.; Wei, K.-L.; Hsieh, Y.-Y.; Huang, H.-C.; Chen, W.-M.; Shen, C.-H.; Lu, C.-H.; Wu, C.-S.; et al. Alpha-Fetoprotein Measurement Benefits Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Am. J. Gastroenterol. 2015, 110, 836–844. [Google Scholar] [CrossRef]
- Mocan, T.; Simão, A.L.; Castro, R.E.; Rodrigues, C.M.P.; Słomka, A.; Wang, B.; Strassburg, C.; Wöhler, A.; Willms, A.G.; Kornek, M. Liquid Biopsies in Hepatocellular Carcinoma: Are We Winning? J. Clin. Med. 2020, 9, 1541. [Google Scholar] [CrossRef] [PubMed]
- Del Poggio, P.; Olmi, S.; Ciccarese, F.; Di Marco, M.; Rapaccini, G.L.; Benvegnù, L.; Borzio, F.; Farinati, F.; Zoli, M.; Giannini, E.G.; et al. Factors That Affect Efficacy of Ultrasound Surveillance for Early Stage Hepatocellular Carcinoma in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1927–1933.e2. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hsu, Y.-C.; Ho, H.J.; Chen, Y.-J.; Lee, T.-Y.; Lin, J.-T. Association between ultrasonography screening and mortality in patients with hepatocellular carcinoma: A nationwide cohort study. Gut 2015, 65, 693–701. [Google Scholar] [CrossRef]
- Trinchet, J.-C.; Chaffaut, C.; Bourcier, V.; Degos, F.; Henrion, J.; Fontaine, H.; Roulot, D.; Mallat, A.; Hillaire, S.; Cales, P.; et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: A randomized trial comparing 3- and 6-month periodicities. Hepatology 2011, 54, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Foerster, F.; Galle, P.R. Ultrasound for Hepatocellular Carcinoma Surveillance: Still Looking for the Fortune Teller. Liver Transplant. 2018, 24, 1167–1168. [Google Scholar] [CrossRef]
- Rapaccini, G.L.; Pompili, M.; Caturelli, E.; Covino, M.; Lippi, M.E.; Beccaria, S.; Cedrone, A.; Riccardi, L.; Siena, D.A.; Gasbarrini, G. 661 Hepatocellular carcinomas <2 cm in diameter complicating cirrhosis: Ultrasound and clinical features in 153 consecutive pa-662 tients. Liver Int. 2004, 24, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Tateishi, R.; Yoshida, H.; Ohki, T.; Masuzaki, R.; Imamura, J.; Goto, T.; Kanai, F.; Obi, S.; Kato, N.; et al. Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C. Hepatol. Int. 2009, 3, 544–550. [Google Scholar] [CrossRef]
- Wu, S.; Tu, R.; Liu, G.; Shi, Y. Dynamic changes in ultrasound characteristics of nodules in cirrhotic liver and their implications in surveillance for malignancy. J. Med. Ultrason. 2013, 41, 165–171. [Google Scholar] [CrossRef]
- Sparchez, Z.; Radu, P.; Zaharia, T.; Kacso, G.; Diaconu, B.; Grigorescu, I.; Badea, R. B-mode and contrast enhanced ultrasound guided biopsy of portal vein thrombosis. Value in the diagnosis of occult hepatocellular carcinoma in liver cirrhosis. Med. Ultrason. 2010, 12. [Google Scholar]
- Caturelli, E.; Solmi, L.; Anti, M.; Fusilli, S.; Roselli, P.; Andriulli, A.; Fornari, F.; Blanco, C.D.V.; De Sio, I. Ultrasound guided fine needle biopsy of early hepatocellular carcinoma complicating liver cirrhosis: A multicentre study. Gut 2004, 53, 1356–1362. [Google Scholar] [CrossRef]
- Rao, P.N. Nodule in Liver: Investigations, Differential Diagnosis and Follow-up. J. Clin. Exp. Hepatol. 2014, 4, S57–S62. [Google Scholar] [CrossRef]
- Roskams, T. Anatomic Pathology of Hepatocellular Carcinoma: Impact on Prognosis and Response to Therapy. Clin. Liver Dis. 2011, 15, 245–259. [Google Scholar] [CrossRef]
- Pateron, D.; Ganne, N.; Trinchet, J.C.; Aurousseau, M.H.; Mal, F.; Meicler, C.; Coderc, E.; Reboullet, P.; Beaugrand, M.; Ganne-Carrié, N. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J. Hepatol. 1994, 20, 65–71. [Google Scholar] [CrossRef]
- Oka, H.; Kurioka, N.; Kim, K.; Kanno, T.; Kuroki, T.; Mizoguchi, Y.; Kobayashi, K. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology 1990, 12, 680–687. [Google Scholar] [CrossRef]
- Bolondi, L.; Sofia, S.; Siringo, S.; Gaiani, S.; Casali, A.; Zironi, G.; Piscaglia, F.; Gramantieri, L.; Zanetti, M.; Sherman, M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: A cost effectiveness analysis. Gut 2001, 48, 251–259. [Google Scholar] [CrossRef] [PubMed]
- SanGiovanni, A.; Del Ninno, E.; Fasani, P.; De Fazio, C.; Ronchi, G.; Romeo, R.; Morabito, A.; De Franchis, R.; Colombo, M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance☆. Gastroenterology 2004, 126, 1005–1014. [Google Scholar] [CrossRef]
- Samoylova, M.L.; Mehta, N.; Roberts, J.P.; Yao, F.Y. Predictors of Ultrasound Failure to Detect Hepatocellular Carcinoma. Liver Transplant. 2018, 24, 1171–1177. [Google Scholar] [CrossRef]
- Colli, A.; Fraquelli, M.; Casazza, G.; Massironi, S.; Colucci, A.; Conte, D.; Duca, P. Accuracy of Ultrasonography, Spiral CT, Magnetic Resonance, and Alpha-Fetoprotein in Diagnosing Hepatocellular Carcinoma: A Systematic Review. CME. Am. J. Gastroenterol. 2006, 101, 513–523. [Google Scholar] [CrossRef]
- Maringhini, A.; Cottone, M.; Sciarrino, E.; Marceno, M.P.; La Seta, F.; Rinaldi, F.; Pagliaro, L. Ultrasonographic and radionuclide detection of hepatocellular carcinoma in cirrhotics with low alpha-fetoprotein levels. Cancer 1984, 54, 2924–2926. [Google Scholar] [CrossRef]
- Tanaka, S.; Kitamura, T.; Ohshima, A.; Umeda, K.; Okuda, S.; Ohtani, T.; Tatsuta, M.; Yamamoto, K. Diagnostic accuracy of ultraso-nography for hepatocellular carcinoma. Cancer 1986, 58, 344–347. [Google Scholar] [CrossRef]
- Gambarin-Gelwan, M.; Wolf, D.C.; Shapiro, R.S.; Schwartz, M.E.; Min, A.D. Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am. J. Gastroenterol. 2000, 95, 1535–1538. [Google Scholar] [CrossRef]
- Rode, A.; Bancel, B.; Douek, P.; Chevallier, M.; Vilgrain, V.; Picaud, G.; Henry, L.; Berger, F.; Bizollon, T.; Gaudin, J.-L.; et al. Small Nodule Detection in Cirrhotic Livers: Evaluation with US, Spiral CT, and MRI and Correlation with Pathologic Examination of Explanted Liver. J. Comput. Assist. Tomogr. 2001, 25, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Lim, J.H.; Lee, W.J. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: Accuracy of ultrasonography in transplant patients. J. Ultrasound Med. 2001, 20, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sang, L.Y.; Lim, Y.-S.; Han, S.; Lee, J.-Y.; Byun, J.H.; Won, H.J.; Lee, S.J.; Lee, H.C.; Lee, Y.S. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017, 3, 456–463. [Google Scholar] [CrossRef]
- Sinn, D.H.; Yi, J.; Choi, M.S.; Choi, D.; Gwak, G.-Y.; Paik, Y.-H.; Lee, J.H.; Koh, K.C.; Paik, S.W.; Yoo, B.C. Incidence and risk factors for surveillance failure in patients with regular hepatocellular carcinoma surveillance. Hepatol. Int. 2013, 7, 1010–1018. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; An, C.; Kim, D.Y.; Aljoqiman, K.S.; Choi, J.-Y.; Kim, M.-J. Failure of hepatocellular carcinoma surveillance: Inadequate echogenic window and macronodular parenchyma as potential culprits. Ultrason 2019, 38, 311–320. [Google Scholar] [CrossRef]
- Simmons, O.; Fetzer, D.T.; Yokoo, T.; Marrero, J.A.; Yopp, A.; Kono, Y.; Parikh, N.D.; Browning, T.; Singal, A.G. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment. Pharmacol. Ther. 2017, 45, 169–177. [Google Scholar] [CrossRef]
- Piñero, F.; Rubinstein, F.; Marciano, S.; Fernández, N.; Silva, J.; Zambelo, Y.; Anders, M.; Zerega, A.; Ridruejo, E.; Miguez, C.; et al. Surveillance for Hepatocellular Carcinoma: Does the Place Where Ultrasound Is Performed Impact Its Effectiveness? Dig. Dis. Sci. 2018, 64, 718–728. [Google Scholar] [CrossRef]
- Khalili, K.; Menezes, R.; Kim, T.K.; Yazdi, L.K.; Jang, H.-J.; Sharma, S.; Feld, J.; Sherman, M. The effectiveness of ultrasound surveillance for hepatocellular carcinoma in a Canadian centre and determinants of its success. Can. J. Gastroenterol. Hepatol. 2015, 29, 267–273. [Google Scholar] [CrossRef]
- Choi, M.H.; Jung, S.E.; Choi, J.-I.; Jeong, W.K.; Kim, H.C.; Kim, Y.; Kim, Y.; Park, B. Quality Management of Ultrasound Surveillance for Hepatocellular Carcinoma Under the Korean National Cancer Screening Program. J. Ultrasound Med. 2018, 37, 245–254. [Google Scholar] [CrossRef]
- Akkus, Z.; Cai, J.; Boonrod, A.; Zeinoddini, A.; Weston, A.D.; Philbrick, K.A.; Erickson, B.J. A Survey of Deep-Learning Applications in Ultrasound: Artificial Intelligence–Powered Ultrasound for Improving Clinical Workflow. J. Am. Coll. Radiol. 2019, 16, 1318–1328. [Google Scholar] [CrossRef]
- Mokrane, F.-Z.; Lu, L.; Vavasseur, A.; Otal, P.; Peron, J.-M.; Luk, L.; Yang, H.; Ammari, S.; Saenger, Y.; Rousseau, H.; et al. Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur. Radiol. 2019, 30, 558–570. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A.; De Micheli, V.; Ghirarduzzi, A.; et al. Comparison between different D-D imer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2015, 38, 42–49. [Google Scholar] [CrossRef]
- Chen, C.-J.; Yang, H.-I.; Su, J.; Jen, C.-L.; You, S.-L.; Lu, S.-N.; Huang, G.-T.; Iloeje, U.H.; for the REVEAL-HBV Study Group. Risk of Hepatocellular Carcinoma Across a Biological Gradient of Serum Hepatitis B Virus DNA Level. JAMA 2006, 295, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.-H.; Chan, H.L.-Y.; Wong, C.K.-Y.; Leung, C.; Chan, C.Y.; Ho, P.P.-L.; Chung, V.C.-Y.; Chan, Z.C.-Y.; Tse, Y.-K.; Chim, A.M.-L.; et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J. Hepatol. 2014, 60, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Dalekos, G.N.; Sypsa, V.; Yurdaydin, C.; Buti, M.; Goulis, J.; Calleja, J.L.; Chi, H.; Manolakopoulos, S.; Mangia, G.; et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J. Hepatol. 2016, 64, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886.e6. [Google Scholar] [CrossRef]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Addissie, B.D.; Mara, K.C.; Harmsen, W.S.; Dai, J.; Zhang, N.; Wongjarupong, N.; Ali, H.M.; Ali, H.A.; Hassan, F.A.; et al. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol. Biomark. Prev. 2019, 28, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Mookerjee, R.P. Individualized care for portal hypertension: Not quite yet. J. Hepatol. 2015, 63, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Morabito, A.; D’Amico, M.; Pasta, L.; Malizia, G.; Rebora, P.; Valsecchi, M.G. Clinical states of cirrhosis and competing risks. J. Hepatol. 2018, 68, 563–576. [Google Scholar] [CrossRef]
- De Franchis, R. Expanding consensus in portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef]
- Bureau, C.; Metivier, S.; Peron, J.-M.; Selves, J.; Robic, M.A.; Gourraud, P.A.; Rouquet, O.; Dupuis, E.; Alric, L.; Vinel, J.-P. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment. Pharmacol. Ther. 2008, 27, 1261–1268. [Google Scholar] [CrossRef]
- Robic, M.A.; Procopet, B.; Métivier, S.; Péron, J.M.; Selves, J.; Vinel, J.P.; Bureau, C. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: A prospective study. J. Hepatol. 2011, 55, 1017–1024. [Google Scholar] [CrossRef]
- Jung, K.S.; Kim, S.U.; Ahn, S.H.; Park, Y.N.; Kim, D.Y.; Park, J.Y.; Chon, C.Y.; Choi, E.H.; Han, K.-H. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology 2010, 53, 885–894. [Google Scholar] [CrossRef]
- Masuzaki, R.; Tateishi, R.; Yoshida, H.; Goto, E.; Sato, T.; Ohki, T.; Imamura, J.; Goto, T.; Kanai, F.; Kato, N.; et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology 2009, 49, 1954–1961. [Google Scholar] [CrossRef]
- Oh, J.H.; Goh, M.J.; Park, Y.; Kim, J.; Kang, W.; Sinn, D.H.; Gwak, G.-Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; et al. Different Performance of Liver Stiffness Measurement According to Etiology and Outcome for the Prediction of Liver-Related Events. Dig. Dis. Sci. 2020, 1–10. [Google Scholar] [CrossRef]
- Schulz, M.; Tacke, F. Identifying High-Risk NASH Patients: What We Know so Far. Hepatic Med. Évid. Res. 2020, 12, 125–138. [Google Scholar] [CrossRef]
- Cassinotto, C.; Boursier, J.; De Lédinghen, V.; Lebigot, J.; Lapuyade, B.; Cales, P.; Hiriart, J.; Michalak, S.; Le Bail, B.; Cartier, V.; et al. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology 2016, 63, 1817–1827. [Google Scholar] [CrossRef]
- Vilana, R.; Forner, A.; Bianchi, L.; García-Criado, Á.; Rimola, J.; De Lope, C.R.; Reig, M.; Ayuso, C.; Brú, C.; Bruix, J. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010, 51, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Wilson, S.R.; Lyshchik, A.; Cosgrove, D.; Dietrich, C.F.; Jang, H.J.; Kim, T.K.; Salvatore, V.; Willmann, J.K.; Sirlin, C.B.; et al. American College of Radiology Contrast Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) for the diagnosis of Hepatocellular Carcinoma: A pictorial essay. Ultraschall Med. Eur. J. Ultrasound 2017, 38, 320–324. [Google Scholar] [CrossRef] [PubMed]
- McGillen, K.L.; Zaidi, S.; Ahmed, A.; Harter, S.; Yee, N.S. Contrast-Enhanced Ultrasonography for Screening and Diagnosis of Hepatocellular Carcinoma: A Case Series and Review of the Literature. Medicines 2020, 7, 51. [Google Scholar] [CrossRef]
- Schellhaas, B.; Bernatik, T.; Bohle, W.; Borowitzka, F.; Chang, J.; Dietrich, C.F.; Dirks, K.; Donoval, R.; Drube, K.; Friedrich-Rust, M.; et al. Contrast-Enhanced Ultrasound Algorithms (CEUS-LIRADS/ESCULAP) for the Noninvasive Diagnosis of Hepatocellular Carcinoma–A Prospective Multicenter DEGUM Study. Ultraschall Der Med. Eur. J. Ultrasound 2020. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Osaki, Y.; Hirooka, M.; Imai, Y.; Aso, K.; Numata, K.; Kitano, M.; Kumada, T.; Izumi, N.; et al. B-Mode Ultrasonography versus Contrast-Enhanced Ultrasonography for Surveillance of Hepatocellular Carcinoma: A Prospective Multicenter Randomized Controlled Trial. Liver Cancer 2019, 8, 271–280. [Google Scholar] [CrossRef]
- Park, J.H.; Park, M.-S.; Lee, S.J.; Jeong, W.K.; Lee, J.Y.; Park, M.J.; Lee, S.S.; Han, K.; Nam, C.M.; Park, S.H.; et al. Contrast-enhanced US with Perfluorobutane for Hepatocellular Carcinoma Surveillance: A Multicenter Diagnostic Trial (SCAN). Radiology 2019, 292, 638–646. [Google Scholar] [CrossRef]
- Pocha, C.; Dieperink, E.; McMaken, K.A.; Knott, A.; Thuras, P.; Ho, S.B. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography—A randomised study. Aliment. Pharmacol. Ther. 2013, 38, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Van Thiel, D.H.; Yong, S.; Li, S.D.; Kennedy, M.; Brems, J. The development of de novo hepatocellular carcinoma in patients on a liver transplant list: Frequency, size, and assessment of current screening methods. Liver Transplant. 2004, 10, 631–637. [Google Scholar] [CrossRef]
- Renzulli, M.; Golfieri, R.; Bologna Liver Oncology Group (BLOG). Proposal of a new diagnostic algorithm for hepatocellular carcinoma based on the Japanese guidelines but adapted to the Western world for patients under surveillance for chronic liver disease. J. Gastroenterol. Hepatol. 2015, 31, 69–80. [Google Scholar] [CrossRef]
- Yu, N.C.; Chaudhari, V.; Raman, S.S.; Lassman, C.; Tong, M.J.; Busuttil, R.W.; Lu, D.S. CT and MRI Improve Detection of Hepatocellular Carcinoma, Compared With Ultrasound Alone, in Patients With Cirrhosis. Clin. Gastroenterol. Hepatol. 2011, 9, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, B.; Kim, S.Y.; Shim, Y.S.; Kim, J.H.; Huh, J.; Kim, H.J.; Kim, K.W.; Lee, S.S. Abbreviated MRI with optional multiphasic CT as an alternative to full-sequence MRI: LI-RADS validation in a HCC-screening cohort. Eur. Radiol. 2019, 30, 2302–2311. [Google Scholar] [CrossRef]
- Khatri, G.; Pedrosa, I.; Ananthakrishnan, L.; De Leon, A.D.; Fetzer, D.T.; Leyendecker, J.; Singal, A.G.; Xi, Y.; Yopp, A.; Yokoo, T. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS Vj. Magn. Reson. Imaging 2020, 51, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.V.; McDonald, S.J.; Ong, Y.-Y.; Mastrocostas, K.; Ho, E.; Huo, Y.R.; Santhakumar, C.; Lee, A.U.; Yang, J. HCC screening: Assessment of an abbreviated non-contrast MRI protocol. Eur. Radiol. Exp. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- An, C.; Kim, D.Y.; Choi, J.-Y.; Han, K.H.; Roh, Y.H.; Kim, M.-J. Noncontrast magnetic resonance imaging versus ultrasonography for hepatocellular carcinoma surveillance (MIRACLE-HCC): Study protocol for a prospective randomized trial. BMC Cancer 2018, 18, 915. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, Y.K.; Park, H.J.; Park, M.J.; Lee, W.J.; Choi, D. Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma. Magn. Reson. Imaging 2014, 32, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Besa, C.; Lewis, S.; Pandharipande, P.V.; Chhatwal, J.; Kamath, A.; Cooper, N.; Knight-Greenfield, A.; Babb, J.S.; Boffetta, P.; Padron, N.; et al. Hepatocellular carcinoma detection: Diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom. Radiol. 2017, 42, 179–190. [Google Scholar] [CrossRef] [PubMed]

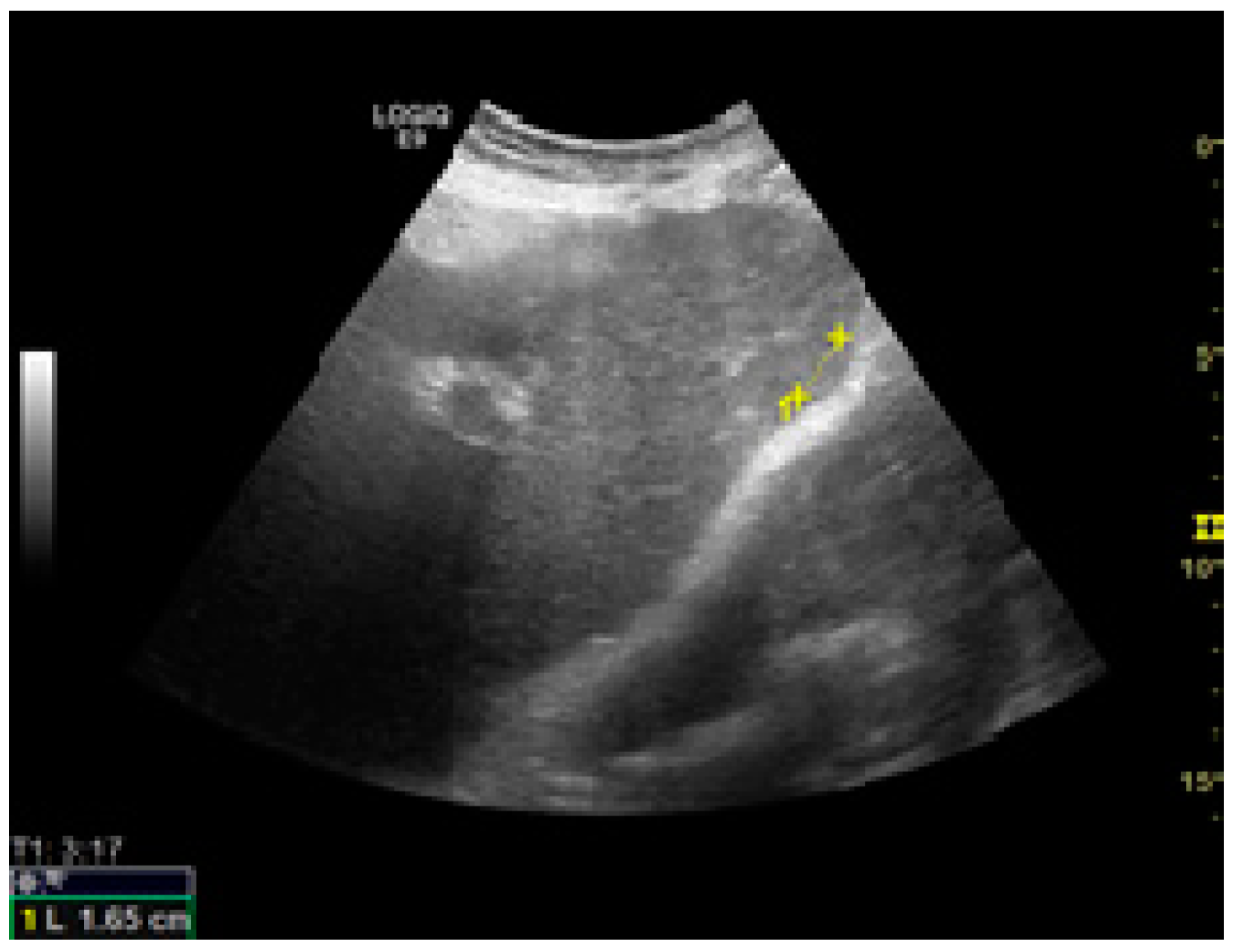

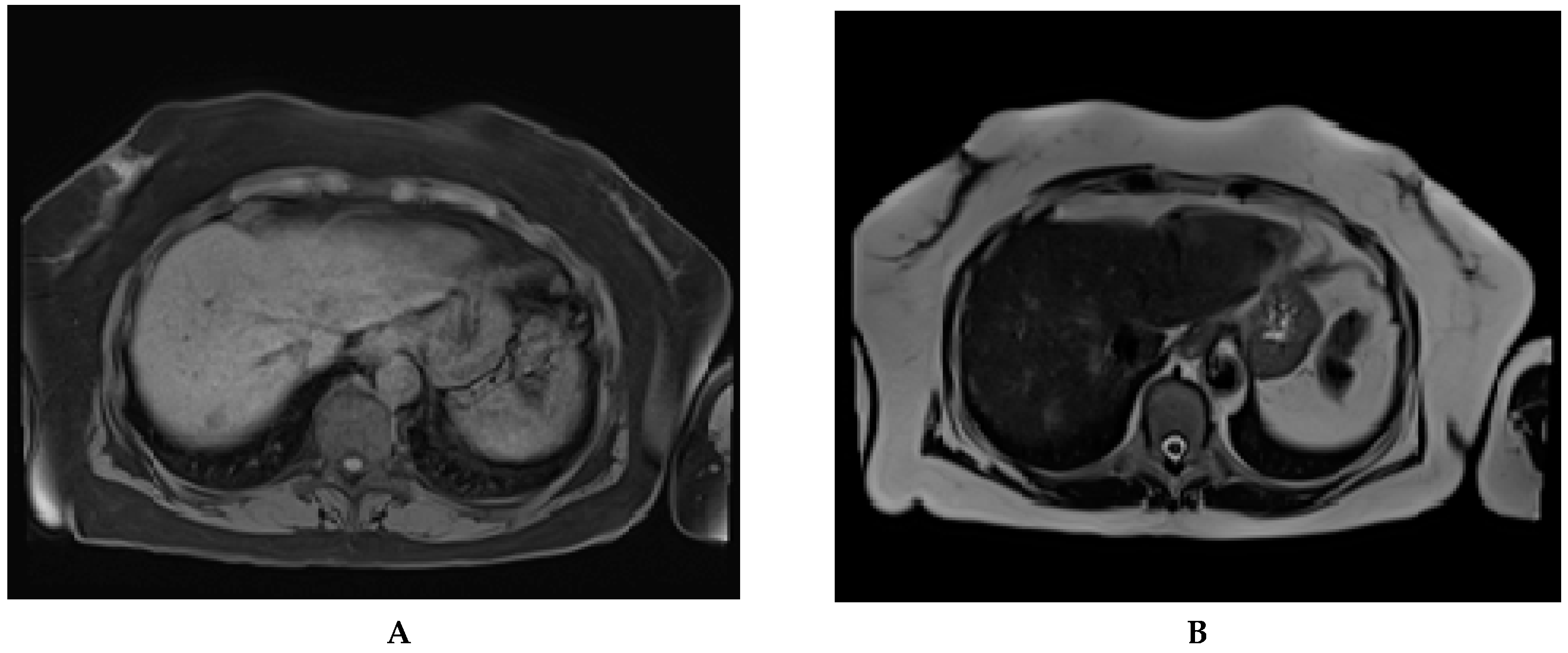
| Any Stage HCC | Early HCC | ||||||
|---|---|---|---|---|---|---|---|
| Author and Year | Period | Studies Included | Patients Included | Se %, (95 CI) | Sp % (95 CI) | Se% (95 CI) | Sp% (95 CI) |
| Colli et al. 2006 [47] | 1984–2003 | 14 | 7347 | 60.5 (44–76) | 96.6 (95–98) | NA | NA |
| Singal et al. 2009 [7] | 1985–2003 | 6 * 13 ** | 2984 * 3567 ** | 95 (89–98) | 91 (77–97) | 69 (50–83) | NA |
| Tzartzeva et al. 2018 [53] | 1990–2016 | 31 * 15 ** | 12997 | 84 (76–92) | NA | 47 (33–61) | NA |
| 1. Lesion (size, margins, echogenicity, location) |
| 2. Underlying liver disease (the aspect of parenchyma, etiology, severity) |
| 3. Patient status (bodyweight, abdominal fat, intra-abdominal gas, previous surgery) |
| 4. US expertise |
| 5. Quality of US machines |
| 6. Modality of screening (visit frequency) |
| Key Morphological Feature | Standard Image |
|---|---|
| Vasculature |
|
| |
| Biliary structures |
|
| |
| Left liver lobe |
|
| |
| Right liver lobe |
|
| |
| |
|
| Elements of the Screening Program | Current Recommendations | Possible Ways for Improvement [Sherman 2019] |
|---|---|---|
| Population at risk | Patients with advanced fibrosis or cirrhosis (Child A and B), regardless of etiology; Child C cirrhotics awaiting liver transplantation; HBV+ patients with intermediate /high PAGE-B risk scores [14] | Refine the risk assessment (when should screening start); Refine/individualize risk scores according to clinical scenarios (consider the effect of etiology/geography); Develop reliable (universal) biomarkers; |
| Screening tests | B mode ultrasound (US); [14,15,16,24] AFP (≥200 ng/dl) [16]; DCP, AFP-L3 [15] | Make use of technical advances in US assessment and emerging US-based examination modalities (elastography and contrast-enhanced US—CEUS); Determine the optimal level of the screening tests’ sensitivity that would impact cure rates and survival; |
| Screening interval | 6 months [14,15,16,24] 3–4 months (extremely high-risk patients) [15] | Individualize according to risk and clinical scenario; |
| Recall procedures | Improvement and standardization of confirmatory tests (cross-sectional imaging and/or biopsy) |
| Score | Author, Year | Clinical and Laboratory Parameters | Other Parameters |
|---|---|---|---|
| REACH-B | Cheng et al., 2006 [64] | age, gender, serum levels of ALT, HBe antigen status, and HBV DNA level | |
| CU-HCC | Wong et al., 2014 [65] | age, albumin, bilirubin, HBV DNA | Liver Stiffness Measurement (Fibroscan) |
| PAGE-B | Papatheodoridis et al., 2016 [66] | age, gender, platelets | |
| GALAD | Berhane et al., 2016 [67] | age, gender, AFP-L3, AFP, and DCP | |
| aMAP | Fan et al., 2020 [68] | age, gender, albumin, bilirubin, platelets |
| Etiology | Liver Stiffness Measurement Intervals (kPa) | Hazard Risk for Developing Hepatocellular Carcinoma |
|---|---|---|
| Hepatitis B | 8 | 3.0 |
| 13 | 4.6 | |
| 18 | 5.5 | |
| 23 | 6.6 | |
| Hepatitis C | 10 | 16.7 |
| 15 | 20.9 | |
| 20 | 25.6 | |
| 25 | 45.5 |
| Study | Year | Technique | Sensitivity | Specificity |
|---|---|---|---|---|
| Kim, et al. [95] | 2014 | Non-contrast MRI | 91% | 77% |
| Pocha, et al. [87] | 2013 | Contrast-enhanced CT | 87% | 87% |
| Van Thiel, et al. [88] | 2004 | Contrast-enhanced CT | 70% | 100% |
| Kim, et al. [54] | 2017 | Liver-specific contrast-enhanced MRI | 83% | Not available |
| Yu, et al. [90] | 2011 | Contrast-enhanced CT | 65% | 96% |
| Yu, et al. [90] | 2011 | Contrast-enhanced MRI | 72% | 87% |
| Chan, et al. [93] | 2019 | Non-contrast, abbreviated MRI | 85% | 93% |
| Besa, et al. [96] | 2017 | Contrast-enhanced abbreviated MRI | 80% | 87% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sparchez, Z.; Craciun, R.; Caraiani, C.; Horhat, A.; Nenu, I.; Procopet, B.; Sparchez, M.; Stefanescu, H.; Mocan, T. Ultrasound or Sectional Imaging Techniques as Screening Tools for Hepatocellular Carcinoma: Fall Forward or Move Forward? J. Clin. Med. 2021, 10, 903. https://doi.org/10.3390/jcm10050903
Sparchez Z, Craciun R, Caraiani C, Horhat A, Nenu I, Procopet B, Sparchez M, Stefanescu H, Mocan T. Ultrasound or Sectional Imaging Techniques as Screening Tools for Hepatocellular Carcinoma: Fall Forward or Move Forward? Journal of Clinical Medicine. 2021; 10(5):903. https://doi.org/10.3390/jcm10050903
Chicago/Turabian StyleSparchez, Zeno, Rares Craciun, Cosmin Caraiani, Adelina Horhat, Iuliana Nenu, Bogdan Procopet, Mihaela Sparchez, Horia Stefanescu, and Tudor Mocan. 2021. "Ultrasound or Sectional Imaging Techniques as Screening Tools for Hepatocellular Carcinoma: Fall Forward or Move Forward?" Journal of Clinical Medicine 10, no. 5: 903. https://doi.org/10.3390/jcm10050903
APA StyleSparchez, Z., Craciun, R., Caraiani, C., Horhat, A., Nenu, I., Procopet, B., Sparchez, M., Stefanescu, H., & Mocan, T. (2021). Ultrasound or Sectional Imaging Techniques as Screening Tools for Hepatocellular Carcinoma: Fall Forward or Move Forward? Journal of Clinical Medicine, 10(5), 903. https://doi.org/10.3390/jcm10050903







