Sleeve Gastrectomy Is Associated with a Greater Reduction in Plasma Liver Enzymes Than Bypass Surgeries—A Registry-Based Two-Year Follow-Up Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistics
3. Results
3.1. Study Population
3.2. Sleeve Gastrectomy Is Associated with Lower ALT Levels Two Years after Surgery
3.3. Retrospective Matching of Patients Shows That SG Is Superior in Reducing ALT Levels
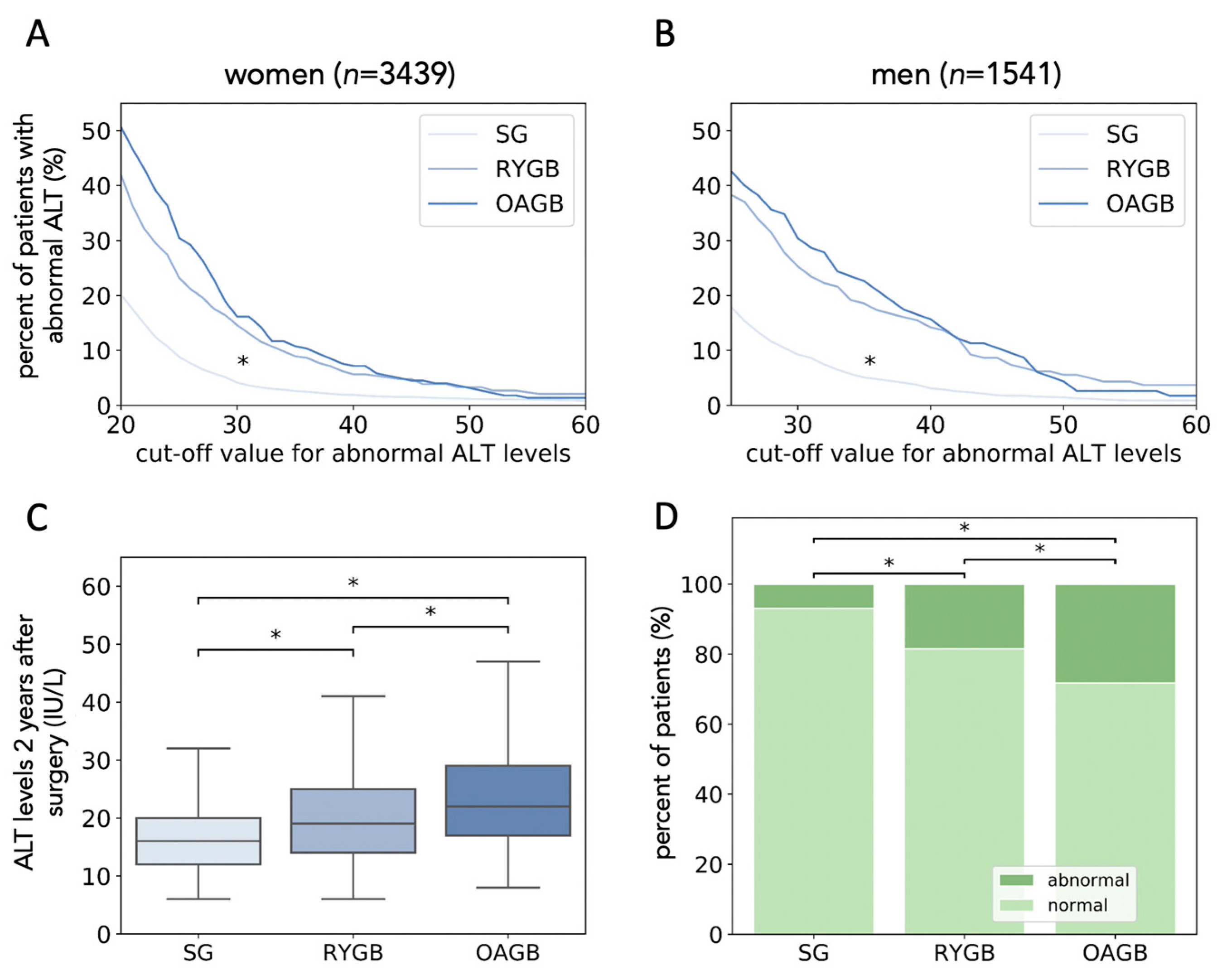
3.4. Surgery Type Has a Strong Effect on the Occurrence of High ALT Levels Two Years after Surgery
3.5. Sleeve Gastrectomy Is Superior to Bypass Surgery in Normalizing ALT Levels
3.6. Bypass Surgery Is Associated with New-Onset of High ALT Levels Despite Weight-Loss
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laursen, T.L.; Hagemann, C.A.; Wei, C.; Kazankov, K.; Thomsen, K.L.; Knop, F.K.; Grønbæk, H. Bariatric surgery in patients with non-alcoholic fatty liver disease—From pathophysiology to clinical effects. World J. Hepatol. 2019, 11, 138–249. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Doumouras, A.G.; Yu, J.; Brar, K.; Banfield, L.; Gmora, S.; Anvari, M.; Hong, D. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1040–1060.e11. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Baud, G.; Verkindt, H.; Ningarhari, M.; Louvet, A.; Leteurtre, E.; Raverdy, V.; Dharancy, S.; Pattou, F.; et al. Bariatric Surgery Provides Long-term Resolution of Nonalcoholic Steatohepatitis and Regression of Fibrosis. Gastroenterology 2020, 159, 1290–1301.e5. [Google Scholar] [CrossRef]
- Kahramanoǧlu Aksoy, E.; Göktaş, Z.; Albuz, Ö.; Akplnar, M.Y.; Öztürk, D.; Buluş, H.; Uzman, M. Effects of sleeve gastrectomy on liver enzymes, non-alcoholic fatty liver disease-related fibrosis and steatosis scores in morbidly obese patients: First year follow-up. J. Lab. Med. 2019, 43, 115–122. [Google Scholar] [CrossRef]
- Hafeez, S.; Ahmed, M.H. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: A future treatment by choice or by chance? J. Obes. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Burza, M.A.; Romeo, S.; Kotronen, A.; Svensson, P.-A.; Sjöholm, K.; Torgerson, J.S.; Lindroos, A.-K.; Sjöström, L.; Carlsson, L.M.S.; Peltonen, M. Long-Term Effect of Bariatric Surgery on Liver Enzymes in the Swedish Obese Subjects (SOS) Study. PLoS ONE 2013, 8, e60495. [Google Scholar] [CrossRef]
- Xourafas, D.; Ardestani, A.; Ashley, S.W.; Tavakkoli, A. Impact of weight-loss surgery and diabetes status on serum ALT levels. Obes. Surg. 2012, 22, 1540–1547. [Google Scholar] [CrossRef][Green Version]
- Caiazzo, R.; Lassailly, G.; Leteurtre, E.; Baud, G.; Verkindt, H.; Raverdy, V.; Buob, D.; Pigeyre, M.; Mathurin, P.; Pattou, F. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: A 5-Year Controlled Longitudinal Study. Ann. Surg. 2014, 260, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Nickel, F.; Tapking, C.; Benner, L.; Sollors, J.; Billeter, A.T.; Kenngott, H.G.; Bokhary, L.; Schmid, M.; von Frankenberg, M.; Fischer, L.; et al. Bariatric Surgery as an Efficient Treatment for Non-Alcoholic Fatty Liver Disease in a Prospective Study with 1-Year Follow-up: BariScan Study. Obes. Surg. 2018, 28, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.S.; Wong, G.L.H.; Chan, R.S.M.; Shu, S.S.T.; Cheung, B.H.K.; Li, L.S.; Chim, A.M.L.; Chan, C.K.M.; Leung, J.K.Y.; Chu, W.C.W.; et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.; Wing, R.R. Randomized Controlled Trial Testing the Effects of Weight Loss on Nonalcoholic Steatohepatitis (NASH). Hepatology 2010, 51, 121. [Google Scholar] [CrossRef]
- Stefater, M.A.; Wilson-Pérez, H.E.; Chambers, A.P.; Sandoval, D.A.; Seeley, R.J. All bariatric surgeries are not created equal: Insights from mechanistic comparisons. Endocr. Rev. 2012, 33, 595–622. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; Brethauer, S.A.; Andalib, A.; Punchai, S.; Mackey, J.; Rodriguez, J.; Rogula, T.; Kroh, M.; Schauer, P.R. Can Sleeve Gastrectomy “Cure” Diabetes? Long-term Metabolic Effects of Sleeve Gastrectomy in Patients With Type 2 Diabetes. Ann. Surg. 2016, 264, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. [Google Scholar] [CrossRef]
- Sjöström, L.; Narbro, K.; Sjöström, C.D.; Karason, K.; Larsson, B.; Wedel, H.; Lystig, T.; Sullivan, M.; Bouchard, C.; Carlsson, B.; et al. Effects of Bariatric Surgery on Mortality in Swedish Obese Subjects. N. Engl. J. Med. 2007, 357, 741–752. [Google Scholar] [CrossRef]
- Sjostrom, L. Review of the key results from the Swedish Obese Subjects (SOS) trial—A prospective controlled intervention study of bariatric surgery. J. Intern. Med. 2013, 273, 219–234. [Google Scholar] [CrossRef]
- Salman, M.A.; Salman, A.A.; Omar, H.S.E.; Abdelsalam, A.; Mostafa, M.S.; Tourky, M.; Sultan, A.A.E.A.; Elshafey, M.H.; Abdelaty, W.R.; Salem, A.; et al. Long-term effects of one-anastomosis gastric bypass on liver histopathology in NAFLD cases: A prospective study. Surg. Endosc. 2020, 1–6. [Google Scholar] [CrossRef]
- Spivak, H.; Munz, Y.; Rubin, M.; Raz, I.; Shohat, T.; Blumenfeld, O. Omega-loop gastric bypass is more effective for weight loss but negatively impacts liver enzymes: A registry-based comprehensive first-year analysis. Surg. Obes. Relat. Dis. 2018, 14, 175–180. [Google Scholar] [CrossRef]
- Kaplan, U.; Romano-Zelekha, O.; Goitein, D.; Keren, D.; Gralnek, I.M.; Boker, L.K.; Sakran, N. Trends in Bariatric Surgery: A 5-Year Analysis of the Israel National Bariatric Surgery Registry. Obes. Surg. 2020, 30, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, O.; Goitein, D.; Liverant-Taub, S.; Diker, D.; Sakran, N.; Keinan-Boker, L. The Israel National Bariatric Surgery Registry: The inception process. Surg. Obes. Relat. Dis. 2020, 16, 80–89. [Google Scholar] [CrossRef]
- Kwo, P.Y.; Cohen, S.M.; Lim, J.K. ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries. Am. J. Gastroenterol. 2017, 112, 18–35. [Google Scholar] [CrossRef]
- Newsome, P.N.; Cramb, R.; Davison, S.M.; DIllon, J.F.; Foulerton, M.; Godfrey, E.M.; Hall, R.; Harrower, U.; Hudson, M.; Langford, A.; et al. Guidelines on the management of abnormal liver blood tests. Gut 2018, 67, 6–19. [Google Scholar] [CrossRef]
- Liver Function | Johns Hopkins Diabetes Guide. Available online: https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_Diabetes_Guide/547086/all/Liver_function (accessed on 16 October 2020).
- Liver Test Interpretation—Approach to the Patient with Liver Disease: A Guide to Commonly Used Liver Tests. Available online: https://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/hepatology/guide-to-common-liver-tests/ (accessed on 16 October 2020).
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 8–14 July 2010. [Google Scholar]
- Pedregosa, F.; Michel, V.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Vanderplas, J.; Cournapeau, D.; Pedregosa, F.; Varoquaux, G.; et al. Scikit-Learn: Machine Learning in Python; Packt Publishing Ltd.: Birmingham, UK, 2011; Volume 12. [Google Scholar]
- Billeter, A.T.; Senft, J.; Gotthardt, D.; Knefeli, P.; Nickel, F.; Schulte, T.; Fischer, L.; Nawroth, P.P.; Büchler, M.W.; Müller-Stich, B.P. Combined Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Sleeve Gastrectomy or Gastric Bypass?—A Controlled Matched Pair Study of 34 Patients. Obes. Surg. 2016, 26, 1867–1874. [Google Scholar] [CrossRef]
- Kalinowski, P.; Paluszkiewicz, R.; Ziarkiewicz-Wróblewska, B.; Wróblewski, T.; Remiszewski, P.; Grodzicki, M.; Krawczyk, M. Liver Function in Patients with Nonalcoholic Fatty Liver Disease Randomized to Roux-en-Y Gastric Bypass Versus Sleeve Gastrectomy. Ann. Surg. 2017, 266, 738–745. [Google Scholar] [CrossRef]
- Froylich, D.; Corcelles, R.; Daigle, C.; Boules, M.; Brethauer, S.; Schauer, P. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: A comparative study. In Proceedings of the Surgery for Obesity and Related Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2016; Volume 12, pp. 127–131. [Google Scholar]
- Raj, P.; Gomes, R.; Kumar, S.; Senthilnathan, P.; Karthikeyan, P.; Shankar, A.; Palanivelu, C. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians: “NASHOST” prospective observational trial. Surg. Obes Relat Dis. 2015, 11, 1315–1322. [Google Scholar]
- von Schönfels, W.; Beckmann, J.; Ahrens, M.; Hendricksm, A.; Röcken, C.; Szymczak, S.; Hampe, J.; Schafmayer, C. Histologic improvement of NAFLD in patients with obesity after bariatric surgery based on standardized NAS (NAFLD activity score). Surg. Obes Relat Dis. 2014, 14, 1607–1616. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Kahn, S.E. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef]
- Bhatt, H.B.; Smith, R.J. Fatty liver disease in diabetes mellitus. Hepatobiliary Surg. Nutr. 2015, 4, 101–108. [Google Scholar] [CrossRef]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar] [CrossRef]
- Benjaminov, O.; Beglaibter, N.; Gindy, L.; Spivak, H.; Singer, P.; Wienberg, M.; Stark, A.; Rubin, M. The effect of a low-carbohydrate diet on the nonalcoholic fatty liver in morbidly obese patients before bariatric surgery. Surg. Endosc. Other Interv. Tech. 2007, 21, 1423–1427. [Google Scholar] [CrossRef]
- Keleidari, B.; Mahmoudieh, M.; Gorgi, K.; Sheikhbahaei, E.; Shahabi, S. Hepatic failure after bariatric surgery: A systematic review. Hepat. Mon. 2019, 19, e86078. [Google Scholar] [CrossRef]
- Geerts, A.; Darius, T.; Chapelle, T.; Roeyen, G.; Francque, S.; Libbrecht, L.; Nevens, F.; Pirenne, J.; Troisi, R. The multicenter belgian survey on liver transplantation for hepatocellular failure after bariatric surgery. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2010; Volume 42, pp. 4395–4398. [Google Scholar]
- Eilenberg, M.; Langer, F.B.; Beer, A.; Trauner, M.; Prager, G.; Staufer, K. Significant Liver-Related Morbidity After Bariatric Surgery and Its Reversal—A Case Series. Obes. Surg. 2018, 28, 812–819. [Google Scholar] [CrossRef]
- D’Albuquerque, L.A.C.; Gonzalez, A.M.; Wahle, R.C.; Souza, E.d.O.; Mancero, J.M.P.; e Silva, A.d.O. Liver transplantation for subacute hepatocellular failure due to massive steatohepatitis after bariatric surgery. Liver Transplant. 2008, 14, 881–885. [Google Scholar] [CrossRef]
- Murphy, R.; Tsai, P.; Jullig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2017, 27, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, J.; Zhou, Z. Changes in bile acid profiles after Roux-ex-Y gastric bypass and sleeve gastrectomy. Medicine 2021, 100, e23939. [Google Scholar] [CrossRef]
- Thulin, P.; Rafter, I.; Stockling, K.; Tomkiewicz, C.; Norjavaara, E.; Aggerbeck, M.; Hellmond, H.; Ehrenborg, E.; Andersson, U.; Cotgreave, I.; et al. PPARalpha regulates the hepatotoxic biomarker alanine aminotransferase (ALT1) gene expression in human hepatocytes. Toxicol. Appl. Pharmacol. 2008, 234, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.; Ammerpohl, O.; von Schönfels, W.; Kolarova, J.; Bens, S.; Itzel, T.; Teufel, A.; Herrmann, A.; Brosch, M.; Hinrichsen, V.; et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013, 18, 296–302. [Google Scholar] [CrossRef]
- Ben-Zvi, D.; Meoli, L.; Abidi, W.; Nestoridi, E.; Panciotti, C.; Castillo, E.; Pizarro, P.; Shirley, E.; Gourash, W.; Thompson, C.; et al. Time-Dependent Molecular Responses Differ between Gastric Bypass and Dieting, but Are Conserved across Species. Cell Metab. 2018, 28, 310–323. [Google Scholar] [CrossRef]
- McLaren, L. Socioeconomic status and obesity. Epidemiol. Rev. 2007, 29, 29–48. [Google Scholar] [CrossRef]
- Sobal, J.; Stunkard, A.J. Socioeconomic Status and Obesity: A Review of the Literature. Psychol. Bull. 1989, 105, 260–275. [Google Scholar] [CrossRef]

| Parameter | SG (n = 4144) | RYGB (n = 498) | OAGB (n = 338) |
|---|---|---|---|
| ALT (IU/L) pre-surgery, Female | 22 (17–32) | 23 (16–33) | 22 (16–28) |
| ALT (IU/L) 2 years post-surgery, Female * | 14 (11–19) | 19 (14–25) | 21 (16.5–28) |
| ALT (IU/L) pre-surgery, Male | 33 (25–48) | 30 (22–39.75) | 32 (23.5–43.5) |
| ALT (IU/L) 2 years post-surgery, Male * | 18 (14–23) | 22 (17–30.75) | 24 (19.5–33) |
| AST (IU/L) pre-surgery | 22 (18–29) | 23 (18–29) | 22 (19–28) |
| AST (IU/L) 2 years post-surgery ** | 18 (15–22) | 22 (18–27) | 24 (20–30) |
| Patients with abnormal ALT levels Pre-surgery | 1741 (42%) | 202 (41%) | 133 (39%) |
| Patients with abnormal ALT levels 2 years post-surgery * | 336 (8%) | 113 (23%) | 96 (28%) |
| Normal ALT Levels Post-Surgery (n = 4435) | Abnormal ALT Levels Post-Surgery (n = 545) | p Value | |
|---|---|---|---|
| Age (years) | 44.98 (35.33–54.87) | 49.53 (38.09–57.29) | <0.001 |
| Sex—female | 3029 (69%) | 400 (73%) | 0.01 |
| Ethnicity (Jewish) | 3705 (84%) | 488 (90%) | <0.001 |
| BMI (kg/m2) pre-surgery | 41.14 (38.61–44.46) | 40.86 (37.96–44.52) | 0.1 |
| A1C (%) pre-surgery | 5.9 (5.5–6.6) | 6 (5.6–6.8) | <0.001 |
| ALT (IU/L) pre-surgery | 25 (18–36) | 31 (22–45) | <0.001 |
| TG (mg/dL) | 147 (108–203) | 152 (110–211.7) | 0.07 |
| Surgery type (SG, RYGB, OAGB) | 3797 (86%) 384 (9%) 241 (5%) | 336 (61.7%) 113 (20.7%) 96 (17.6%) | <0.001 |
| Alcohol consumption | 96 (22%) | 14 (26%) | 0.52 |
| Smoking | 823 (19%) | 108 (20%) | 0.47 |
| Hypertension | 1644 (37%) | 230 (42%) | 0.02 |
| OSA | 740 (17%) | 98 (18%) | 0.44 |
| β | SE β | Wald’s χ2 | p-Value | OR | CI | |
|---|---|---|---|---|---|---|
| Constant | −3.39 | 0.42 | 64.63 | <1 × 10−5 | NA | NA |
| RYGB vs. SG | 1.11 | 0.26 | 17.78 | <1 × 10−5 | 3.05 | (1.82, 5.16) |
| OAGB vs. SG | 1.69 | 0.25 | 44.01 | <1 × 10−5 | 5.40 | (3.28, 8.89) |
| OAGB vs. RYGB | 0.57 | 0.19 | 8.94 | <0.05 | 1.77 | (1.22, 2.58) |
| Age (years) | 0.02 | 0.01 | 4.98 | <0.05 | 1.02 | (1.00, 1.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azulai, S.; Grinbaum, R.; Beglaibter, N.; Eldar, S.M.; Rubin, M.; Ben-Haroush Schyr, R.; Romano-Zelekha, O.; Ben-Zvi, D. Sleeve Gastrectomy Is Associated with a Greater Reduction in Plasma Liver Enzymes Than Bypass Surgeries—A Registry-Based Two-Year Follow-Up Analysis. J. Clin. Med. 2021, 10, 1144. https://doi.org/10.3390/jcm10051144
Azulai S, Grinbaum R, Beglaibter N, Eldar SM, Rubin M, Ben-Haroush Schyr R, Romano-Zelekha O, Ben-Zvi D. Sleeve Gastrectomy Is Associated with a Greater Reduction in Plasma Liver Enzymes Than Bypass Surgeries—A Registry-Based Two-Year Follow-Up Analysis. Journal of Clinical Medicine. 2021; 10(5):1144. https://doi.org/10.3390/jcm10051144
Chicago/Turabian StyleAzulai, Shira, Ronit Grinbaum, Nahum Beglaibter, Shai Meron Eldar, Moshe Rubin, Rachel Ben-Haroush Schyr, Orly Romano-Zelekha, and Danny Ben-Zvi. 2021. "Sleeve Gastrectomy Is Associated with a Greater Reduction in Plasma Liver Enzymes Than Bypass Surgeries—A Registry-Based Two-Year Follow-Up Analysis" Journal of Clinical Medicine 10, no. 5: 1144. https://doi.org/10.3390/jcm10051144
APA StyleAzulai, S., Grinbaum, R., Beglaibter, N., Eldar, S. M., Rubin, M., Ben-Haroush Schyr, R., Romano-Zelekha, O., & Ben-Zvi, D. (2021). Sleeve Gastrectomy Is Associated with a Greater Reduction in Plasma Liver Enzymes Than Bypass Surgeries—A Registry-Based Two-Year Follow-Up Analysis. Journal of Clinical Medicine, 10(5), 1144. https://doi.org/10.3390/jcm10051144






