Hodgkin Lymphoma—Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients
Abstract
1. Introduction
2. Epidemiology and Risk Factors
3. Pathophysiology
4. Diagnosis and Staging
5. Treatment Strategies
5.1. Classical Hodgkin Lymphoma (cHL)
5.1.1. First-Line Treatment
Early-Stage Favorable cHL
Early-Stage Unfavorable cHL
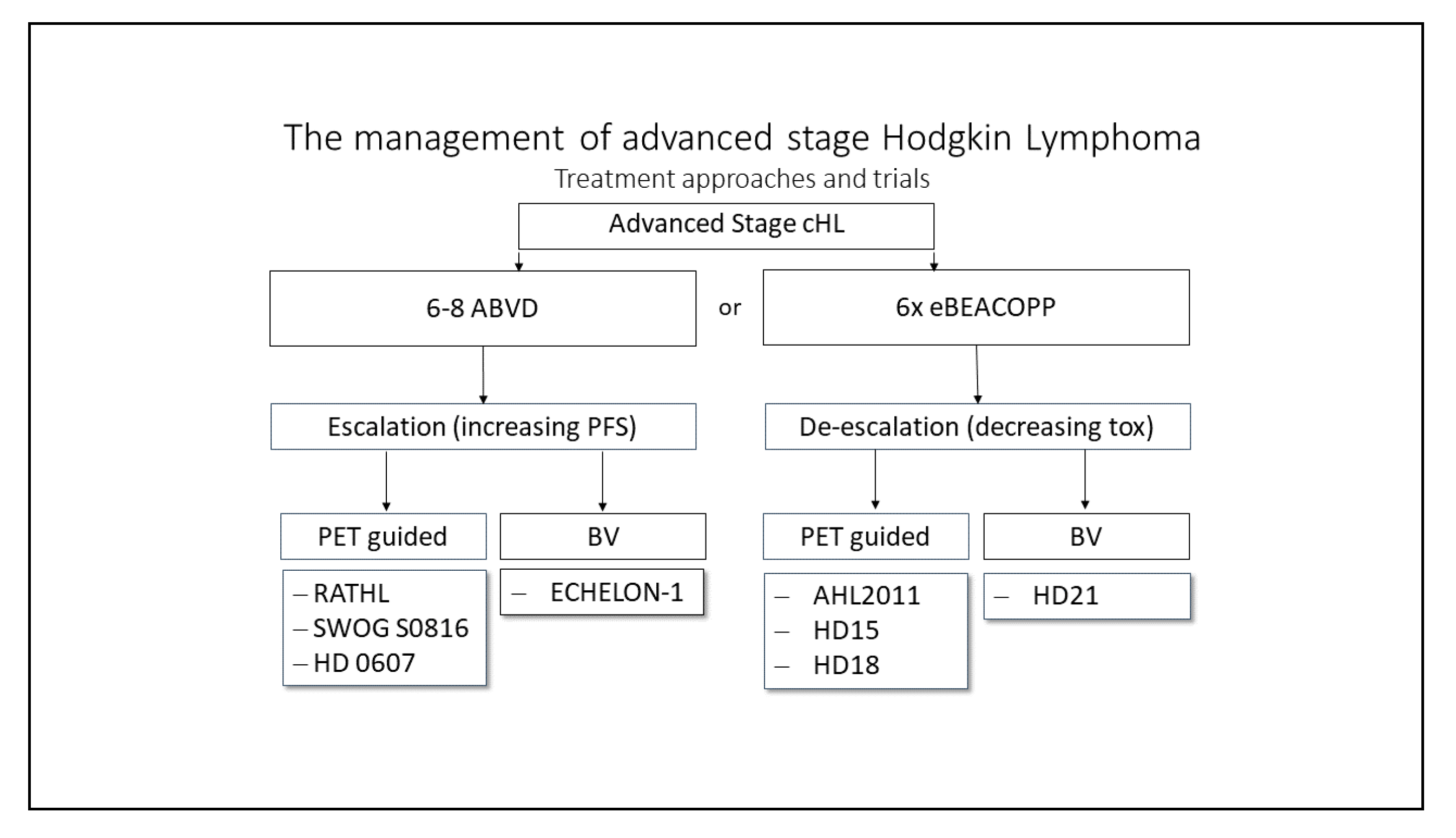
Advanced-Stage cHL
Deescalating Strategies
Escalating Approaches
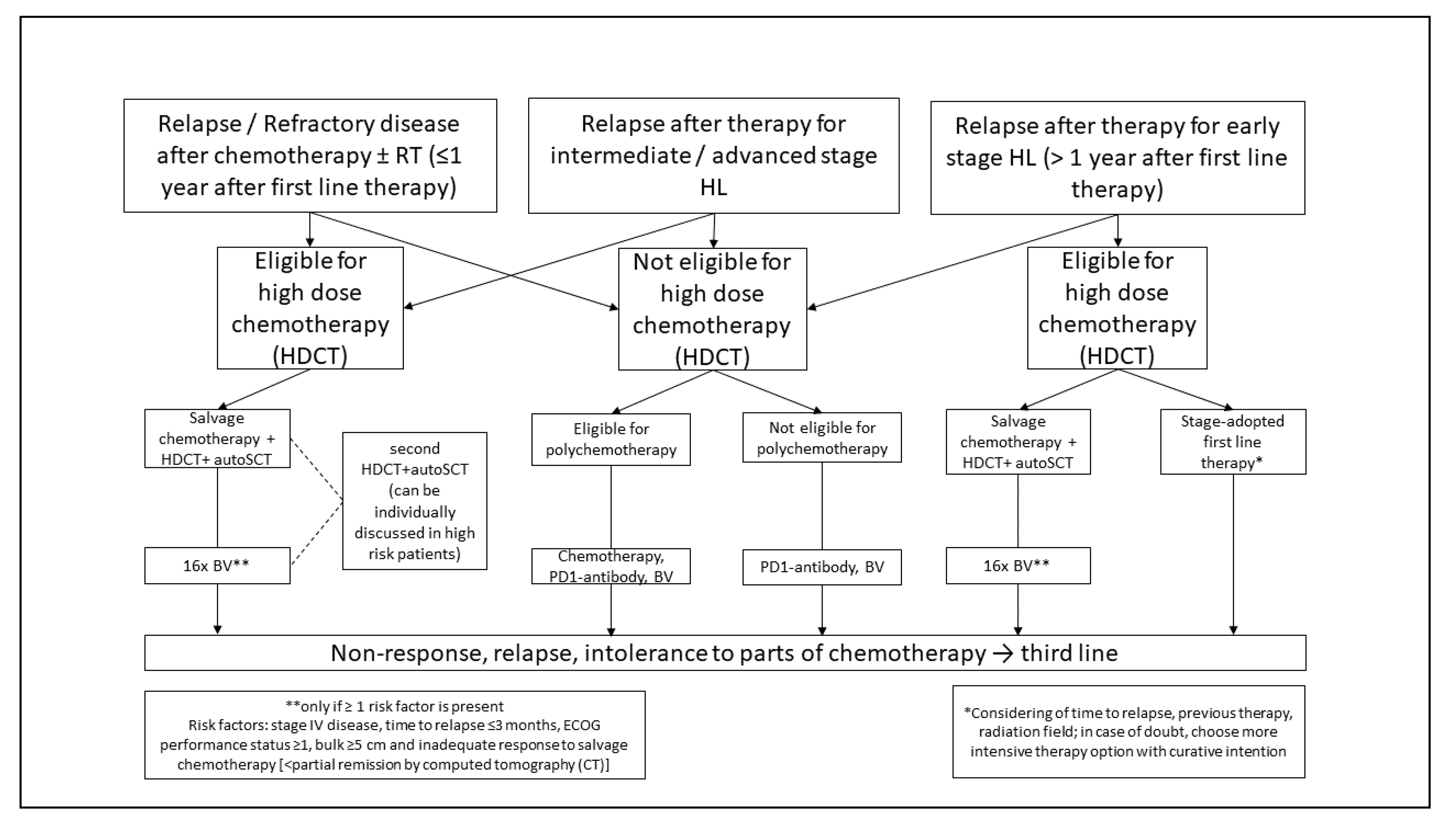
5.1.2. Relapsed or Refractory cHL
Second Line Treatment
Relapse After Second Line Treatment
5.2. Nodular Lymphocyte-Predominant HL (NLPHL)
5.2.1. First Line Treatment
5.2.2. Relapsed NLPHL
5.3. Treatment of Elderly Patients
6. Future Treatment Approaches
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975–2013. Available online: http://seer.cancer.gov/csr/1975_2013 (accessed on 22 October 2016).
- Mack, T.M.; Cozen, W.; Shibata, D.K.; Weiss, L.M.; Nathwani, B.N.; Hernandez, A.M.; Taylor, C.R.; Hamilton, A.S.; Deapen, D.M.; Rappaport, E.B. Concordance for Hodgkin’s disease in identical twins suggesting genetic susceptibility to the young-adult form of the disease. N. Engl. J. Med. 1995, 332, 413–418. [Google Scholar] [CrossRef]
- Weiss, L.M.; Strickler, J.G.; Warnke, R.A.; Purtilo, D.T.; Sklar, J. Epstein-Barr viral DNA in tissues of Hodgkin’s disease. Am. J. Pathol. 1987, 129, 86–91. [Google Scholar]
- Kowalkowski, M.A.; Mims, M.P.; Amiran, E.S.; Lulla, P.; Chiao, E.Y. Effect of immune reconstitution on the incidence of HIV-related Hodgkin lymphoma. PLoS ONE 2013, 8, e77409. [Google Scholar] [CrossRef]
- Patel, P.; Hanson, D.L.; Sullivan, P.S.; Novak, R.M.; Moorman, A.C.; Tong, T.C.; Holmberg, S.D.; Brooks, J.T. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann. Intern. Med. 2008, 148, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R.; Engert, A.; Hansmann, M.-L. Hodgkin lymphoma. J. Clin. Investig. 2012, 122, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, J.; Halthur, C.; Kristinsson, S.Y.; Landgren, O.; Nygell, U.A.; Dickman, P.W.; Bjorkholm, M. Progress in Hodgkin lymphoma: A population-based study on patients diagnosed in Sweden from 1973–2009. Blood 2012, 119, 990–996. [Google Scholar] [CrossRef]
- Engert, A.; Diehl, V.; Franklin, J.; Lohri, A.; Dorken, B.; Ludwig, W.-D.; Koch, P.; Hanel, M.; Pfreundschuh, M.; Wilhelm, M.; et al. Escalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4548–4554. [Google Scholar] [CrossRef]
- Sasse, S.; Brockelmann, P.J.; Goergen, H.; Plutschow, A.; Muller, H.; Kreissl, S.; Buerkle, C.; Borchmann, S.; Fuchs, M.; Borchmann, P.; et al. Long-Term Follow-Up of Contemporary Treatment in Early-Stage Hodgkin Lymphoma: Updated Analyses of the German Hodgkin Study Group HD7, HD8, HD10, and HD11 Trials. J. Clin. Oncol. 2017, 35, 1999–2007. [Google Scholar] [CrossRef]
- Akpek, G.; Ambinder, R.F.; Piantadosi, S.; Abrams, R.A.; Brodsky, R.A.; Vogelsang, G.B.; Zahurak, M.L.; Fuller, D.; Miller, C.B.; Noga, S.J.; et al. Long-term results of blood and marrow transplantation for Hodgkin’s lymphoma. J. Clin. Oncol. 2001, 19, 4314–4321. [Google Scholar] [CrossRef]
- Rapoport, A.P.; Guo, C.; Badros, A.; Hakimian, R.; Akpek, G.; Kiggundu, E.; Meisenberg, B.; Mannuel, H.; Takebe, N.; Fenton, R.; et al. Autologous stem cell transplantation followed by consolidation chemotherapy for relapsed or refractory Hodgkin’s lymphoma. Bone Marrow Transplant. 2004, 34, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Storm, H.H.; Klint, A.; Tryggvadottir, L.; Gislum, M.; Engholm, G.; Bray, F.; Hakulinen, T. Trends in the survival of patients diagnosed with malignant neoplasms of lymphoid, haematopoietic, and related tissue in the Nordic countries 1964-2003 followed up to the end of 2006. Acta Oncol. 2010, 49, 694–712. [Google Scholar] [CrossRef]
- Swerdlow, A.J. Epidemiology of Hodgkin’s disease and non-Hodgkin’s lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30 (Suppl. 1), S3–S12. [Google Scholar] [CrossRef]
- Clarke, C.A.; Glaser, S.L.; Keegan, T.H.M.; Stroup, A. Neighborhood socioeconomic status and Hodgkin’s lymphoma incidence in California. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2005, 14, 1441–1447. [Google Scholar] [CrossRef]
- Pallesen, G.; Hamilton-Dutoit, S.J.; Rowe, M.; Young, L.S. Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin’s disease. Lancet 1991, 337, 320–322. [Google Scholar] [CrossRef]
- Hernández-Ramírez, R.U.; Shiels, M.S.; Dubrow, R.; Engels, E.A. Cancer risk in HIV-infected people in the USA from 1996 to 2012: A population-based, registry-linkage study. Lancet HIV 2017, 4, e495–e504. [Google Scholar] [CrossRef]
- Biggar, R.J.; Jaffe, E.S.; Goedert, J.J.; Chaturvedi, A.; Pfeiffer, R.; Engels, E.A. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood 2006, 108, 3786–3791. [Google Scholar] [CrossRef]
- Küppers, R.; Rajewsky, K.; Zhao, M.; Simons, G.; Laumann, R.; Fischer, R.; Hansmann, M.L. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc. Natl. Acad. Sci. USA 1994, 91, 10962–10966. [Google Scholar] [CrossRef]
- Klien, U.; Goasens, T.; Fischer, M.; Kanzler, H.; Braeuninger, A.; Rajewsky, K.; Küppers, R. Somatic hypermutation in normal and transformed human B cells. Immunol. Rev. 1998, 162, 261–280. [Google Scholar] [CrossRef]
- Kanzler, H.; Küppers, R.; Hansmann, M.L.; Rajewsky, K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J. Exp. Med. 1996, 4, 1495–1505. [Google Scholar] [CrossRef]
- Schwering, I.; Brauninger, A.; Klein, U.; Jungnickel, B.; Tinguely, M.; Diehl, V.; Hansmann, M.-L.; Dalla-Favera, R.; Rajewsky, K.; Kuppers, R. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood 2003, 101, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.R.; Shipp, M.A. Signaling pathways and immune evasion mechanisms in classical Hodgkin lymphoma. Blood 2017, 130, 2265–2270. [Google Scholar] [CrossRef] [PubMed]
- Steidl, C.; Shah, S.P.; Woolcock, B.W.; Rui, L.; Kawahara, M.; Farinha, P.; Johnson, N.A.; Zhao, Y.; Telenius, A.; Neriah, S.B.; et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature 2011, 471, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Reichel, J.B.; McCormick, J.; Fromm, J.R.; Elemento, O.; Cesarman, E.; Roshal, M. Flow-sorting and Exome Sequencing of the Reed-Sternberg Cells of Classical Hodgkin Lymphoma. J. Vis. Exp. JoVE 2017, 54399. [Google Scholar] [CrossRef]
- Kessler, J.; Reiners, K.S.; Sauer, M.; Engert, A.; von Strandmann, E.P. NK Cells in Hodgkin Lymphoma Are Impaired but Can Be Activated. Blood 2011, 118, 2182. [Google Scholar] [CrossRef]
- Cader, F.Z.; Schackmann, R.C.J.; Hu, X.; Wienand, K.; Redd, R.; Chapuy, B.; Ouyang, J.; Paul, N.; Gjini, E.; Lipschitz, M.; et al. Mass cytometry of Hodgkin lymphoma reveals a CD4(+) regulatory T-cell-rich and exhausted T-effector microenvironment. Blood 2018, 132, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.D.; Gusenleitner, D.; Lipschitz, M.; Roemer, M.G.M.; Stack, E.C.; Gjini, E.; Hu, X.; Redd, R.; Freeman, G.J.; Neuberg, D.; et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017, 130, 2420–2430. [Google Scholar] [CrossRef]
- Hehn, S.T.; Grogan, T.M.; Miller, T.P. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J. Clin. Oncol. off. J. Am. Soc. Clin. Oncol. 2004, 22, 3046–3052. [Google Scholar] [CrossRef]
- Hutchings, M.; Loft, A.; Hansen, M.; Pedersen, L.M.; Berthelsen, A.K.; Keiding, S.; D’Amore, F.; Boesen, A.M.; Roemer, L.; Specht, L. Position emission tomography with or without computed tomography in the primary staging of Hodgkin’s lymphoma. Haematologica 2006, 91, 482–489. [Google Scholar]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Mueller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- El-Galaly, T.C.; d’Amore, F.; Mylam, K.J.; Brown, P.d.N.; Bøgsted, M.; Bukh, A.; Specht, L.; Loft, A.; Iyer, V.; Hjorthaug, K.; et al. Routine Bone Marrow Biopsy Has Little or No Therapeutic Consequence for Positron Emission Tomography/Computed Tomography–Staged Treatment-Naive Patients with Hodgkin Lymphoma. J. Clin. Oncol. 2012, 30, 4508–4514. [Google Scholar] [CrossRef]
- Weiler-Sagie, M.; Kagna, O.; Dann, E.J.; Ben-Barak, A.; Israel, O. Characterizing bone marrow involvement in Hodgkin’s lymphoma by FDG-PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1133–1140. [Google Scholar] [CrossRef]
- Chen-Liang, T.H.; Martin-Santos, T.; Jerez, A.; Senent, L.; Orero, M.T.; Remigia, M.J.; Muiña, B.; Romera, M.; Fernandez-Muñoz, H.; Raya, J.M.; et al. The role of bone marrow biopsy and FDG-PET/CT in identifying bone marrow infiltration in the initial diagnosis of high grade non-Hodgkin B-cell lymphoma and Hodgkin lymphoma. Accuracy in a multicenter series of 372 patients. Am. J. Hematol. 2015, 90, 686–690. [Google Scholar] [CrossRef]
- Bröckelmann, P.J.; Engert, A. The GHSG Approach to Treating Hodgkin’s Lymphoma. Curr. Hematol. Malig. Rep. 2015, 10, 256–265. [Google Scholar] [CrossRef]
- Aleman, B.M.P.; van den Belt-Dusebout, A.W.; Bruin, M.L.d.; van ‘t Veer, M.B.; Baaijens, M.H.A.; Boer, J.P.d.; Hart, A.A.M.; Klokman, W.J.; Kuenen, M.A.; Ouwens, G.M.; et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007, 109, 1878–1886. [Google Scholar] [CrossRef]
- Andrea, K.; Bernardo, M.V.P.; Weller, E.; Backstrand, K.; Silver, B.; Marcus, K.C.; Tarbell, N.J.; Stevenson, M.A.; Friedberg, J.W.; Mauch, P.M. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: Long-term risks and risk factors. Blood 2002, 100, 1989–1996. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Aleman, B.M.P.; Andre, M.; Federico, M.; Hutchings, M.; Illidge, T.; Engert, A.; Ladetto, M.; Committee, E.G. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv19–iv29. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Vander Els, N.; Straus, D.J.; Gomez, E.G.; Leung, D.; Portlock, C.S.; Yahalom, J. Effect of ABVD chemotherapy with and without mantle or mediastinal irradiation on pulmonary function and symptoms in early-stage Hodgkin’s disease. J. Clin. Oncol. off. J. Am. Soc. Clin. Oncol. 1996, 14, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Behringer, K.; Mueller, H.; Goergen, H.; Thielen, I.; Eibl, A.D.; Stumpf, V.; Wessels, C.; Wiehlputz, M.; Rosenbrock, J.; Halbsguth, T.; et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Behringer, K.; Wildt, L.; Mueller, H.; Mattle, V.; Ganitis, P.; van den Hoonaard, B.; Ott, H.W.; Hofer, S.; Pluetschow, A.; Diehl, V.; et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced-stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann. Oncol. 2010, 21, 2052–2060. [Google Scholar] [CrossRef]
- Engert, A.; Plutschow, A.; Eich, H.T.; Lohri, A.; Dorken, B.; Borchmann, P.; Berger, B.; Greil, R.; Willborn, K.C.; Wilhelm, M.; et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N. Engl. J. Med. 2010, 363, 640–652. [Google Scholar] [CrossRef]
- Andre, M.P.E.; Girinsky, T.; Federico, M.; Reman, O.; Fortpied, C.; Gotti, M.; Casasnovas, O.; Brice, P.; van der Maazen, R.; Re, A.; et al. Early Positron Emission Tomography Response-Adapted Treatment in Stage I and II Hodgkin Lymphoma: Final Results of the Randomized EORTC/LYSA/FIL H10 Trial. J. Clin. Oncol. 2017, 35, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Goergen, H.; Kobe, C.; Eich, H.; Baues, C.; Greil, R.; Sasse, S.; Zijlstra, J.M.; Lohri, A.; Rosenwald, A.; et al. PET-Guided Treatment of Early-Stage Favorable Hodgkin Lymphoma: Final Results of the International, Randomized Phase 3 Trial HD16 By the German Hodgkin Study Group. Blood 2018, 132, 925. [Google Scholar] [CrossRef]
- Schaapveld, M.; Aleman, B.M.; van Eggermond, A.M.; Janus, C.P.; Krol, A.D.; van der Maazen, R.W.; Roesink, J.; Raemaekers, J.M.; de Boer, J.P.; Zijlstra, J.M.; et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 2499–2511. [Google Scholar] [CrossRef]
- Behringer, K.; Goergen, H.; Hitz, F.; Zijlstra, J.M.; Greil, R.; Markova, J.; Sasse, S.; Fuchs, M.; Topp, M.S.; Soekler, M.; et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): An open-label, randomised, non-inferiority trial. Lancet 2015, 385, 1418–1427. [Google Scholar] [CrossRef]
- Radford, J.; Illidge, T.; Counsell, N.; Hancock, B.; Pettengell, R.; Johnson, P.; Wimperis, J.; Culligan, D.; Popova, B.; Smith, P.; et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 1598–1607. [Google Scholar] [CrossRef]
- Engert, A.; Schiller, P.; Josting, A.; Herrmann, R.; Koch, P.; Sieber, M.; Boissevain, F.; Wit, M.d.; Mezger, J.; Duhmke, E.; et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: Results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 3601–3608. [Google Scholar] [CrossRef]
- Viviani, S.; Zinzani, P.L.; Rambaldi, A.; Brusamolino, E.; Levis, A.; Bonfante, V.; Vitolo, U.; Pulsoni, A.; Liberati, A.M.; Specchia, G.; et al. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N. Engl. J. Med. 2011, 365, 203–212. [Google Scholar] [CrossRef]
- Ferme, C.; Thomas, J.; Brice, P.; Casasnovas, O.; Vranovsky, A.; Bologna, S.; Lugtenburg, P.J.; Bouabdallah, R.; Carde, P.; Sebban, C.; et al. ABVD or BEACOPPbaseline along with involved-field radiotherapy in early-stage Hodgkin Lymphoma with risk factors: Results of the European Organisation for Research and Treatment of Cancer (EORTC)-Groupe d’Etude des Lymphomes de l’Adulte (GELA) H9-U intergroup randomised trial. Eur. J. Cancer 2017, 81, 45–55. [Google Scholar] [CrossRef]
- Tresckow, B.v.; Plutschow, A.; Fuchs, M.; Klimm, B.; Markova, J.; Lohri, A.; Kral, Z.; Greil, R.; Topp, M.S.; Meissner, J.; et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: Final analysis of the German Hodgkin Study Group HD14 trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 907–913. [Google Scholar] [CrossRef]
- Gillessen, S.; Plütschow, A.; Fuchs, M.; Markova, J.; Greil, R.; Topp, M.S.; Meissner, J.; Zijlstra, J.M.; Eichenauer, D.A.; Diehl, V.; et al. Dose-Intensification in Early Unfavorable Hodgkin Lymphoma: Long-Term Follow up of the German Hodgkin Study Group (GHSG) HD14 Trial. Blood 2019, 134, 129. [Google Scholar] [CrossRef]
- Borchmann, P. Positron Emission Tomography Guided Omission of Radiotherapy in Early-Stage Unfavorable Hodgkin Lymphoma: Final Results of The International, Randomized Phase III HD17 Trial By the GHSG. Abstract Book: 25th Congress of the European Hematology Association Virtual Edition, 2020. HemaSphere 2020, 4. [Google Scholar] [CrossRef]
- Gallamini, A.; Rossi, A.; Patti, C.; Picardi, M.; Romano, A.; Cantonetti, M.; Oppi, S.; Viviani, S.; Bolis, S.; Trentin, L.; et al. Consolidation Radiotherapy Could Be Safely Omitted in Advanced Hodgkin Lymphoma With Large Nodal Mass in Complete Metabolic Response After ABVD: Final Analysis of the Randomized GITIL/FIL HD0607 Trial. J. Clin. Oncol. 2020, 38, 3905–3913. [Google Scholar] [CrossRef]
- Skoetz, N.; Will, A.; Monsef, I.; Brillant, C.; Engert, A.; von Tresckow, B. Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst. Rev. 2017, 5, CD007941. [Google Scholar] [CrossRef] [PubMed]
- Skoetz, N.; Trelle, S.; Rancea, M.; Haverkamp, H.; Diehl, V.; Engert, A.; Borchmann, P. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: A systematic review and network meta-analysis. Lancet Oncol. 2013, 14, 943–952. [Google Scholar] [CrossRef]
- Hoppe, R.T.; Advani, R.H.; Ai, W.Z.; Ambinder, R.F.; Armand, P.; Bello, C.M.; Benitez, C.M.; Bierman, P.J.; Boughan, K.M.; Dabaja, B.; et al. Hodgkin Lymphoma, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 755–781. [Google Scholar] [CrossRef]
- Borchmann, P.; Goergen, H.; Kobe, C.; Lohri, A.; Greil, R.; Eichenauer, D.A.; Zijlstra, J.M.; Markova, J.; Meissner, J.; Feuring-Buske, M.; et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): Final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2018, 390, 2790–2802. [Google Scholar] [CrossRef]
- Casasnovas, R.O.; Bouabdallah, R.; Brice, P.; Lazarovici, J.; Ghesquieres, H.; Stamatoullas, A.; Dupuis, J.; Gac, A.C.; Gastinne, T.; Joly, B.; et al. PET-adapted treatment for newly diagnosed advanced Hodgkin lymphoma (AHL2011): A randomised, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2019, 20, 202–215. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Plütschow, A.; Kreissl, S.; Sökler, M.; Hellmuth, J.C.; Meissner, J.; Mathas, S.; Topp, M.S.; Behringer, K.; Klapper, W.; et al. Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin’s lymphoma: Final analysis of a phase 2 randomised trial by the German Hodgkin Study Group. Lancet Oncol. 2017, 18, 1680–1687. [Google Scholar] [CrossRef]
- Gallamini, A.; Patti, C.; Viviani, S.; Rossi, A.; Fiore, F.; Di Raimondo, F.; Cantonetti, M.; Stelitano, C.; Feldman, T.; Gavarotti, P.; et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br. J. Haematol. 2011, 152, 551–560. [Google Scholar] [CrossRef]
- Stephens, D.M.; Li, H.; Schoder, H.; Straus, D.J.; Moskowitz, C.H.; Leblanc, M.L.; Rimsza, L.M.; Bartlett, N.L.; Evens, A.M.; LaCasce, A.S.; et al. Long-Term Follow-up of SWOG S0816: Response-Adapted Therapy for Stage III/IV Hodgkin Lymphoma Demonstrates Limitations of PET-Adapted Approach. Blood 2018, 132, 929. [Google Scholar] [CrossRef]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illes, A.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef]
- Linch, D.C.; Winfield, D.; Goldstone, A.H.; Moir, D.; Hancock, B.; McMillan, A.; Chopra, R.; Milligan, D.; Hudson, G.V. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: Results of a BNLI randomised trial. Lancet 1993, 341, 1051–1054. [Google Scholar] [CrossRef]
- Rancea, M.; von Tresckow, B.; Monsef, I.; Engert, A.; Skoetz, N. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed or refractory Hodgkin lymphoma: A systematic review with meta-analysis. Crit. Rev. Oncol. Hematol. 2014, 92, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N.; Pfistner, B.; Sextro, M.; Sieber, M.; Carella, A.M.; Haenel, M.; Boissevain, F.; Zschaber, R.; Müller, P.; Kirchner, H.; et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: A randomised trial. Lancet 2002, 359, 2065–2071. [Google Scholar] [CrossRef]
- Adams, H.J.; Nievelstein, R.A.; Kwee, T.C. Prognostic value of interim FDG-PET in Hodgkin lymphoma: Systematic review and meta-analysis. Br. J. Haematol. 2015, 170, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Brockelmann, P.J.; Muller, H.; Casasnovas, O.; Hutchings, M.; von Tresckow, B.; Jurgens, M.; McCall, S.J.; Morschhauser, F.; Fuchs, M.; Borchmann, P.; et al. Risk factors and a prognostic score for survival after autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma. Ann. Oncol. 2017, 28, 1352–1358. [Google Scholar] [CrossRef]
- Garcia-Sanz, R.; Sureda, A.; de la Cruz, F.; Canales, M.; Gonzalez, A.P.; Pinana, J.L.; Rodriguez, A.; Gutierrez, A.; Domingo-Domenech, E.; Sanchez-Gonzalez, B.; et al. Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO Group). Ann. Oncol. 2019, 30, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Hagenbeek, A.; Zijlstra, J.M.; Plattel, W.J.; Morschhauser, F.; Lugtenburg, P.J.; Brice, P.; Hutchings, M.; Gastinne, T.; Liu, R.D.; Burggraaf, C.N.; et al. Combining Brentuximab Vedotin with DHAP as Salvage Treatment in Relapsed/Refractory Hodgkin Lymphoma: The Phase II HOVON/LLPC Transplant BRaVE study. Blood 2018, 132, 2923. [Google Scholar] [CrossRef]
- LaCasce, A.S.; Bociek, R.G.; Sawas, A.; Caimi, P.; Agura, E.; Matous, J.; Ansell, S.M.; Crosswell, H.E.; Islas-Ohlmayer, M.; Behler, C.; et al. Brentuximab vedotin plus bendamustine: A highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood 2018, 132, 40–48. [Google Scholar] [CrossRef]
- Herrera, A.F.; Moskowitz, A.J.; Bartlett, N.L.; Vose, J.M.; Ramchandren, R.; Feldman, T.A.; LaCasce, A.S.; Ansell, S.M.; Moskowitz, C.H.; Fenton, K.; et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018, 131, 1183–1194. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Nademanee, A.; Masszi, T.; Agura, E.; Holowiecki, J.; Abidi, M.H.; Chen, A.I.; Stiff, P.; Gianni, A.M.; Carella, A.; et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 385, 1853–1862. [Google Scholar] [CrossRef]
- Boll, B.; Gorgen, H.; Fuchs, M.; Pluetschow, A.; Eich, H.T.; Bargetzi, M.J.; Weidmann, E.; Junghanss, C.; Greil, R.; Scherpe, A.; et al. ABVD in older patients with early-stage Hodgkin lymphoma treated within the German Hodgkin Study Group HD10 and HD11 trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1522–1529. [Google Scholar] [CrossRef]
- Crump, M. Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am. Soc. Hematol. Educ. Program 2008, 326–333. [Google Scholar] [CrossRef]
- Younes, A.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Ramchandren, R.; Bartlett, N.L.; Cheson, B.D.; Vos, S.D.; et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2183–2189. [Google Scholar] [CrossRef]
- Chen, R.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Connors, J.M.; Engert, A.; Larsen, E.K.; Huebner, D.; et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016, 128, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Bröckelmann, P.J.; Zagadailov, E.A.; Corman, S.L.; Chirikov, V.; Johnson, C.; Macahilig, C.; Seal, B.; Dalal, M.R.; Illidge, T. Brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma who are Ineligible for autologous stem cell transplant: A Germany and United Kingdom retrospective study. Eur. J. Haematol. 2017, 99, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; Johnson, N.; Melnichenko, V.; Fogliatto, L.M.; Goncalves, I.; Oliveira, J.d.; et al. KEYNOTE-204: Randomized, open-label, phase III study of pembrolizumab (pembro) versus brentuximab vedotin (BV) in relapsed or refractory classic Hodgkin lymphoma (R/R cHL). J. Clin. Oncol. 2020, 38, 8005. [Google Scholar] [CrossRef]
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J. Clin. Oncol. 2018, 36, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef]
- Carreau, N.A.; Pail, O.; Armand, P.; Merryman, R.W.; Advani, R.H.; Spinner, M.A.; Herrera, A.F.; Chen, R.W.; Tomassetti, S.; Ramchandren, R.; et al. Checkpoint Blockade Therapy May Sensitize Hodgkin Lymphoma to Subsequent Therapy. Blood 2018, 2018, 1626. [Google Scholar] [CrossRef]
- Cohen, J.B.; Kuruvilla, J.; Engert, A.; Ansell, S.M.; Younes, A.; Lee, H.J.; Trněný, M.; Savage, K.J.; Ramchandren, R.; Collins, G.P.; et al. Nivolumab Treatment Beyond Investigator-Assessed Progression: Extended Follow-up in Patients with Relapsed/Refractory Classical Hodgkin Lymphoma from the Phase 2 CheckMate 205 Study. Blood 2018, 132, 2932. [Google Scholar] [CrossRef]
- Fatobene, G.; Rocha, V.; St Martin, A.; Hamadani, M.; Robinson, S.; Bashey, A.; Boumendil, A.; Brunstein, C.; Castagna, L.; Dominietto, A.; et al. Nonmyeloablative Alternative Donor Transplantation for Hodgkin and Non-Hodgkin Lymphoma: From the LWP-EBMT, Eurocord, and CIBMTR. J. Clin. Oncol. 2020, 38, 1518–1526. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Engert, A. Nodular lymphocyte-predominant Hodgkin lymphoma: A unique disease deserving unique management. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 324–328. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Plütschow, A.; Fuchs, M.; von Tresckow, B.; Böll, B.; Behringer, K.; Diehl, V.; Eich, H.T.; Borchmann, P.; Engert, A. Long-Term Course of Patients With Stage IA Nodular Lymphocyte-Predominant Hodgkin Lymphoma: A Report From the German Hodgkin Study Group. J. Clin. Oncol. 2015, 33, 2857–2862. [Google Scholar] [CrossRef]
- Chen, R.C.; Chin, M.S.; Ng, A.K.; Feng, Y.; Neuberg, D.; Silver, B.; Pinkus, G.S.; Stevenson, M.A.; Mauch, P.M. Early-stage, lymphocyte-predominant Hodgkin’s lymphoma: Patient outcomes from a large, single-institution series with long follow-up. J. Clin. Oncol. 2010, 28, 136–141. [Google Scholar] [CrossRef]
- Savage, K.J.; Skinnider, B.; Al-Mansour, M.; Sehn, L.H.; Gascoyne, R.D.; Connors, J.M. Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood 2011, 118, 4585–4590. [Google Scholar] [CrossRef] [PubMed]
- Eichenauer, D.A.; Plütschow, A.; Fuchs, M.; Sasse, S.; Baues, C.; Böll, B.; von Tresckow, B.; Diehl, V.; Borchmann, P.; Engert, A. Long-Term Follow-Up of Patients With Nodular Lymphocyte-Predominant Hodgkin Lymphoma Treated in the HD7 to HD15 Trials: A Report From the German Hodgkin Study Group. J. Clin. Oncol. 2020, 38, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Eichenauer, D.A. Pet-2-Guided Escalated BEACOPP For Patients With Advanced Nodular Lymphocyte-Predominant Hodgkin Lymphoma: An Analysis From The German Hodgkin Study Group. Abstract Book: 25th Congress of the European Hematology Association Virtual Edition, 2020. HemaSphere 2020, 4. [Google Scholar] [CrossRef]
- Fanale, M.A.; Cheah, C.Y.; Rich, A.; Medeiros, L.J.; Lai, C.M.; Oki, Y.; Romaguera, J.E.; Fayad, L.E.; Hagemeister, F.B.; Samaniego, F.; et al. Encouraging activity for R-CHOP in advanced stage nodular lymphocyte-predominant Hodgkin lymphoma. Blood 2017, 130, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Prusila, R.E.I.; Haapasaari, K.M.; Marin, K.; Pollari, M.; Soini, Y.; Vornanen, M.; Karjalainen-Lindsberg, M.L.; Turpeenniemi-Hujanen, T.; Kuittinen, O. R-Bendamustine in the treatment of nodular lymphocyte-predominant Hodgkin lymphoma. Acta Oncol. 2018, 57, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.H.; Connors, J.M.; Lai, A.; Al-Mansour, M.; Sehn, L.H.; Villa, D.; Klasa, R.; Shenkier, T.; Gascoyne, R.D.; Skinnider, B.; et al. Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: A matched pair outcome analysis. Blood 2014, 123, 3567–3573. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Plütschow, A.; Schröder, L.; Fuchs, M.; Böll, B.; von Tresckow, B.; Diehl, V.; Borchmann, P.; Engert, A. Relapsed and refractory nodular lymphocyte-predominant Hodgkin lymphoma: An analysis from the German Hodgkin Study Group. Blood 2018, 132, 1519–1525. [Google Scholar] [CrossRef]
- Eichenauer, D.A.; Goergen, H.; Plütschow, A.; Wongso, D.; Behringer, K.; Kreissl, S.; Thielen, I.; Halbsguth, T.; Bröckelmann, P.J.; Fuchs, M.; et al. ofatumumab in relapsed nodular lymphocyte-predominant Hodgkin lymphoma: Results of a phase II study from the German Hodgkin study group. Leukemia 2016, 30, 1425–1427. [Google Scholar] [CrossRef]
- Akhtar, S.; Montoto, S.; Boumendil, A.; Finel, H.; Masszi, T.; Jindra, P.; Nemet, D.; Fuhrmann, S.; Beguin, Y.; Castagna, L.; et al. High dose chemotherapy and autologous stem cell transplantation in nodular lymphocyte-predominant Hodgkin lymphoma: A retrospective study by the European society for blood and marrow transplantation-lymphoma working party. Am. J. Hematol. 2018, 93, 40–46. [Google Scholar] [CrossRef]
- Proctor, S.J.; Wilkinson, J.; Jones, G.; Watson, G.C.; Lucraft, H.H.; Mainou-Fowler, T.; Culligan, D.; Galloway, M.J.; Wood, K.M.; McNally, R.J.; et al. Evaluation of treatment outcome in 175 patients with Hodgkin lymphoma aged 60 years or over: The SHIELD study. Blood 2012, 119, 6005–6015. [Google Scholar] [CrossRef]
- Engert, A.; Ballova, V.; Haverkamp, H.; Pfistner, B.; Josting, A.; Duhmke, E.; Muller-Hermelink, K.; Diehl, V. Hodgkin’s lymphoma in elderly patients: A comprehensive retrospective analysis from the German Hodgkin’s Study Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Enblad, G.; Glimelius, B.; Sundstrom, C. Treatment outcome in Hodgkin’s disease in patients above the age of 60: A population-based study. Ann. Oncol. 1991, 2, 297–302. [Google Scholar] [CrossRef]
- Wongso, D.; Fuchs, M.; Plutschow, A.; Klimm, B.; Sasse, S.; Hertenstein, B.; Maschmeyer, G.; Vieler, T.; Duhrsen, U.; Lindemann, W.; et al. Treatment-related mortality in patients with advanced-stage hodgkin lymphoma: An analysis of the german hodgkin study group. J. Clin. Oncol. 2013, 31, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Helenowski, I.; Ramsdale, E.; Nabhan, C.; Karmali, R.; Hanson, B.; Parsons, B.; Smith, S.; Larsen, A.; McKoy, J.M.; et al. A retrospective multicenter analysis of elderly Hodgkin lymphoma: Outcomes and prognostic factors in the modern era. Blood 2012, 119, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Borchmann, S.; Engert, A.; Böll, B. Hodgkin lymphoma in elderly patients. Curr. Opin. Oncol. 2018, 30, 308–316. [Google Scholar] [CrossRef]
- Boll, B.; Bredenfeld, H.; Gorgen, H.; Halbsguth, T.; Eich, H.T.; Soekler, M.; Markova, J.; Keller, U.; Graeven, U.; Kremers, S.; et al. Phase 2 study of PVAG (prednisone, vinblastine, doxorubicin, gemcitabine) in elderly patients with early unfavorable or advanced stage Hodgkin lymphoma. Blood 2011, 118, 6292–6298. [Google Scholar] [CrossRef] [PubMed]
- Bröckelmann, P.J.; Goergen, H.; Keller, U.; Meissner, J.; Ordemann, R.; Halbsguth, T.V.; Sasse, S.; Sökler, M.; Kerkhoff, A.; Mathas, S.; et al. Efficacy of Nivolumab and AVD in Early-Stage Unfavorable Classic Hodgkin Lymphoma: The Randomized Phase 2 German Hodgkin Study Group NIVAHL Trial. JAMA Oncol. 2020, 6, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Ramchandren, R.; Domingo-Domenech, E.; Rueda, A.; Trneny, M.; Feldman, T.A.; Lee, H.J.; Provencio, M.; Sillaber, C.; Cohen, J.B.; Savage, K.J.; et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J. Clin. Oncol. 2019, JCO1900315. [Google Scholar] [CrossRef] [PubMed]
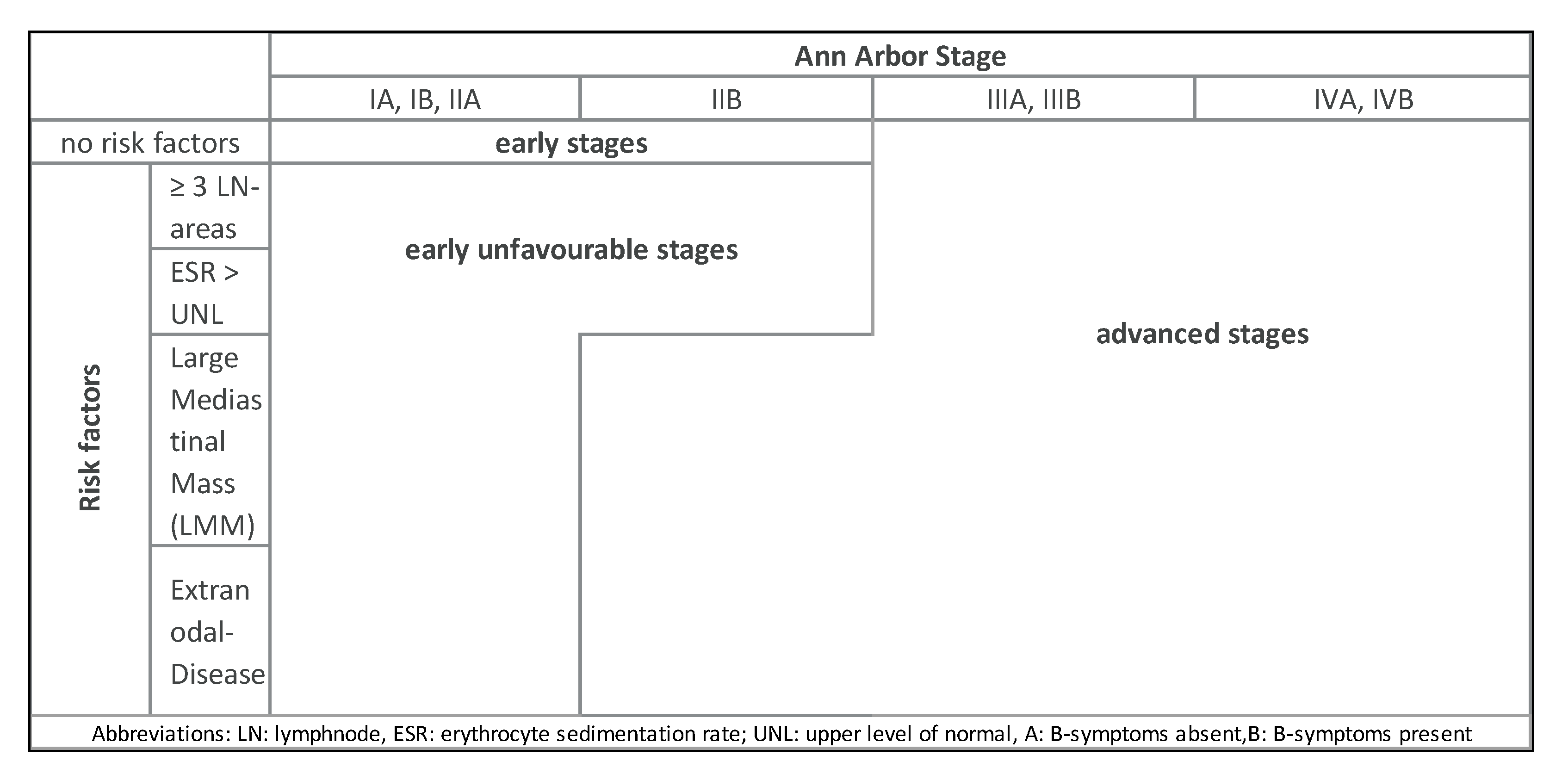
| Stage | Explanation |
|---|---|
| I | Involvement of one lymph node region or a single localized involvement outside the lymphatic system |
| II | Involvement of two or more lymph node regions on the same side of the diaphragm or localized involvement outside the lymphatic system and lymph node regions on the same side of the diaphragm |
| III | Involvement of two or more lymph node regions or organs outside the lymphatic system on both sides of the diaphragm |
| IV | Diffuse or disseminated infestation of one or more extralymphatic organs with or without infestation of lymphoid tissue |
| A | No B-symptoms |
| B | B symptoms: fever, drenching night sweats, and/or unexplained loss of body weight >10% within the preceding 6 months. |
| Risk Factors | GHSG | EORTC | NCIC/ECOG | NCCN |
|---|---|---|---|---|
| Large mediastinal mass | Yes 1, ratio ≥ 1/3 | Yes, ratio ≥ 0.35 | No | Yes, ratio > 1/3 |
| Extranodal disease | Yes 1 | No | No | Yes |
| Nodal areas | Yes, ≥3 areas | Yes, ≥4 areas | Yes, ≥4 areas | Yes, ≥3 regions |
| ESR | Yes, ≥50 (A) or ≥30 (B) | Yes, ≥50 (A) or ≥30 (B) | Yes, ≥50 | Yes, ≥50 (A) |
| B-symptoms | No | No | No | Yes |
| Bulk | No | No | No | Yes, >10 cm |
| Age | No | Yes, ≥50 years | Yes, ≥40 years | No |
| Histology other than LP/NS | No | No | Yes | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Momotow, J.; Borchmann, S.; Eichenauer, D.A.; Engert, A.; Sasse, S. Hodgkin Lymphoma—Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients. J. Clin. Med. 2021, 10, 1125. https://doi.org/10.3390/jcm10051125
Momotow J, Borchmann S, Eichenauer DA, Engert A, Sasse S. Hodgkin Lymphoma—Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients. Journal of Clinical Medicine. 2021; 10(5):1125. https://doi.org/10.3390/jcm10051125
Chicago/Turabian StyleMomotow, Jesko, Sven Borchmann, Dennis A. Eichenauer, Andreas Engert, and Stephanie Sasse. 2021. "Hodgkin Lymphoma—Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients" Journal of Clinical Medicine 10, no. 5: 1125. https://doi.org/10.3390/jcm10051125
APA StyleMomotow, J., Borchmann, S., Eichenauer, D. A., Engert, A., & Sasse, S. (2021). Hodgkin Lymphoma—Review on Pathogenesis, Diagnosis, Current and Future Treatment Approaches for Adult Patients. Journal of Clinical Medicine, 10(5), 1125. https://doi.org/10.3390/jcm10051125





