A Time-Based Meta-Analysis on the Incidence of New Onset Diabetes after Liver Transplantation
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria and Data Extraction
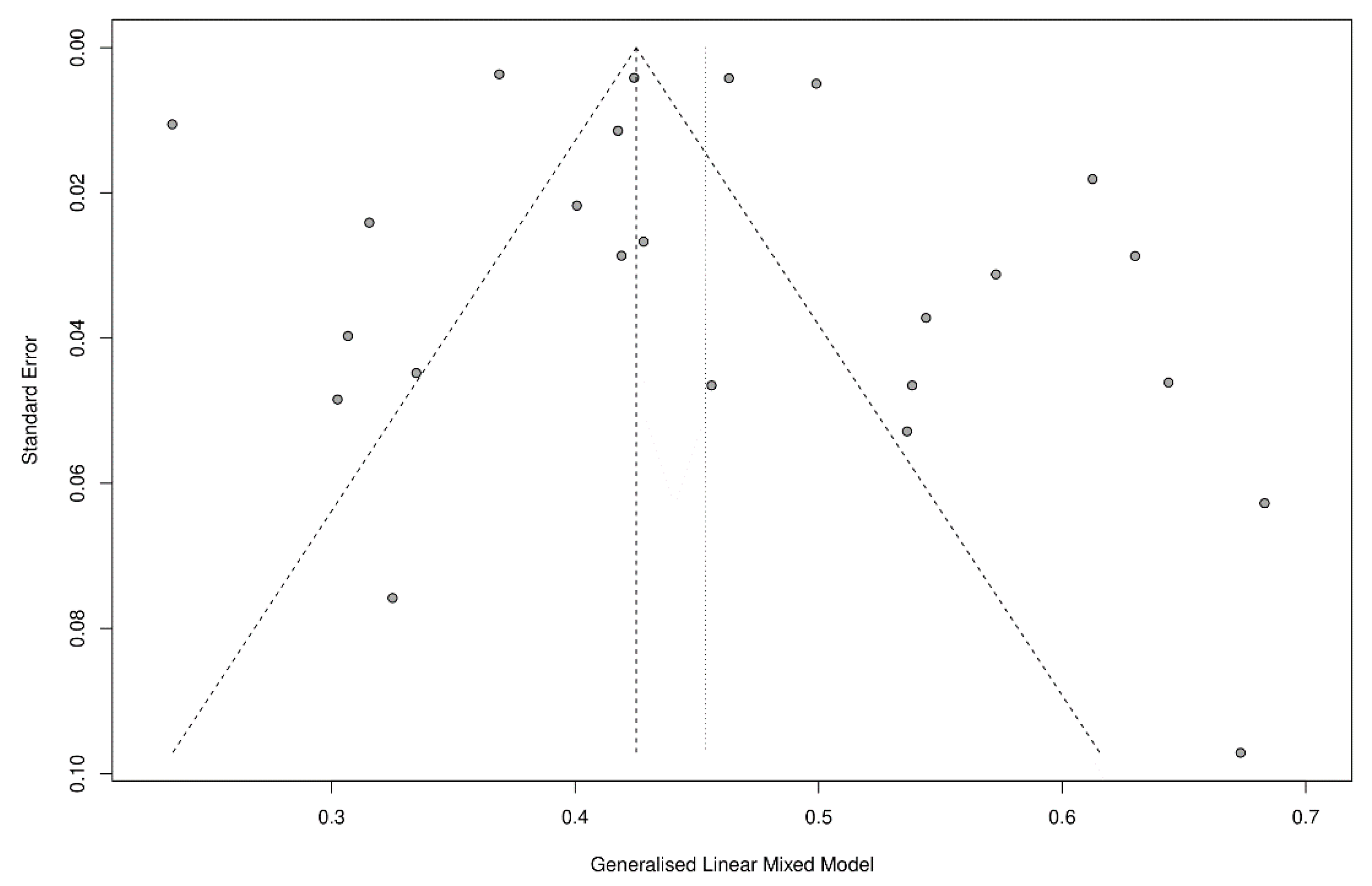
2.3. Statistical Analysis and Quality Assessment
3. Results
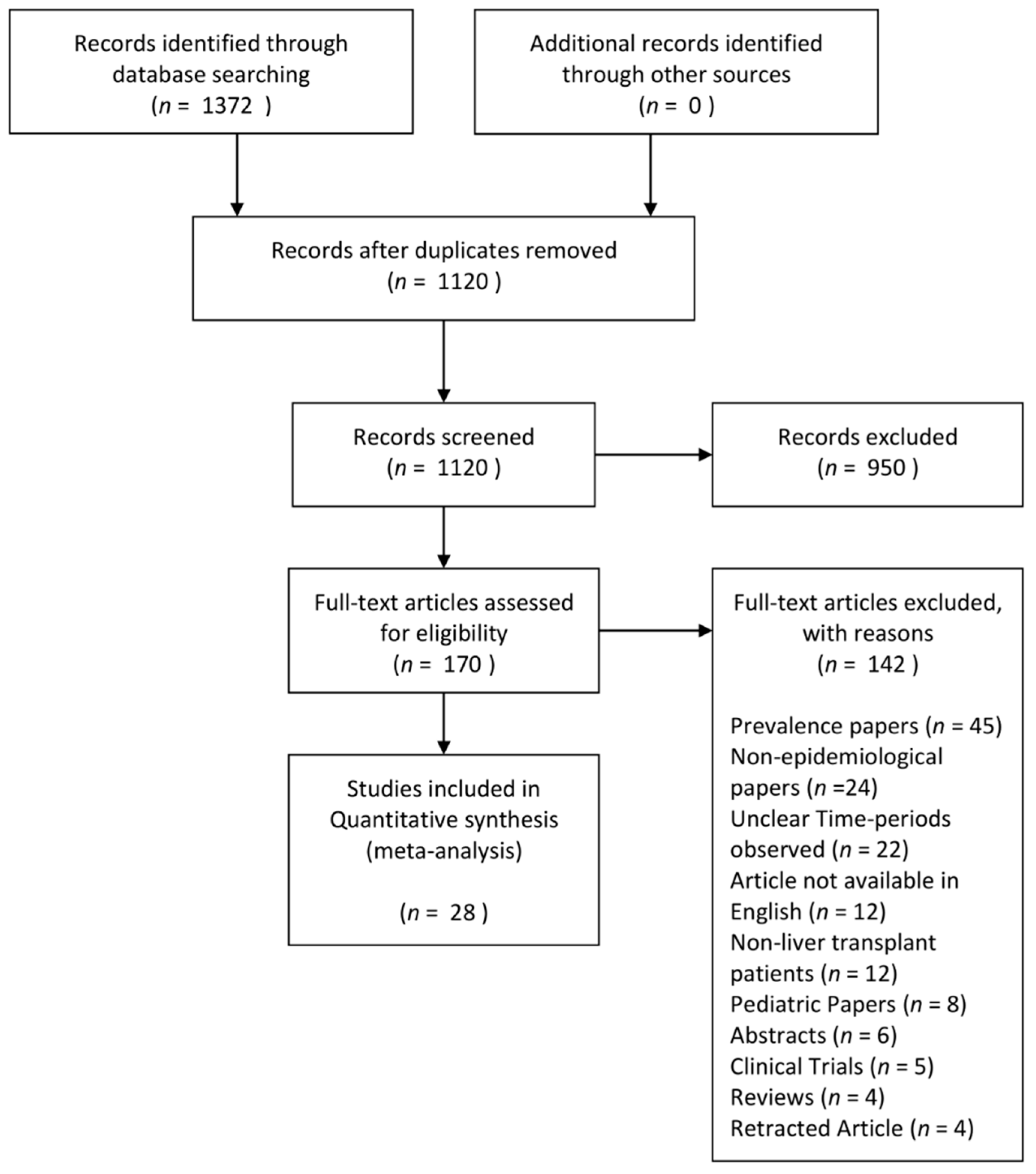
3.1. Search Results and Study Characteristics
3.2. Definition of NODAT
3.3. Overall Incidence of NODAT
4. Discussion
Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schuppan, D.; Afdhal, N.H. Liver cirrhosis. Lancet 2008, 371, 838–851. [Google Scholar] [CrossRef]
- Marchetti, P. New-onset diabetes after liver transplantation: From pathogenesis to management. Liver Transpl. 2005, 11, 612–620. [Google Scholar] [CrossRef]
- Watt, K.D.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of causes and risk factors for mortality post-liver transplant: Results of the NIDDK long-term follow-up study. Am. J. Transpl. 2010, 10, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, G.A.; Goldberg, D.S.; Hwang, W.T.; Judy, R.; Thomasson, A.; Kimmel, S.E.; Forde, K.A.; Lewis, J.D.; Yang, Y.X. Sustained posttransplantation diabetes is associated with long-term major cardiovascular events following liver transplantation. Am. J. Transpl. 2018, 18, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhang, Y.; Chen, X.; Huang, X.; Xue, M.; Sun, Q.; Wang, T.; Liang, J.; He, S.; Gao, J.; et al. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J. Diabetes 2015, 7, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Xu, X.; Xie, H.; Wang, K.; Xiang, P.; Zhuang, R.; Shen, T.; Wu, J.; Wang, W.; Zheng, S. New-onset diabetes after liver transplantation: A national report from China Liver Transplant Registry. Liver Int. 2016, 36, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Navasa, M.; Bustamante, J.; Marroni, C.; González, E.; Andreu, H.; Esmatjes, E.; García-Valdecasas, J.C.; Grande, L.; Cirera, I.; Rimola, A.; et al. Diabetes mellitus after liver transplantation: Prevalence and predictive factors. J. Hepatol. 1996, 25, 64–71. [Google Scholar] [CrossRef]
- Moon, J.I.; Barbeito, R.; Faradji, R.N.; Gaynor, J.J.; Tzakis, A.G. Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: Long-term follow up. Transplantation 2006, 82, 1625–1628. [Google Scholar] [CrossRef]
- Davidson, J.; Wilkinson, A.; Dantal, J.; Dotta, F.; Haller, H.; Hernandez, D.; Kasiske, B.L.; Kiberd, B.; Krentz, A.; Legendre, C.; et al. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation 2003, 75, SS3–S24. [Google Scholar]
- Pham, P.-T.T.; Pham, P.-M.T.; Pham, S.V.; Pham, P.-A.T.; Pham, P.-C.T. New onset diabetes after transplantation (NODAT): An overview. Diabetes Metab. Syndr. Obes. 2011, 4, 175–186. [Google Scholar] [CrossRef]
- Kuo, H.T.; Sampaio, M.S.; Ye, X.; Reddy, P.; Martin, P.; Bunnapradist, S. Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing database. Transplantation 2010, 89, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-W.; Lu, T.-F.; Hua, X.-W.; Dai, H.-J.; Cui, X.-L.; Zhang, J.-J.; Xia, Q. Risk factors for new onset diabetes mellitus after liver transplantation: A meta-analysis. World J. Gastroenterol. 2015, 21, 6329–6340. [Google Scholar] [CrossRef]
- Li, Z.; Sun, F.; Hu, Z.; Xiang, J.; Zhou, J.; Yan, S.; Wu, J.; Zhou, L.; Zheng, S. New-onset diabetes mellitus in liver transplant recipients with hepatitis C: Analysis of the national database. Transpl. Proc. 2016, 48, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.D.; Chang, Y.-H.; Aqel, B.A.; Byrne, T.J.; Chakkera, H.A.; Douglas, D.D.; Mulligan, D.C.; Rakela, J.; Vargas, H.E.; Carey, E.J. New onset diabetes mellitus in living donor versus deceased donor liver transplant recipients: Analysis of the UNOS/OPTN database. J. Transpl. 2013, 2013, 269096. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Lv, C.; Chen, X.; Liang, J.; Zhao, C.; Zhang, Y.; Huang, X.; Sun, Q.; Wang, T.; Gao, J.; et al. Donor liver steatosis: A risk factor for early new-onset diabetes after liver transplantation. J. Diabetes Investig. 2017, 8, 181–187. [Google Scholar] [CrossRef]
- Kelava, T.; Turcic, P.; Markotic, A.; Ostojic, A.; Sisl, D.; Mrzljak, A. Importance of genetic polymorphisms in liver transplantation outcomes. World J. Gastroenterol. 2020, 26, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Sulanc, E.; Lane, J.T.; Puumala, S.E.; Groggel, G.C.; Wrenshall, L.E.; Stevens, R.B. New-onset diabetes after kidney transplantation: An application of 2003 International Guidelines. Transplantation 2005, 80, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Peláez-Jaramillo, M.J.; Cárdenas-Mojica, A.A.; Gaete, P.V.; Mendivil, C.O. Post-liver transplantation diabetes mellitus: A review of relevance and approach to treatment. Diabetes Ther. 2018, 9, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Heisel, O.; Heisel, R.; Balshaw, R.; Keown, P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: A systematic review and meta-analysis. Am. J. Transpl. 2004, 4, 583–595. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Schwarzer, G.; Chemaitelly, H.; Abu-Raddad, L.J.; Rücker, G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res. Synth. Methods 2019, 10, 476–483. [Google Scholar] [CrossRef]
- Ye, Q.; Zou, B.; Yeo, Y.H.; Li, J.; Huang, D.Q.; Wu, Y.; Yang, H.; Liu, C.; Kam, L.Y.; Tan, X.X.E.; et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 739–752. [Google Scholar] [CrossRef]
- Fairbrass, K.M.; Costantino, S.J.; Gracie, D.J.; Ford, A.C. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 1053–1062. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G.; Carpenter, J.R.; Schumacher, M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med. Res. Methodol. 2008, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.H.; Ng, C.H.; Lee, M.H.; Koh, J.W.H.; Kiew, J.; Yang, S.P.; Sundar, G.; Khoo, C.M. Prevalence of thyroid eye disease in Graves’ disease: A meta-analysis and systematic review. Clin. Endocrinol. 2020, 93, 363–374. [Google Scholar] [CrossRef]
- Koo, C.H.; Chang, J.H.E.; Syn, N.L.; Wee, I.J.Y.; Mathew, R. Systematic review and meta-analysis on colorectal cancer findings on colonic evaluation after CT-confirmed acute diverticulitis. Dis. Colon. Rectum. 2020, 63, 701–709. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, j.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis; Ottawa Hospital Research Institute: Ottawa, ON, USA, 2000. [Google Scholar]
- Jain, A.B.; Kashyap, R.; Rakela, J.; Starzl, T.E.; Fung, J.J. Primary adult liver transplantation under tacrolimus: More than 90 months actual follow-up survival and adverse events. Liver Transpl. Surg. 1999, 5, 144–150. [Google Scholar] [CrossRef]
- Driscoll, C.J.; Cashion, A.K.; Hathaway, D.K.; Thompson, C.; Conley, Y.; Gaber, O.; Vera, S.; Shokouh-Amiri, H. Posttransplant diabetes mellitus in liver transplant recipients. Prog. Transpl. 2006, 16, 110–116. [Google Scholar] [CrossRef]
- Lieber, S.R.; Lee, R.-A.; Jiang, Y.; Reuter, C.; Watkins, R.; Szempruch, K.; Gerber, D.A.; Desai, C.S.; DeCherney, G.S.; Barritt, A.S.T. The impact of post-transplant diabetes mellitus on liver transplant outcomes. Clin. Transplant. 2019, 33, e13554. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Saab, S.; Trimble, G.; Mishra, A.; Henry, L. The association of hepatitis C virus infection and post-liver transplant diabetes: Data from 17 000 HCV-infected transplant recipients. Aliment. Pharmacol. Ther. 2015, 41, 209–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stepanova, M.; Henry, L.; Garg, R.; Kalwaney, S.; Saab, S.; Younossi, Z. Risk of de novo post-transplant type 2 diabetes in patients undergoing liver transplant for non-alcoholic steatohepatitis. BMC Gastroenterol. 2015, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Aqel, B.A.; Byrne, T.J.; Douglas, D.D.; Rakela, J.; Vargas, H.E.; Moss, A.A.; Mulligan, D.C.; Reddy, K.S.; Chakkera, H.A. Pretransplant fasting glucose predicts new-onset diabetes after liver transplantation. J. Transpl. 2012, 2012, 614781. [Google Scholar] [CrossRef]
- Song, J.-L.; Gao, W.; Zhong, Y.; Yan, L.-N.; Yang, J.-Y.; Wen, T.-F.; Li, B.; Wang, W.-T.; Wu, H.; Xu, M.-Q.; et al. Minimizing tacrolimus decreases the risk of new-onset diabetes mellitus after liver transplantation. World J. Gastroenterol. 2016, 22, 2133–2141. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, L.; Li, B.; Zeng, Y.; Wen, T.; Zhao, J.; Wang, W.; Xu, M.; Yang, J.; Ma, Y.; et al. Diabetes mellitus after living donor liver transplantation: Data from mainland China. Transpl. Proc. 2009, 41, 1756–1760. [Google Scholar] [CrossRef]
- Cen, C.; Fang, H.-X.; Yu, S.-F.; Liu, J.-M.; Liu, Y.-X.; Zhou, L.; Yu, J.; Zheng, S.-S. Association between ADIPOQ gene polymorphisms and the risk of new-onset diabetes mellitus after liver transplantation. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 602–609. [Google Scholar] [CrossRef]
- Saliba, F.; Rostaing, L.; Gugenheim, J.; Durand, F.; Radenne, S.; Leroy, V.; Neau-Cransac, M.; Calmus, Y.; Salame, E.; Pageaux, G.-P.; et al. Corticosteroid-sparing and optimization of mycophenolic acid exposure in liver transplant recipients receiving mycophenolate mofetil and tacrolimus: A randomized, multicenter study. Transplantation 2016, 100, 1705–1713. [Google Scholar] [CrossRef]
- Oufroukhi, L.; Kamar, N.; Muscari, F.; Lavayssiere, L.; Guitard, J.; Ribes, D.; Esposito, L.; Alric, L.; Hanaire, H.; Rostaing, L. Predictive factors for posttransplant diabetes mellitus within one-year of liver transplantation. Transplantation 2008, 85, 1436–1442. [Google Scholar] [CrossRef]
- Saliba, F.; Lakehal, M.; Pageaux, G.P.; Roche, B.; Vanlemmens, C.; Duvoux, C.; Dumortier, J.; Salamé, E.; Calmus, Y.; Maugendre, D. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis C infection: An observational multicenter study. Liver Transpl. 2007, 13, 136–144. [Google Scholar] [CrossRef]
- Oommen, T.; Arun, C.S.; Kumar, H.; Nair, V.; Jayakumar, R.V.; Sudhindran, S.; Praveen, V.P.; Abraham, N.B.N.; Menon, U. Incidence of new-onset diabetes and posttransplant metabolic syndrome after liver transplantation—A prospective study from South India. Indian J. Endocrinol. Metab. 2020, 24, 165–169. [Google Scholar] [CrossRef]
- Varghese, J.; Reddy, M.S.; Venugopal, K.; Perumalla, R.; Narasimhan, G.; Arikichenin, O.; Shanmugam, V.; Shanmugam, N.; Srinivasan, V.; Jayanthi, V.; et al. Tacrolimus-related adverse effects in liver transplant recipients: Its association with trough concentrations. Indian J. Gastroenterol. 2014, 33, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Asonuma, K.; Hayashida, S.; Suda, H.; Ohya, Y.; Lee, K.-J.; Yamamoto, H.; Takeichi, T.; Inomata, Y. Incidence and risk factors for new-onset diabetes in living-donor liver transplant recipients. Clin. Transplant. 2013, 27, 426–435. [Google Scholar] [CrossRef]
- Yagi, S.; Kaido, T.; Iida, T.; Yoshizawa, A.; Okajima, H.; Uemoto, S. New-onset diabetes mellitus after living-donor liver transplantation: Association with graft synthetic function. Surg. Today 2017, 47, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Cuervas-Mons, V.; Herrero, J.I.; Gomez, M.A.; Gonzalez-Pinto, I.; Serrano, T.; de la Mata, M.; Fabregat, J.; Gastaca, M.; Bilbao, I.; Varo, E.; et al. Impact of tacrolimus and mycophenolate mofetil regimen vs. a conventional therapy with steroids on cardiovascular risk in liver transplant patients. Clin. Transpl. 2015, 29, 667–677. [Google Scholar] [CrossRef]
- Rubin, A.; Sanchez-Montes, C.; Aguilera, V.; Juan, F.S.; Ferrer, I.; Moya, A.; Montalva, E.; Pareja, E.; Lopez-Andujar, R.; Prieto, M.; et al. Long-term outcome of ‘long-term liver transplant survivors’. Transpl. Int. 2013, 26, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Aravinthan, A.D.; Fateen, W.; Doyle, A.C.; Venkatachalapathy, S.V.; Issachar, A.; Galvin, Z.; Sapisochin, G.; Cattral, M.S.; Ghanekar, A.; McGilvray, I.D.; et al. The impact of preexisting and post-transplant diabetes mellitus on outcomes following liver transplantation. Transplantation 2019, 103, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.S.; Alsabaawy, M.; Afify, S.; El-Azab, G.; Omar, N. Incidence and risk factors for diabetes, hypertension and hyperlipidemia after liver transplantation. J. Gastroenterol. Hepatol. Res. 2020, 9, 3077–3081. [Google Scholar] [CrossRef]
- Gebhardt, S.; Jara, M.; Malinowski, M.; Seehofer, D.; Puhl, G.; Pratschke, J.; Stockmann, M. Risk factors of metabolic disorders after liver transplantation: An analysis of data from fasted patients. Transplantation 2015, 99, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, Z.; Azarpira, N.; Kohan, L.; Darai, M.; Kazemi, K.; Parvizi, M.M. Association between E23K variant in KCNJ11 gene and new-onset diabetes after liver transplantation. Mol. Biol. Rep. 2014, 41, 6063–6069. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, M.J.; Choe, E.Y.; Jung, C.H.; Joo, D.J.; Kim, M.S.; Cha, B.S.; Park, J.-Y.; Kang, E.S. Statin therapy is associated with the development of new-onset diabetes after transplantation in liver recipients with high fasting plasma glucose levels. Liver Transpl. 2014, 20, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-C.; Lin, J.-R.; Chen, H.-P.; Tsai, Y.-F.; Yu, H.-P. Prevalence, predictive factors, and survival outcome of new-onset diabetes after liver transplantation: A population-based cohort study. Medicine 2016, 95, e3829. [Google Scholar] [CrossRef]
- Hartog, H.; May, C.J.H.; Corbett, C.; Phillips, A.; Tomlinson, J.W.; Mergental, H.; Isaac, J.; Bramhall, S.; Mirza, D.F.; Muiesan, P.; et al. Early occurrence of new-onset diabetes after transplantation is related to type of liver graft and warm ischaemic injury. Liver Int. 2015, 35, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- John, P.R.; Thuluvath, P.J. Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl. 2002, 8, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.; Davidson, J.; Dotta, F.; Home, P.D.; Keown, P.; Kiberd, B.; Jardine, A.; Levitt, N.; Marchetti, P.; Markell, M.; et al. Guidelines for the treatment and management of new-onset diabetes after transplantation. Clin. Transpl. 2005, 19, 291–298. [Google Scholar] [CrossRef]
- Jain, A.; Reyes, J.; Kashyap, R.; Dodson, SF.; Demetris, AJ.; Ruppert, K.; Abu-Elmagd, K.; Marsh, W.; Madariaga, J.; Mazariegos, G.; et al. Long-term survival after liver transplantation in 4000 consecutive patients at a single center. Ann. Surg. 2000, 23, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ogura, Y.; Kiuchi, T.; Inomata, Y.; Uemoto, S.; Furukawa, H. Living donor liver transplantation: Eastern experiences. HPB (Oxf.) 2004, 6, 88–94. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sgourakis, G.; Radtke, A.; Fouzas, I.; Mylona, S.; Goumas, K.; Gockel, I.; Lang, H.; Karaliotas, C. Corticosteroid-free immunosuppression in liver transplantation: A meta-analysis and meta-regression of outcomes. Transpl. Int. 2009, 22, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Klintmalm, G.B.; Nashan, B. The Role of mTOR inhibitors in liver transplantation: Reviewing the evidence. J. Transpl. 2014, 2014, 845438. [Google Scholar] [CrossRef]
- Tan, P.S.; Muthiah, M.D.; Koh, T.; Teoh, Y.L.; Chan, A.; Kow, A.; Zheng, Q.; Kwon, C.H.D.; Lee, G.H.; Lesmana, C.R.A.; et al. Asian liver transplant network clinical guidelines on immunosuppression in liver transplantation. Transplantation 2019, 103, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Vadeyar, H.; Rela, M.; Shah, S. Liver transplantation: East versus West. J. Clin. Exp. Hepatol. 2013, 3, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; Hecking, M.; de Vries, A.P.J.; Porrini, E.; Hornum, M.; Rasoul-Rockenschaub, S.; Berlakovich, G.; Krebs, M.; Kautzky-Willer, A.; Schernthaner, G.; et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: Recommendations and future directions. Am. J. Transpl. 2014, 14, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Thoefner, L.B.; Rostved, A.A.; Pommergaard, H.C.; Rasmussen, A. Risk factors for metabolic syndrome after liver transplantation: A systematic review and meta-analysis. Transpl. Rev. 2018, 32, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Borges Migliavaca, C.; Stein, C.; Colpani, V.; Barker, T.H.; Munn, Z.; Falavigna, M. How are systematic reviews of prevalence conducted? A methodological study. BMC Med. Res. Methodol. 2020, 20, 96. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Sample Size | Gender (M) | Age | BMI | Immunosuppressive Regimen | NOS Score |
|---|---|---|---|---|---|---|---|
| Jain AB et al. [31] | 1999 | 121 | 0.56 | 46.3 ± 12.3 | - | Tacrolimus, Cyclosporine, Prednisone, Azathioprine | 4 |
| Driscoll et al. [32] | 2006 | 115 | 0.71 | 48.5 ± 10.9 | 27.4 ± 6.1 | Tacrolimus, Cyclosporine, Sirolimus, MMF, Azathioprine | 6 |
| Saliba et al. [42] | 2007 | 211 | 0.71 | 52.7 ± 9.8 | 25.3 ± 4.68 | Tacrolimus, Steroids | 6 |
| Oufroukhi et al. [41] | 2008 | 141 | 0.67 | 52.6 ± 10 | 24.7 ± 5 | Tacrolimus, Steroids, MMF | 5 |
| Zhao et al. [38] | 2009 | 84 | 0.83 | 42.5 ± 9.1 | 22.2 ± 3.2 | Tacrolimus, Cyclosporine | 4 |
| Carey et al. [36] | 2011 | 225 | 0.71 | 51.7 ± 9.8 | 28.1 ± 5.2 | Tacrolimus, Cyclosporine, Sirolimus | 6 |
| Honda et al. [45] | 2013 | 161 | 0.47 | 47.2 ± 12.9 | 22.6 ± 3.8 | Tacrolimus, Cyclosporine, Steroids, MMF | 5 |
| Rubin et al. [48] | 2013 | 158 | 0.67 | 44.75 ± 9.60 | 25.25 ± 3.39 | Cyclosporine, Tacrolimus, Azathioprine, Steroids | 6 |
| Cho et al. [53] | 2014 | 364 | 0.69 | 49.98 ± 9.18 | 23.62 ± 3.23 | Steroids | 6 |
| Parvizi et al. [52] | 2014 | 350 | 0.58 | 39.24 ± 16.24 | 22.70 ± 4.63 | Tacrolimus, Mycophenolate, Prednisone | 4 |
| Varghese et al. [44] | 2014 | 32 | 0.90 | 44.3 ± 12.4 | - | Tacrolimus, MMF | 4 |
| Younossi et al. [34] | 2014 | 18,571 | 0.75 | 54.04 ± 7.37 | 27.98 ± 5.45 | Tacrolimus, MMF, Steroids | 5 |
| Cuervas-Mons et al. [47] | 2015 | 117 | 0.79 | 55.60 ± 8.31 | 27.77 ± 4.48 | Tacrolimus | 4 |
| Gebhardt et al. [51] | 2015 | 81 | 0.70 | 55.10 ± 10.67 | 28.92 ± 6.05 | Tacrolimus, Prednisone | 4 |
| Hartog et al. [55] | 2015 | 430 | 0.8 | 48.25 ± 9.23 | 26.4 ± 5.1 | Tacrolimus, Steroids | 4 |
| Stepanova et al. [35] | 2015 | 17,184 | 0.59 | 51.82 ± 12.54 | 27.27 ± 5.87 | Tacrolimus, Steroids, MMF | 6 |
| Liu et al. [54] | 2016 | 2248 | 0.69 | 43.95 ± 19.14 | - | Tacrolimus, Cyclosporine, MMF, Rapamune, Everolimus | 5 |
| Li et al. [13] | 2016 | 18,741 | 0.67 | 53.62 ± 10.36 | 28.11 ± 5.79 | Tacrolimus, Steroids, MMF | 6 |
| Ling et al. [6] | 2016 | 10,204 | 0.83 | 48.20 ± 10.01 | 22.79 ± 2.91 | Tacrolimus, Cyclosporine, Corticosteroids | 5 |
| Saliba et al. [40] | 2016 | 180 | 0.81 | 54.25 ± 8.42 | - | MMF | 6 |
| Song et al. [37] | 2016 | 528 | 0.85 | 44.93 ± 9.41 | - | Tacrolimus, Corticosteroids, MMF | 5 |
| Yagi et al. [46] | 2016 | 175 | 0.50 | 51 ± 11 | 23.8 ± 0.3 | Tacrolimus, MMF | 5 |
| Cen et al. [39] | 2017 | 256 | 0.84 | 47.92 ± 7.34 | 22.53 ± 3.02 | Tacrolimus, MMF, Steroids | 4 |
| Xue et al. [15] | 2017 | 763 | 0.85 | 48.78 ± 10.23 | 23.06 ± 2.4 | Tacrolimus, MMF, Corticosteroids | 4 |
| Lieber et al. [33] | 2019 | 415 | 0.68 | 54.38 | 28.87 | Tacrolimus, Mycophenolate, Sirolimus | 5 |
| Aravinthan et al. [49] | 2019 | 2209 | 0.67 | 53.67 ± 9.64 | 26.67 ± 5.19 | Tacrolimus, Cyclosporine, Sirolimus, Prednisone, Mycophenolate | 6 |
| Oommen et al. [43] | 2020 | 51 | - | 45.6 ± 9.6 | 24.52 ± 4.63 | Tacrolimus, Glucocorticoids, Azathioprine, Mycophenolate | 4 |
| Hashim et al. [50] | 2020 | 100 | 0.91 | 52 ± 7.7 | 27.2 ± 4.4 | Tacrolimus, Cyclosporine, mTOR, Steroids | 5 |
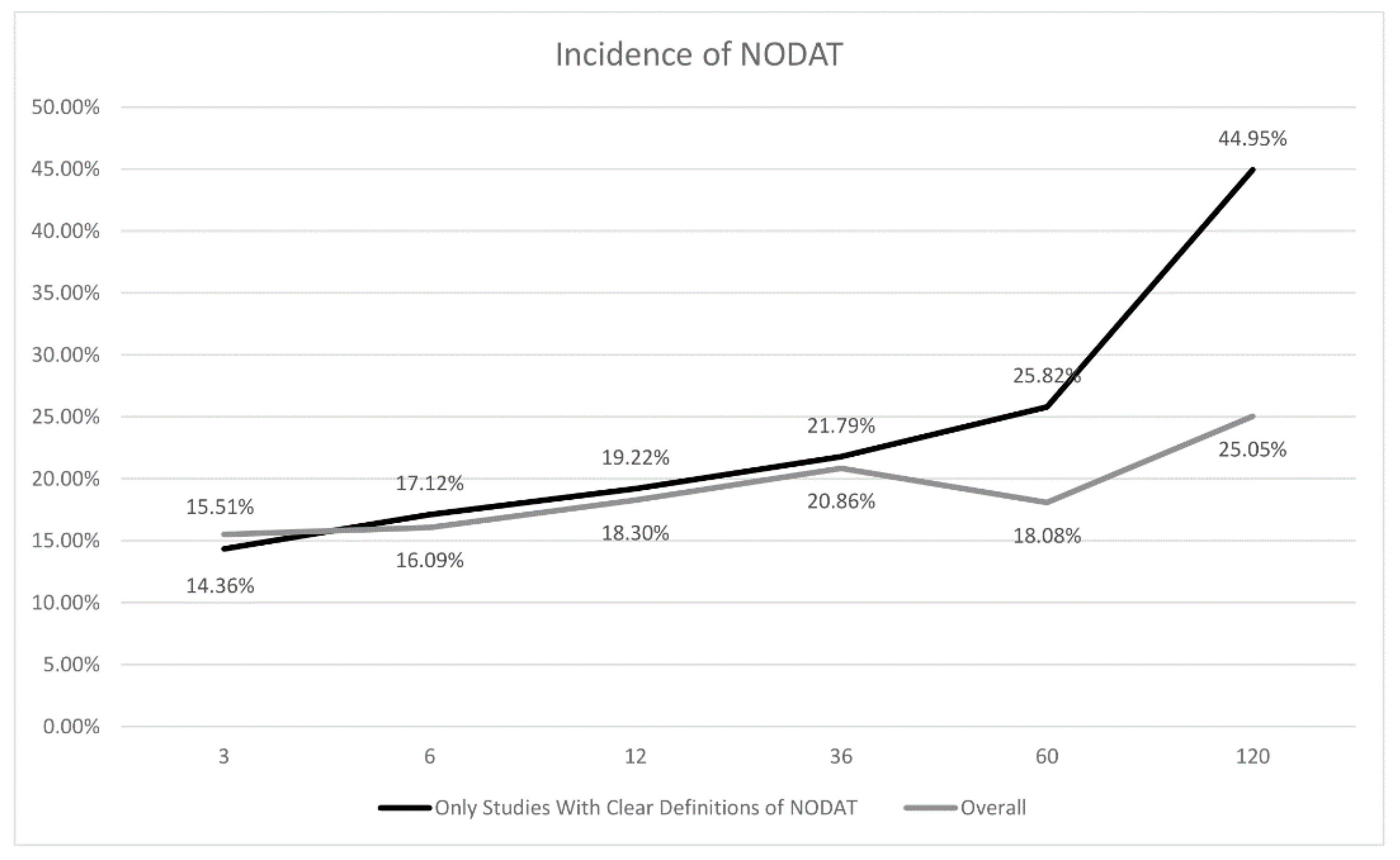
| Study Period | Total Papers | Total Sample Size | Pooled Incidence | Total Sample Size (after Sensitivity Analysis) | Pooled Incidence | Incidence of NODAT from Studies from Predominantly Western Populations | Incidence of NODAT from Studies from Predominantly Asian Populations | p-Value |
|---|---|---|---|---|---|---|---|---|
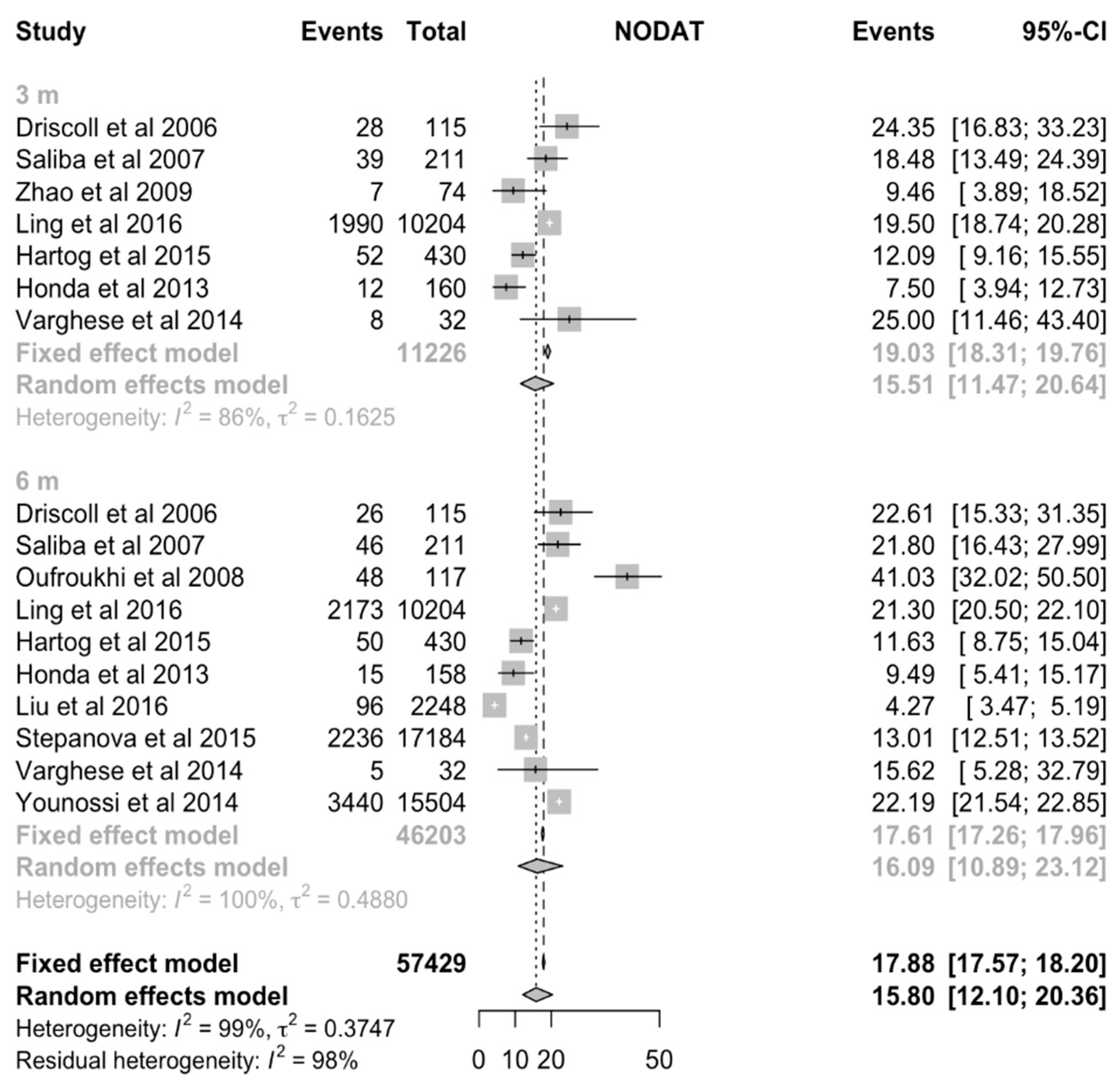
| 3 months | 7 | 11226 | 15.51% (CI:11.47% to 20.65%) | 11367 | 14.36% (CI:11.09% to 18.38%) | 14.65% (CI:10.81% to 19.54%) | 13.97% (CI: 9.65% to 19.81%) | 0.8424 |
| 6 months | 10 | 46203 | 16.09% (CI:10.89% to 23.12%) | 11291 | 17.12% (CI:12.68% to 22.70%) | 15.87% (CI:10.07% to 24.11%) | 17.83% (CI:12.08% to 25.51%) | 0.6926 |
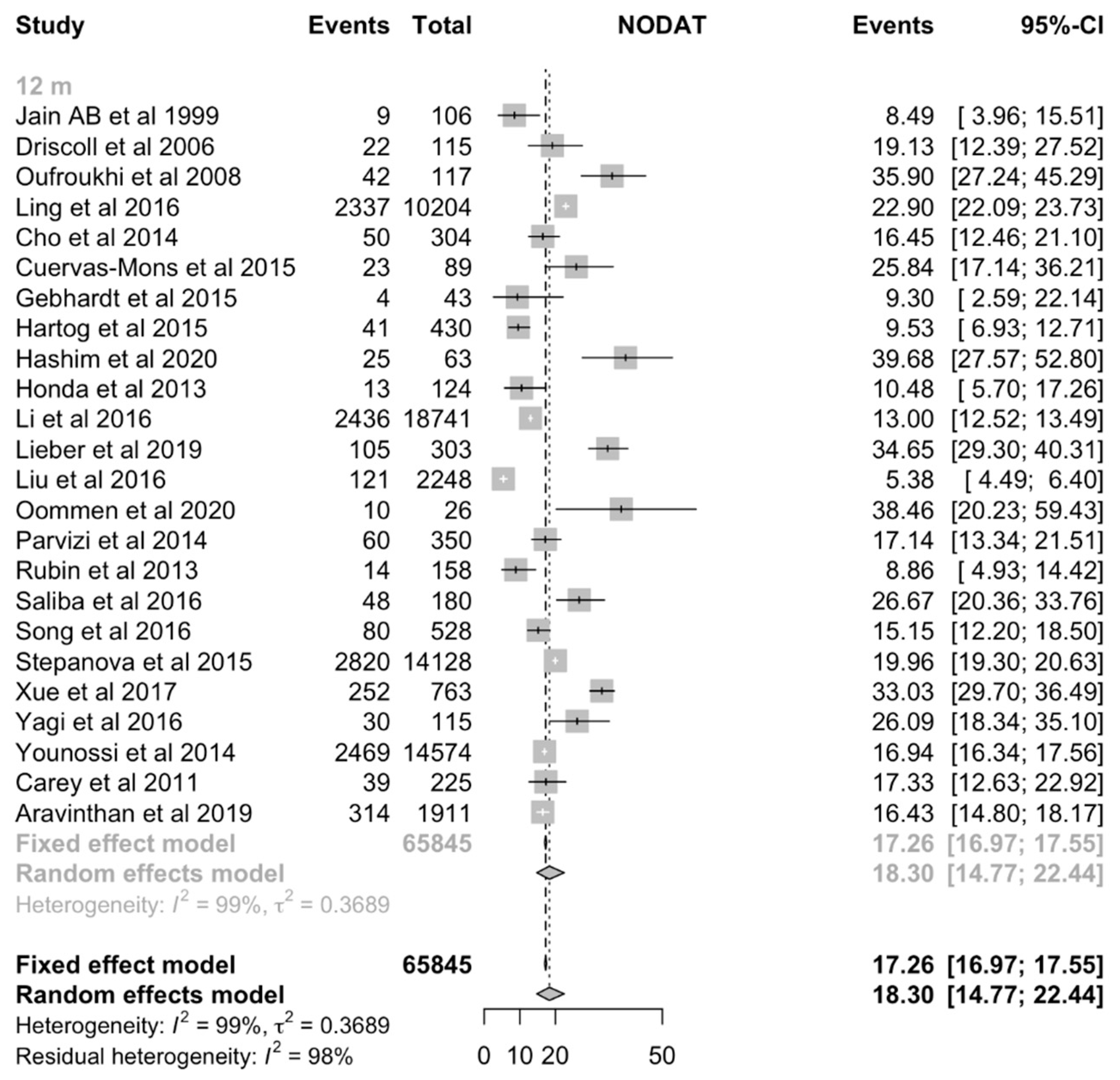
| 1 year | 24 | 65845 | 18.30% (CI:14.78% to 22.44%) | 64298 | 19.22% (CI:15.30% to 23.88%) | 16.39% (CI:12.83% to 20.29%) | 20.04% (CI:15.51% to 25.00%) | 0.2318 |
| 3 years | 10 | 40882 | 20.86% (CI:12.95% to 31.84%) | 20711 | 21.79% (CI:11.19% to 38.12%) | 14.19% (CI:1.62% to 62.46%) | 26.35% (CI:18.44% to 36.16%) | 0.5204 |
| 5 years | 10 | 35680 | 18.08% (CI:10.25% to 29.90%) | 20318 | 25.82% (CI:10.90% to 49.73%) | 20.77% (CI: 2.88% to 69.87%) | 30.94% (CI:16.61% to 50.20%) | 0.6516 |
| 10 years | 4 | 3697 | 25.05% (CI:11.17% to 47.05%) | 1291 | 44.95% (CI:30.56% to 60.24%) | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, Y.H.; Tan, H.Q.M.; Ng, C.H.; Tan, D.J.H.; Lin, S.Y.; Huang, D.Q.; Khoo, C.M.; Muthiah, M.D. A Time-Based Meta-Analysis on the Incidence of New Onset Diabetes after Liver Transplantation. J. Clin. Med. 2021, 10, 1045. https://doi.org/10.3390/jcm10051045
Chin YH, Tan HQM, Ng CH, Tan DJH, Lin SY, Huang DQ, Khoo CM, Muthiah MD. A Time-Based Meta-Analysis on the Incidence of New Onset Diabetes after Liver Transplantation. Journal of Clinical Medicine. 2021; 10(5):1045. https://doi.org/10.3390/jcm10051045
Chicago/Turabian StyleChin, Yip Han, Hon Qin Marcus Tan, Cheng Han Ng, Darren Jun Hao Tan, Snow Yunni Lin, Daniel Q. Huang, Chin Meng Khoo, and Mark Dhinesh Muthiah. 2021. "A Time-Based Meta-Analysis on the Incidence of New Onset Diabetes after Liver Transplantation" Journal of Clinical Medicine 10, no. 5: 1045. https://doi.org/10.3390/jcm10051045
APA StyleChin, Y. H., Tan, H. Q. M., Ng, C. H., Tan, D. J. H., Lin, S. Y., Huang, D. Q., Khoo, C. M., & Muthiah, M. D. (2021). A Time-Based Meta-Analysis on the Incidence of New Onset Diabetes after Liver Transplantation. Journal of Clinical Medicine, 10(5), 1045. https://doi.org/10.3390/jcm10051045






