The Effect of Ventilation with Individualized Positive End-Expiratory Pressure on Postoperative Atelectasis in Patients Undergoing Robot-Assisted Radical Prostatectomy: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
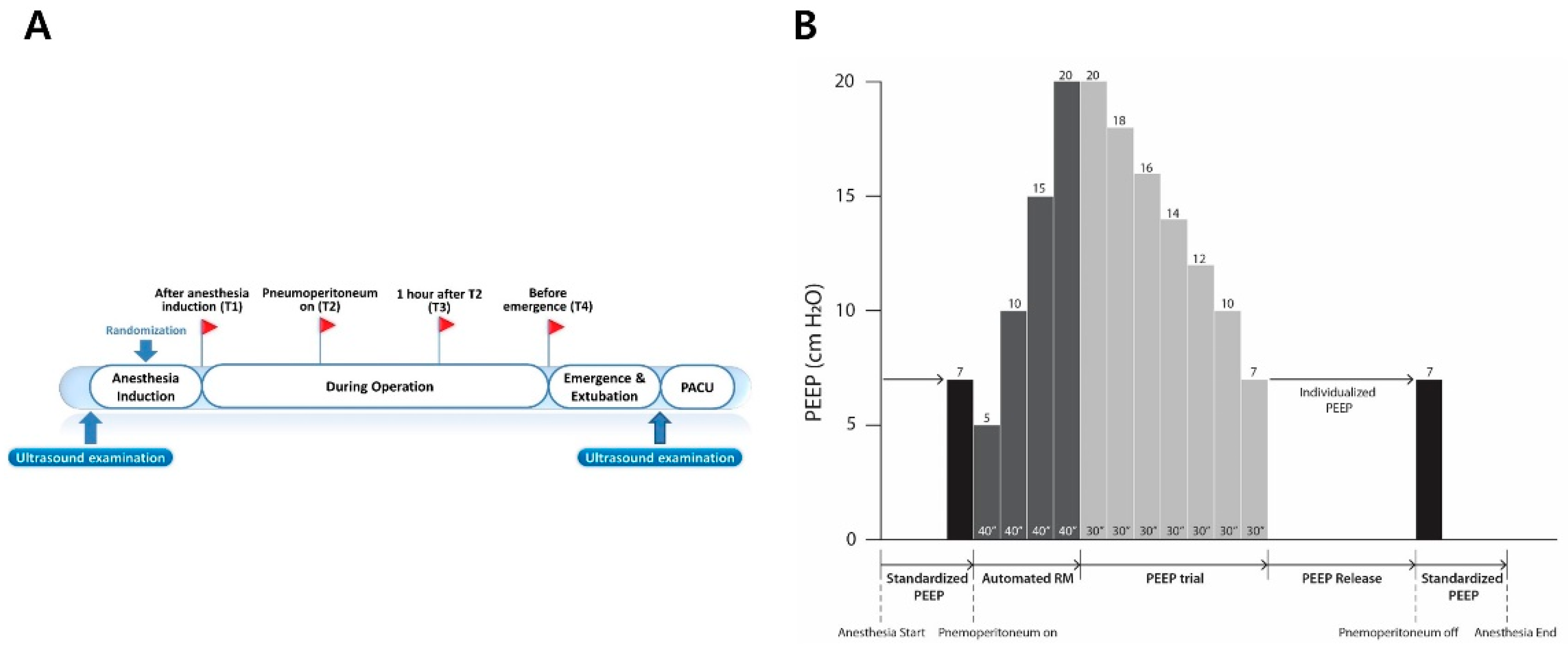
2.1. Trial Design and Participants
2.2. Anesthesia and Study Interventions
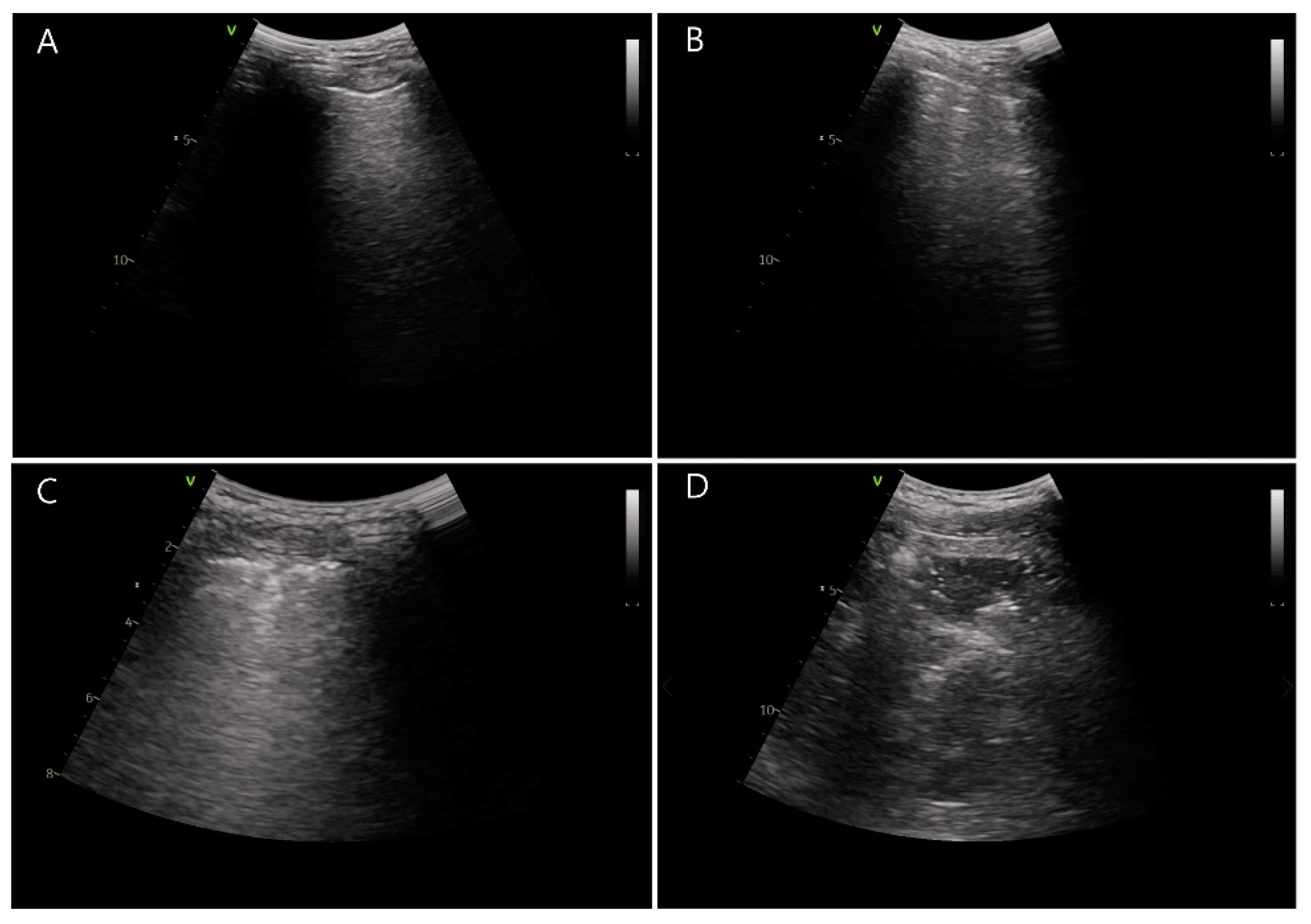
2.3. Study Outcomes
2.4. Statistical Analysis
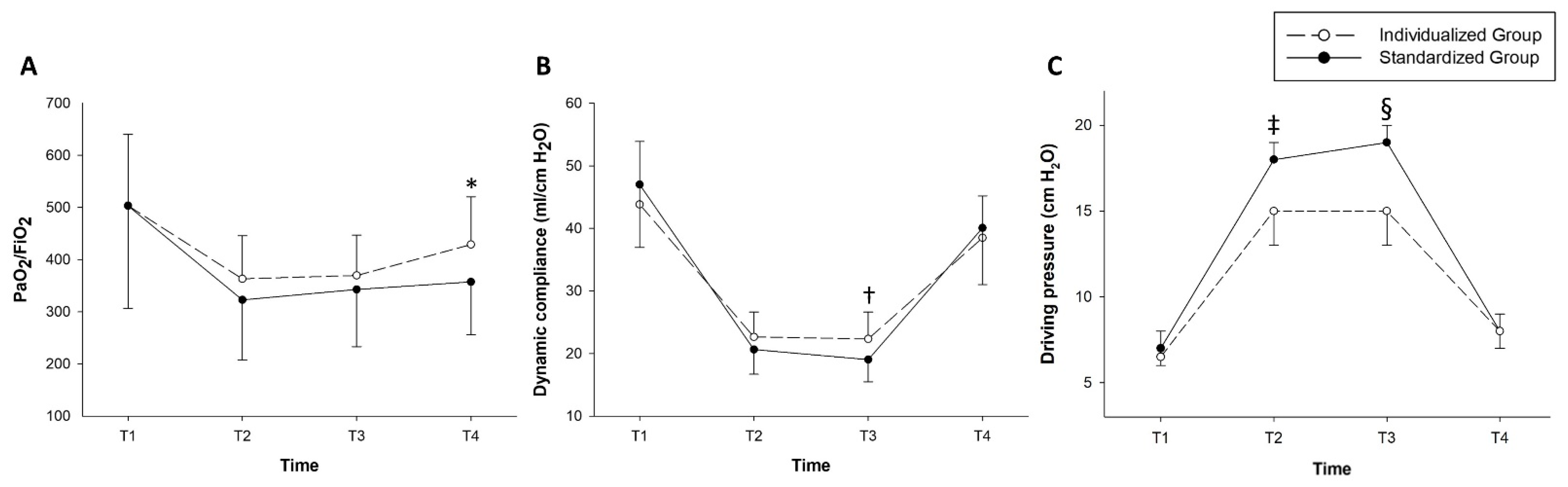
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bill-Axelson, A.; Holmberg, L.; Ruutu, M.; Garmo, H.; Stark, J.R.; Busch, C.; Nordling, S.; Häggman, M.; Andersson, S.-O.; Bratell, S.; et al. Radical Prostatectomy versus Watchful Waiting in Early Prostate Cancer. N. Engl. J. Med. 2011, 364, 1708–1717. [Google Scholar] [CrossRef]
- Trinh, Q.-D.; Sammon, J.; Sun, M.; Ravi, P.; Ghani, K.R.; Bianchi, M.; Jeong, W.; Shariat, S.F.; Hansen, J.; Schmitges, J.; et al. Perioperative Outcomes of Robot-Assisted Radical Prostatectomy Compared With Open Radical Prostatectomy: Results From the Nationwide Inpatient Sample. Eur. Urol. 2012, 61, 679–685. [Google Scholar] [CrossRef]
- Phong, S.V.N.; Koh, L.K.D. Anaesthesia for Robotic-Assisted Radical Prostatectomy: Considerations for Laparoscopy in the Trendelenburg Position. Anaesth. Intensive Care 2007, 35, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Strang, C.M.; Hachenberg, T.; Fredén, F.; Hedenstierna, G. Development of atelectasis and arterial to end-tidal Pco2-difference in a porcine model of pneumoperitoneum. Br. J. Anaesth. 2009, 103, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.E.; Bååth, M.; Thörne, A.; Aspelin, P.; Odeberg-Wernerman, S. Effect of Carbon Dioxide Pneumoperitoneum on Development of Atelectasis during Anesthesia, Examined by Spiral Computed Tomography. J. Am. Soc. Anesthesiol. 2005, 102, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Rubini, A.; Del Monte, D.; Catena, V. Effects of the pneumoperitoneum and Trendelenburg position on respiratory mechanics in the rats by the end-inflation occlusion method. Ann. Thorac. Med. 2012, 7, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Ahn, E.J.; Ko, D.D.; Kang, H.; Shin, H.Y.; Baek, C.H.; Jung, Y.H.; Woo, Y.C.; Kim, J.Y.; Koo, G.H. Effects of pneumoperitoneal pressure and position changes on respiratory mechanics during laparoscopic colectomy. Korean J. Anesthesiol. 2012, 63, 419–424. [Google Scholar] [CrossRef]
- Duggan, M.; Kavanagh, B.P.; Warltier, D.C. Pulmonary Atelectasis. Anesthesiology 2005, 102, 838–854. [Google Scholar] [CrossRef]
- Futier, E.; Constantin, J.M.; Jaber, S. Protective lung ventilation in operating room: A systematic review. Minerva Anestesiol. 2013, 80, 726–735. [Google Scholar]
- Young, C.C.; Harris, E.M.; Vacchiano, C.; Bodnar, S.; Bukowy, B.; Elliott, R.R.D.; Migliarese, J.; Ragains, C.; Trethewey, B.; Woodward, A.; et al. Lung-protective ventilation for the surgical patient: International expert panel-based consensus recommendations. Br. J. Anaesth. 2019, 123, 898–913. [Google Scholar] [CrossRef]
- Ferrando, C.; Mugarra, A.; Gutierrez, A.; Carbonell, J.A.; García, M.; Soro, M.; Tusman, G.; Belda, F.J. Setting Individualized Positive End-Expiratory Pressure Level with a Positive End-Expiratory Pressure Decrement Trial After a Recruitment Maneuver Improves Oxygenation and Lung Mechanics During One-Lung Ventilation. Anesth. Analg. 2014, 118, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Bustamante, A.; Sprung, J.; Parker, R.A.; Bartels, K.; Weingarten, T.N.; Kosour, C.; Thompson, B.T.; Melo, M.F.V. Individualized PEEP to optimise respiratory mechanics during abdominal surgery: A pilot randomised controlled trial. Br. J. Anaesth. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ruszkai, Z.; Kiss, E.; László, I.; Bokrétás, G.P.; Vizserálek, D.; Vámossy, I.; Surány, E.; Buzogány, I.; Bajory, Z.; Molnár, Z. Effects of intraoperative positive end-expiratory pressure optimization on respiratory mechanics and the inflammatory response: A randomized controlled trial. J. Clin. Monit. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Pellikka, P.A. Ultrasound of extravascular lung water: A new standard for pulmonary congestion. Eur. Heart J. 2016, 37, 2097–2104. [Google Scholar] [CrossRef]
- Acosta, C.M.; Maidana, G.A.; Jacovitti, D.; Belaunzarán, A.; Cereceda, S.; Rae, E.; Molina, A.; Gonorazky, S.; Bohm, S.H.; Tusman, G. Accuracy of Transthoracic Lung Ultrasound for Diagnosing Anesthesia-induced Atelectasis in Children. Anesthesiology 2014, 120, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Monastesse, A.; Girard, F.; Massicotte, N.; Chartrand-Lefebvre, C.; Girard, M. Lung Ultrasonography for the Assessment of Perioperative Atelectasis. Anesth. Analg. 2017, 124, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.-E.; Ji, S.-H.; Kim, E.-H.; Lee, J.-H.; Kim, J.-T.; Kim, H.-S. Effect of regular alveolar recruitment on intraoperative atelectasis in paediatric patients ventilated in the prone position: A randomised controlled trial. Br. J. Anaesth. 2020, 124, 648–655. [Google Scholar] [CrossRef]
- Généreux, V.; Chassé, M.; Girard, F.; Massicotte, N.; Chartrand-Lefebvre, C.; Girard, M. Effects of positive end-expiratory pressure/recruitment manoeuvres compared with zero end-expiratory pressure on atelectasis during open gynaecological surgery as assessed by ultrasonography: A randomised controlled trial. Br. J. Anaesth. 2020, 124, 101–109. [Google Scholar] [CrossRef]
- Song, I.-K.; Kim, E.-H.; Lee, J.-H.; Kang, P.; Kim, H.-S.; Kim, J.-T. Utility of Perioperative Lung Ultrasound in Pediatric Cardiac Surgery. Anesthesiology 2018, 128, 718–727. [Google Scholar] [CrossRef]
- Song, I.-K.; Kim, E.-H.; Lee, J.-H.; Ro, S.; Kim, H.-S.; Kim, J.-T. Effects of an alveolar recruitment manoeuvre guided by lung ultrasound on anaesthesia-induced atelectasis in infants: A randomised, controlled trial. Anaesth. 2017, 72, 214–222. [Google Scholar] [CrossRef]
- Soummer, A.; Perbet, S.; Brisson, H.; Arbelot, C.; Constantin, J.-M.; Qin Lung Ultrasound Study Group; Rouby, J.-J. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress*. Crit. Care Med. 2012, 40, 2064–2072. [Google Scholar] [CrossRef]
- Volpicelli, G.; International Liaison Committee on Lung Ultrasound (ILC-LUS) for the International Consensus Conference on Lung Ultrasound (ICC-LUS); Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Nestler, C.; Simon, P.; Petroff, D.; Hammermüller, S.; Kamrath, D.; Wolf, S.; Dietrich, A.; Camilo, L.M.; Beda, A.; Carvalho, A.R.; et al. Individualized positive end-expiratory pressure in obese patients during general anaesthesia: A randomized controlled clinical trial using electrical impedance tomography. Br. J. Anaesth. 2017, 119, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Lundquist, H.; Hedenstierna, G.; Strandberg, Å.; Tokics, L.; Brismar, B. CT-Assessment of Dependent Lung Densities in Man during General Anaesthesia. Acta Radiol. 1995, 36, 626–632. [Google Scholar] [CrossRef]
- Gunnarsson, M.L.; Tokics, M.L.; Gustavsson, B.H.; Hedenstierna, M.G. Influence of age on atelectasis formation and gas exchange impairment during general anaesthesia. Br. J. Anaesth. 1991, 66, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hedenstierna, G.; Tokics, L.; Strandberg, Å.; Lundquist, H.; Brismar, B. Correlation of gas exchange impairment to development of atelectasis during anaesthesia and muscle paralysis. Acta Anaesthesiol. Scand. 1986, 30, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, A.F.; Foubert, L.; Hendrickx, J.F.A.; Mottrie, A.; Absalom, A.; Mortier, E.P.; Struys, M.M.R.F. Influence of steep Trendelenburg position and CO2 pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br. J. Anaesth. 2010, 104, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.C.; Brandstetter, R.D.; Brensilver, J.M.; Jung, L.D. Cardiopulmonary Physiology and Pathophysiology as a Consequence of Laparoscopic Surgery. Chest 1996, 110, 810–815. [Google Scholar] [CrossRef]
- Lestar, M.; Gunnarsson, L.; Lagerstrand, L.; Wiklund, P.; Odeberg-Wernerman, S. Hemodynamic Perturbations During Robot-Assisted Laparoscopic Radical Prostatectomy in 45° Trendelenburg Position. Anesth. Analg. 2011, 113, 1069–1075. [Google Scholar] [CrossRef]
- Van Hecke, D.; Bidgoli, J.S.; Van Der Linden, P. Does Lung Compliance Optimization Through PEEP Manipulations Reduce the Incidence of Postoperative Hypoxemia in Laparoscopic Bariatric Surgery? A Randomized Trial. Obes. Surg. 2019, 29, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, C.; Soro, M.; Unzueta, C.; Suarez-Sipmann, F.; Canet, J.; Librero, J.; Pozo, N.; Peiró, S.; Llombart, A.; León, I.; et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): A randomised controlled trial. Lancet Respir. Med. 2018, 6, 193–203. [Google Scholar] [CrossRef]
- Brandão, J.C.; Lessa, M.A.; Motta-Ribeiro, G.; Hashimoto, S.; Paula, L.F.; Torsani, V.; Le, L.; Bao, X.; Eikermann, M.; Dahl, D.M.; et al. Global and Regional Respiratory Mechanics During Robotic-Assisted Laparoscopic Surgery. Anesth. Analg. 2019, 129, 1564–1573. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, K.S.; Jeong, J.S.; Shim, J.C.; Cho, E.S. Optimal positive end-expiratory pressure during robot-assisted laparoscopic radical prostatectomy. Korean J. Anesthesiol. 2013, 65, 244–250. [Google Scholar] [CrossRef][Green Version]
- Östberg, E.; Thorisson, A.; Enlund, M.; Zetterström, H.; Hedenstierna, G.; Edmark, L. Positive End-expiratory Pressure Alone Minimizes Atelectasis Formation in Nonabdominal Surgery. Anesthesiology 2018, 128, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Severgnini, P.; Selmo, G.; Lanza, C.; Chiesa, A.; Frigerio, A.; Bacuzzi, A.; Dionigi, G.; Novario, R.; Gregoretti, C.; De Abreu, M.G.; et al. Protective Mechanical Ventilation during General Anesthesia for Open Abdominal Surgery Improves Postoperative Pulmonary Function. Anesthesiology 2013, 118, 1307–1321. [Google Scholar] [CrossRef]
- Pereira, S.M.; Tucci, M.R.; Morais, C.C.A.; Simões, C.M.; Tonelotto, B.F.F.; Pompeo, M.S.; Kay, F.U.; Pelosi, P.; Vieira, J.E.; Amato, M.B.P. Individual Positive End-expiratory Pressure Settings Optimize Intraoperative Mechanical Ventilation and Reduce Postoperative Atelectasis. Anesthesiology 2018, 129, 1070–1081. [Google Scholar] [CrossRef]
- Girrbach, F.; Petroff, D.; Schulz, S.; Hempel, G.; Lange, M.; Klotz, C.; Scherz, S.; Giannella-Neto, A.; Beda, A.; Jardim-Neto, A.; et al. Individualised positive end-expiratory pressure guided by electrical impedance tomography for robot-assisted laparoscopic radical prostatectomy: A prospective, randomised controlled clinical trial. Br. J. Anaesth. 2020, 125, 373–382. [Google Scholar] [CrossRef]
- Mazzinari, G.; Diaz-Cambronero, O.; Alonso-Iñigo, J.M.; Garcia-Gregorio, N.; Ayas-Montero, B.; Ibañez, J.L.; Neto, A.S.; Ball, L.; De Abreu, M.G.; Pelosi, P.; et al. Intraabdominal Pressure Targeted Positive End-expiratory Pressure during Laparoscopic Surgery. Anesthesiology 2020, 132, 667–677. [Google Scholar] [CrossRef]
- Park, M.; Ahn, H.J.; Kim, J.A.; Yang, M.; Heo, B.Y.; Choi, J.W.; Kim, Y.R.; Lee, S.H.; Jeong, H.; Choi, S.J.; et al. Driving Pressure during Thoracic Surgery. Anesthesiology 2019, 130, 385–393. [Google Scholar] [CrossRef]
- The DESIGNATION–investigators Driving Pressure During General Anesthesia for Open Abdominal Surgery (DESIGNATION): Study protocol of a randomized clinical trial. Trials 2020, 21, 1–15. [CrossRef]

| Characteristic | Individualized Group (n = 30) | Standardized Group (n = 30) | Standardized Differences | p-Values |
|---|---|---|---|---|
| Demographic data | ||||
| Age, year | 64.5 ± 6.6 | 67.3 ± 6.4 | 0.419 | 0.131 |
| Height, cm | 166.8 ± 4.2 | 166.8 ± 5.7 | 0.019 | 0.951 |
| Weight, cm | 69.1 ± 10.1 | 70.5 ± 9.7 | 0.145 | 0.576 |
| Body mass index, kg/m2 | 24.8 ± 3.2 | 25.3 ± 2.9 | 0.160 | 0.537 |
| Baseline medical status | ||||
| Current smoker, n | 0 (0.0) | 1 (3.3) | 0.263 | 0.999 |
| Hypertension, n | 14 (46.7) | 11 (36.7) | 0.204 | 0.432 |
| Diabetes mellitus, n | 3 (10.0) | 10 (33.3) | 0.591 | 0.028 |
| Cardiac disease, n | 2 (6.7) | 2 (6.7) | <0.001 | 0.999 |
| Neurologic disease, n | 1 (3.3) | 1 (3.3) | <0.001 | 0.999 |
| Thyroid disease, n | 1 (3.3) | 1 (3.3) | <0.001 | 0.999 |
| Dyslipidemia, n | 3 (10.0) | 4 (13.3) | 0.104 | 0.999 |
| Chronic liver disease, n | 3 (10.0) | 2 (6.7) | 0.121 | 0.999 |
| Preoperative laboratory findings | ||||
| Hemoglobin, g/dL | 14.2 ± 1.0 | 14.0 ± 0.9 | 0.213 | 0.412 |
| Hematocrit, % | 42.4 ± 2.7 | 41.3 ± 2.6 | 0.385 | 0.141 |
| Albumin, g/dL | 4.3 (4.2–4.5) | 4.4 (4.2–4.6) | 0.143 | 0.371 |
| Glucose, mg/dL | 105.0 (98.0–117.0) | 110.5 (102.0–143.0) | 0.412 | 0.195 |
| Serum creatinine, mg/dL | 0.92 (0.82–1.02) | 0.93 (0.78–1.02) | 0.079 | 0.871 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 82.0 (72.8–93.9) | 81.5 (72.4–97.3) | 0.096 | 0.982 |
| C-reactive protein, mg/dL | 0.09 (0.04–0.19) | 0.07 (0.04–0.13) | 0.204 | 0.406 |
| Prostate size, mL | 37.1 (33.8–42.6) | 35.7 (29.7–43.0) | 0.190 | 0.469 |
| Characteristic | Individualized Group (n = 30) | Standardized Group (n = 30) | Standardized Differences | p-Values |
|---|---|---|---|---|
| Surgeons | ||||
| A/B/C/D | 5/4/7/14 | 0/1/8/21 | 0.799 | 0.041 |
| Intraoperative variables | ||||
| Operation time, min | 120.0 (95.0–165.0) | 100.0 (90.0–125.0) | 0.672 | 0.023 |
| Anesthesia time, min | 153.5 (130.0–210.0) | 137.5 (125.0–150.0) | 0.690 | 0.024 |
| Duration of pneumoperitoneum, min | 90.0 (75.0–140.0) | 77.5 (70.0–95.0) | 0.522 | 0.106 |
| Administered crystalloid, mL | 1270.0 ± 486.6 | 838.3 ± 347.8 | 1.021 | <0.001 |
| Dose of anesthetics | ||||
| Propofol, mg | 1237.0 (941.0–1391.0) | 1100.0 (1000.0–1288.0) | 0.288 | 0.482 |
| Remifentanil, ng | 1329.9 ± 431.6 | 1221.9 ± 254.5 | 0.305 | 0.244 |
| Estimated blood loss, mL | 240.0 (200.0–300.0) | 175.0 (100.0–300.0) | 0.287 | 0.144 |
| Use of vasopressor | ||||
| Total, % | 27 (90) | 17 (56.7) | 0.800 | 0.004 |
| Ephedrine, % | 25 (83.3) | 17 (56.7) | 0.608 | 0.024 |
| Ephedrine dose, mg | 10.0 (5.0–20.0) | 5.0 (0.0–10.0) | 0.806 | 0.006 |
| Phenylephrine, % | 4 (13.3) | 3 (10.0) | 0.104 | 0.999 |
| Phenylephrine dose, μg | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.215 | 0.701 |
| Intraoperative desaturation, n | 1 (3.3) | 4 (13.3) | 0.119 | 0.353 |
| Characteristic | Individualized Group (n = 30) | Standardized Group (n = 30) | p-Value | Mean or Median or Risk Difference (95% Confidence Interval) |
|---|---|---|---|---|
| Lung ultrasound score | ||||
| Preoperative baseline | 6.0 (4.0–11.0) | 4.5 (3.0–9.0) | 0.143 | −1.0 (−4.0 to 0.0) |
| After extubation | 8.1 ± 5.7 | 12.2 ± 4.2 | 0.002 | −4.13 (−6.74 to –1.53) |
| Difference | −0.5 ± 2.7 | 6.0 ± 2.9 | <0.001 | −6.53 (−8.00 to –5.07) |
| Postoperative laboratory findings (POD #1) | ||||
| Hemoglobin, g/dL | 12.8 ± 1.0 | 12.9 ± 1.0 | 0.608 | −0.13 (−0.65 to 0.38) |
| Hematocrit, % | 38.1 (37.5–39.4) | 38.3 (36.3–40.0) | 0.918 | 0.05 (–1.30 to 1.30) |
| Albumin, g/dL | 3.7 ± 0.2 | 3.8 ± 0.3 | 0.023 | −0.15 (−0.28 to −0.22) |
| Glucose, mg/dL | 112.0 (105.0–120.0) | 118.5 (106.0–129.0) | 0.311 | 5.00 (−4.00 to 13.00) |
| Serum creatinine, mg/dL | 0.84 (0.77–0.96) | 0.87 (0.74–0.98) | 0.947 | 0.00 (−0.08 to 0.09) |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 89.9 ± 18.2 | 89.4 ± 19.1 | 0.926 | 0.45 (−9.19 to 10.09) |
| C-reactive protein, mg/dL | 3.6 ± 1.6 | 3.7 ± 1.3 | 0.766 | −0.11 (−0.85 to 0.63) |
| Postoperative respiratory complications | ||||
| Total | 10 (33.3) | 15 (50.0) | 0.190 | 0.50 (0.18 to 1.42) |
| Hypoxemia, n | 6 (20.0) | 11 (36.7) | 0.152 | 0.43 (0.14 to 1.38) |
| Bronchospasm, n | 0 (0.0) | 0 (0.0) | NA | NA |
| Laryngospasm, n | 0 (0.0) | 0 (0.0) | NA | NA |
| Pneumothorax, n | 0 (0.0) | 0 (0.0) | NA | NA |
| Pleural effusion, n | 0 (0.0) | 0 (0.0) | NA | NA |
| Atelectasis, n | 6 (20.0) | 5 (16.7) | 0.739 | 1.25 (0.34 to 4.64) |
| Pulmonary infiltration, n | 0 (0.0) | 2 (6.7) | 0.492 | 0.48 (0.37 to 0.63) |
| Postoperative other complications | ||||
| Total | 2 (6.7) | 3 (10.0) | 0.999 | 0.64 (0.10 to 4.15) |
| Anastomosis site leakage, n | 1 (3.3) | 1 (3.3) | ||
| Acute kidney injury, n | 1 (3.3) | 1 (3.3) | ||
| Variant angina, n | 0 (0.0) | 1 (3.3) | ||
| Subcutaneous emphysema, n | 2 (6.7) | 3 (10.0) | 0.999 | 0.64 (0.10 to 4.15) |
| Length of hospital stay, day | 5.0 (5.0–5.0) | 5.0 (5.0–5.0) | 0.410 | 0.00 (0.00 to 0.00) |
| ICU admission, n | 0 (0.0) | 0 (0.0) | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.-K.; Kim, B.R.; Yoon, S.; Jeong, Y.H.; Ku, J.H.; Kim, W.H. The Effect of Ventilation with Individualized Positive End-Expiratory Pressure on Postoperative Atelectasis in Patients Undergoing Robot-Assisted Radical Prostatectomy: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 850. https://doi.org/10.3390/jcm10040850
Yoon H-K, Kim BR, Yoon S, Jeong YH, Ku JH, Kim WH. The Effect of Ventilation with Individualized Positive End-Expiratory Pressure on Postoperative Atelectasis in Patients Undergoing Robot-Assisted Radical Prostatectomy: A Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(4):850. https://doi.org/10.3390/jcm10040850
Chicago/Turabian StyleYoon, Hyun-Kyu, Bo Rim Kim, Susie Yoon, Young Hyun Jeong, Ja Hyeon Ku, and Won Ho Kim. 2021. "The Effect of Ventilation with Individualized Positive End-Expiratory Pressure on Postoperative Atelectasis in Patients Undergoing Robot-Assisted Radical Prostatectomy: A Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 4: 850. https://doi.org/10.3390/jcm10040850
APA StyleYoon, H.-K., Kim, B. R., Yoon, S., Jeong, Y. H., Ku, J. H., & Kim, W. H. (2021). The Effect of Ventilation with Individualized Positive End-Expiratory Pressure on Postoperative Atelectasis in Patients Undergoing Robot-Assisted Radical Prostatectomy: A Randomized Controlled Trial. Journal of Clinical Medicine, 10(4), 850. https://doi.org/10.3390/jcm10040850






