Gestational Diabetes Mellitus and Infant Adiposity at Birth: A Systematic Review and Meta-Analysis of Therapeutic Interventions
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Quality Assessment
2.5. Data Extraction
2.6. Data Analysis
3. Results
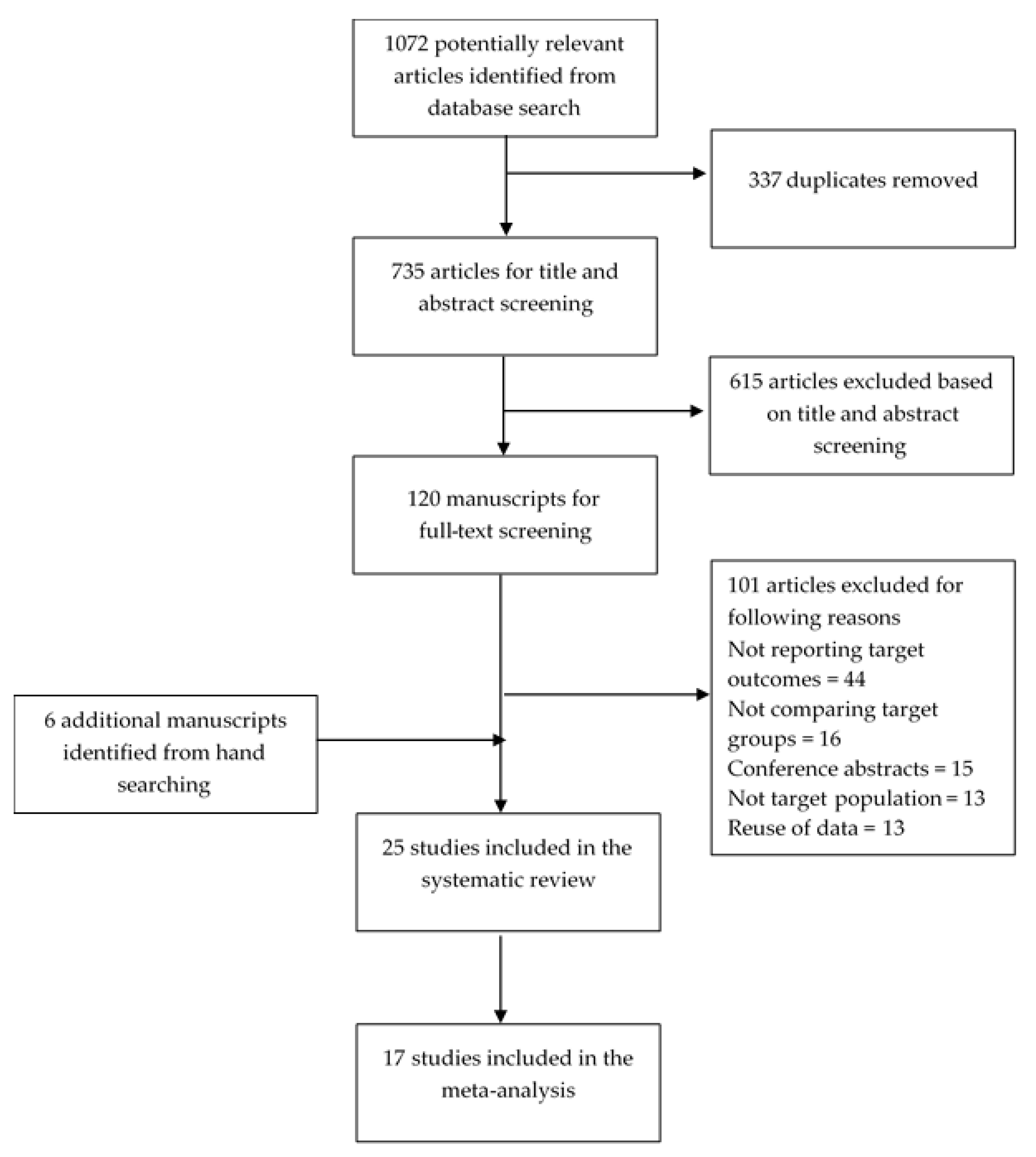
3.1. Study Selection
3.2. Description of the Studies
3.2.1. Treatments Used to Control GDM and Level of Glycaemic Control
3.2.2. Adiposity Assessment Techniques Used in the Studies
3.3. Quality Assessment
3.4. Effects of Treatments for GDM on Infant Adiposity
3.4.1. Treated GDM vs. No Treatment for GDM
3.4.2. Different Treatment Regimens for GDM
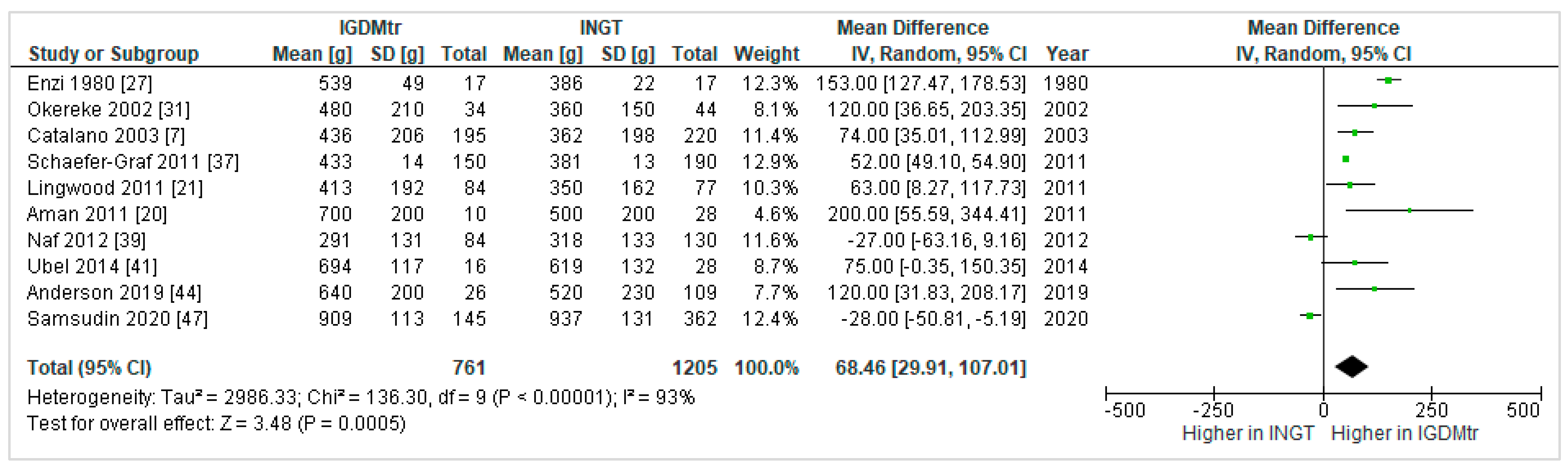
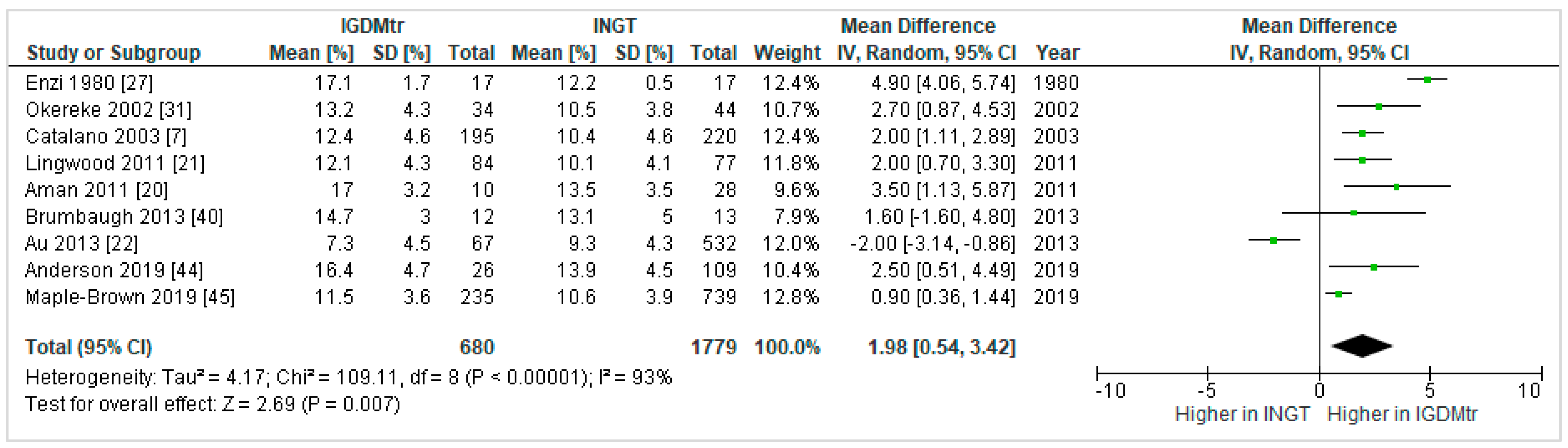
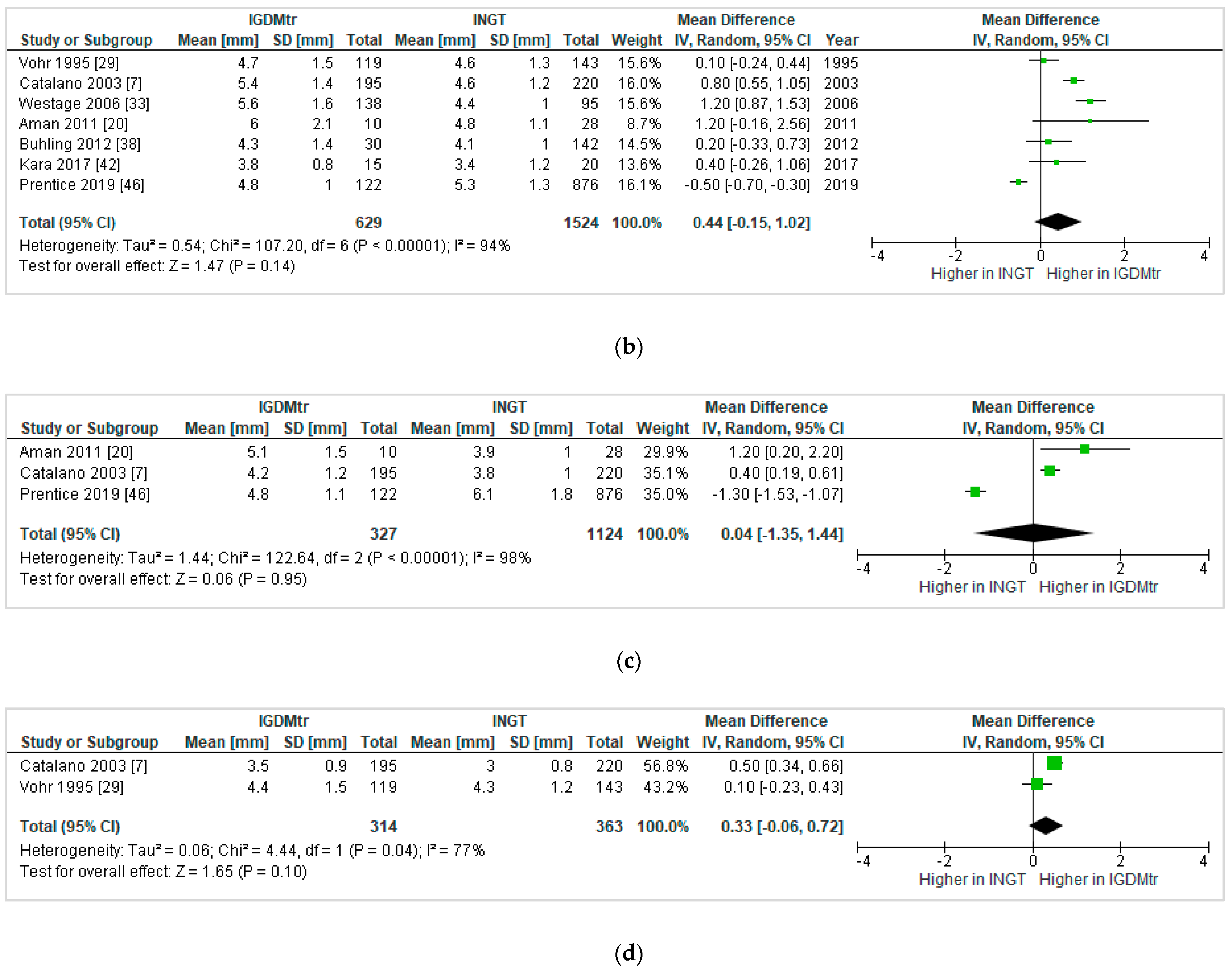
3.4.3. Treated GDM vs. NGT
Fat Mass (FM)
Percent Fat Mass (%FM)
Skinfold Thickness (SFT)
Heterogeneity between the Studies That Compared GDMtr vs. NGT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gyasi-Antwi, P.; Walker, L.; Moody, C.; Okyere, S.; Salt, K.; Anang, L.; Eduful, E.; Laryea, D.; Ottie-Boakye, D.; Asah-Opoku, K. Global Prevalence of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. New Am. J. Med. 2020, 1, 1–10. [Google Scholar]
- Catalano, P.M. 10 Short and long term effects of gestational obesity: Clinical observations. Perinat. Program. 2011, 97–106. [Google Scholar] [CrossRef]
- Nijs, H.; Benhalima, K. Gestational Diabetes Mellitus and the Long-Term Risk for Glucose Intolerance and Overweight in the Offspring: A Narrative Review. J. Clin. Med. 2020, 9, 599. [Google Scholar] [CrossRef]
- Isganaitis, E. Developmental Programming of Body Composition: Update on Evidence and Mechanisms. Curr. Diabetes Rep. 2019, 19, 60. [Google Scholar] [CrossRef]
- Pedersen, J.; Osler, M. Hyperglycemia as the cause of characteristic features of the foetus and newborn of diabetic mothers. Dan. Med. Bull. 1961, 8, 78–83. [Google Scholar]
- Desoye, G.; Nolan, C.J. The fetal glucose steal: An underappreciated phenomenon in diabetic pregnancy. Diabetology 2016, 59, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Thomas, A.; Huston-Presley, L.; Amini, S.B. Increased fetal adiposity: A very sensitive marker of abnormal in utero development. Am. J. Obstet. Gynecol. 2003, 189, 1698–1704. [Google Scholar] [CrossRef]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 37, S81–S90. [CrossRef]
- American Diabetes Association 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes—2019. Diabetes Care 2018, 42, S165–S172. [Google Scholar] [CrossRef]
- American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [PubMed]
- The HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with Neonatal Anthropometrics. Diabetes 2008, 58, 453–459. [CrossRef]
- Chi, C.; Loy, S.L.; Chan, S.-Y.; Choong, C.; Cai, S.; Soh, S.E.; Tan, K.H.; Yap, F.; Gluckman, P.D.; Godfrey, K.M.; et al. Impact of adopting the 2013 World Health Organization criteria for diagnosis of gestational diabetes in a multi-ethnic Asian cohort: A prospective study. BMC Pregnancy Childbirth 2018, 18, 69. [Google Scholar] [CrossRef]
- Daneshmand, S.S.; Stortz, S.; Morrisey, R.; Faksh, A. Bridging Gaps and Understanding Disparities in Gestational Diabetes Mellitus to Improve Perinatal Outcomes. Diabetes Spectr. 2019, 32, 317–323. [Google Scholar] [CrossRef]
- American Diabetes Association 13. Management of Diabetes in Pregnancy:Standards of Medical Care in Diabetes—2018. Diabetes Care 2017, 41, S137–S143. [Google Scholar] [CrossRef]
- Hartling, L.; Dryden, D.M.; Guthrie, A.; Muise, M.; Vandermeer, B.; Donovan, L. Benefits and Harms of Treating Gestational Diabetes Mellitus: A Systematic Review and Meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann. Intern. Med. 2013, 159, 123–129. [Google Scholar] [CrossRef]
- Yamamoto, J.M.; Kellett, J.E.; Balsells, M.; García-Patterson, A.; Hadar, E.; Solà, I.; Gich, I.; Van Der Beek, E.M.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Gestational Diabetes Mellitus and Diet: A Systematic Review and Meta-analysis of Randomized Controlled Trials Examining the Impact of Modified Dietary Interventions on Maternal Glucose Control and Neonatal Birth Weight. Diabetes Care 2018, 41, 1346–1361. [Google Scholar] [CrossRef] [PubMed]
- García-Patterson, A.; Balsells, M.; Yamamoto, J.M.; Kellett, J.E.; Solà, I.; Gich, I.; Van Der Beek, E.M.; Hadar, E.; Castañeda-Gutiérrez, E.; Heinonen, S.; et al. Usual dietary treatment of gestational diabetes mellitus assessed after control diet in randomized controlled trials: Subanalysis of a systematic review and meta-analysis. Acta Diabetol. 2018, 56, 237–240. [Google Scholar] [CrossRef]
- Poolsup, N.; Suksomboon, N.; Amin, M. Effect of Treatment of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e92485. [Google Scholar] [CrossRef]
- Aisa, M.C.; Cappuccini, B.; Barbati, A.; Clerici, G.; Torlone, E.; Gerli, S.; Di Renzo, G.C. Renal Consequences of Gestational Diabetes Mellitus in Term Neonates: A Multidisciplinary Approach to the DOHaD Perspective in the Prevention and Early Recognition of Neonates of GDM Mothers at Risk of Hypertension and Chronic Renal Diseases in Later Life. J. Clin. Med. 2019, 8, 429. [Google Scholar] [CrossRef]
- Åman, J.; Hansson, U.; Östlund, I.; Wall, K.; Persson, B. Increased Fat Mass and Cardiac Septal Hypertrophy in Newborn Infants of Mothers with Well-Controlled Diabetes during Pregnancy. Neonatology 2011, 100, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, B.E.; Henry, A.M.; D’Emden, M.C.; Fullerton, A.-M.; Mortimer, R.H.; Colditz, P.B.; Le Cao, K.-A.; Callaway, L.K. Determinants of Body Fat in Infants of Women with Gestational Diabetes Mellitus Differ with Fetal Sex. Diabetes Care 2011, 34, 2581–2585. [Google Scholar] [CrossRef] [PubMed]
- Au, C.P.; Raynes-Greenow, C.H.; Turner, R.M.; Carberry, A.E.; Jeffery, H.E. Body Composition Is Normal in Term Infants Born to Mothers with Well-Controlled Gestational Diabetes Mellitus. Diabetes Care 2012, 36, 562–564. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 25 March 2020).
- Kennedy, C.E.; Fonner, V.A.; Armstrong, K.A.; Denison, J.A.; Yeh, P.T.; O’Reilly, K.R.; Sweat, M.D. The Evidence Project risk of bias tool: Assessing study rigor for both randomized and non-randomized intervention studies. Syst. Rev. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Review Manager (RevMan) [Computer Program]. Version 5.4, The Cochrane Collaboration; The Nordic Cochrane Centre: Copenhagen, Denmark, 2020. [Google Scholar]
- Enzi, G.; Inelmen, E.M.; Caretta, F.; Villani, F.; Zanardo, V.; DeBiasi, F. Development of Adipose Tissue in Newborns of Gestational-diabetic and Insulin-dependent Diabetic Mothers. Diabetes 1980, 29, 100–104. [Google Scholar] [CrossRef]
- Stevenson, D.K.; Ochikubo, C.G.; Rodgers, P.A.; Kerner, J.A., Jr. Anthropometry and bilirubin production. J. Perinatol. 1991, 11, 340–342. [Google Scholar]
- Vohr, B.R.; McGarvey, S.T.; Coll, C.G. Effects of Maternal Gestational Diabetes and Adiposity on Neonatal Adiposity and Blood Pressure. Diabetes Care 1995, 18, 467–475. [Google Scholar] [CrossRef]
- Simmons, D.; Robertson, S. Influence of maternal insulin treatment on the infants of women with gestational diabetes. Diabet. Med. 1997, 14, 762–765. [Google Scholar] [CrossRef]
- Okereke, N.C.; Uvena-Celebrezze, J.; Hutson-Presley, L.; Amini, S.B.; Catalano, P.M. The effect of gender and gestational diabetes mellitus on cord leptin concentration. Am. J. Obstet. Gynecol. 2002, 187, 798–803. [Google Scholar] [CrossRef]
- Ng, P.C.; Lee, C.H.; Lam, C.W.K.; Wong, E.; Chan, I.H.S.; Fok, T.F. Plasma Ghrelin and Resistin Concentrations Are Suppressed in Infants of Insulin-Dependent Diabetic Mothers. J. Clin. Endocrinol. Metab. 2004, 89, 5563–5568. [Google Scholar] [CrossRef]
- Westgate, J.A.; Lindsay, R.S.; Beattie, J.; Pattison, N.S.; Gamble, G.; Mildenhall, L.F.; Breier, B.H.; Johnstone, F.D. Hyperinsulinemia in Cord Blood in Mothers with Type 2 Diabetes and Gestational Diabetes Mellitus in New Zealand. Diabetes Care 2006, 29, 1345–1350. [Google Scholar] [CrossRef]
- Rowan, J.A.; Hague, W.M.; Gao, W.; Battin, M.R.; Moore, M.P.; Mi, G.T.I. Metformin versus Insulin for the Treatment of Gestational Diabetes. N. Engl. J. Med. 2008, 358, 2003–2015. [Google Scholar] [CrossRef]
- Lain, K.Y.; Garabedian, M.J.; Daftary, A.; Jeyabalan, A. Neonatal adiposity following maternal treatment of gestational diabetes with glyburide compared with insulin. Am. J. Obstet. Gynecol. 2009, 200, 501.e1–501.e6. [Google Scholar] [CrossRef] [PubMed]
- Landon, M.B.; Spong, C.Y.; Thom, E.; Carpenter, M.W.; Ramin, S.M.; Casey, B.; Wapner, R.J.; Varner, M.W.; Rouse, D.J.; Thorp, J.M.; et al. A Multicenter, Randomized Trial of Treatment for Mild Gestational Diabetes. N. Engl. J. Med. 2009, 361, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Graf, U.M.; Meitzner, K.; Ortega-Senovilla, H.; Graf, K.; Vetter, K.; Abou-Dakn, M.; Herrera, E. Differences in the implications of maternal lipids on fetal metabolism and growth between gestational diabetes mellitus and control pregnancies. Diabet. Med. 2011, 28, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Buhling, K.J.; Doll, I.; Siebert, G.; Catalano, P.M. Relationship between sonographically estimated fetal subcutaneous adipose tissue measurements and neonatal skinfold measurements. Ultrasound Obstet. Gynecol. 2012, 39, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Näf, S.; Escote, X.; Yañez, R.E.; Ballesteros, M.; Simón, I.; Gil, P.; Megía, A.; Vendrell, J. Zinc-α2-Glycoprotein Is Unrelated to Gestational Diabetes: Anthropometric and Metabolic Determinants in Pregnant Women and Their Offspring. PLoS ONE 2012, 7, e47601. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brumbaugh, D.E.; Tearse, P.; Cree-Green, M.; Fenton, L.Z.; Brown, M.; Scherzinger, A.; Reynolds, R.; Alston, M.; Hoffman, C.; Pan, Z.; et al. Intrahepatic Fat Is Increased in the Neonatal Offspring of Obese Women with Gestational Diabetes. J. Pediatr. 2013, 162, 930–936.e1. [Google Scholar] [CrossRef]
- Uebel, K.; Pusch, K.; Gedrich, K.; Schneider, K.-T.M.; Hauner, H.; Bader, B.L. Effect of maternal obesity with and without gestational diabetes on offspring subcutaneous and preperitoneal adipose tissue development from birth up to year-1. BMC Pregnancy Childbirth 2014, 14, 138. [Google Scholar] [CrossRef]
- Kara, M.; Orbak, Z.; Döneray, H.; Ozkan, B.; Akcay, F. The Relationship Between Skinfold Thickness and Leptin, Ghrelin, Adiponectin, and Resistin Levels in Infants of Diabetic Mothers. Fetal Pediatr. Pathol. 2016, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D.; Jacqueminet, S.; Nizard, J.; Tanguy, M.-L.; Ciangura, C.; Lacorte, J.-M.; De Carne, C.; L’Hélias, L.F.; Chavatte-Palmer, P.; Charles, M.-A.; et al. Effect of maternal obesity on birthweight and neonatal fat mass: A prospective clinical trial. PLoS ONE 2017, 12, e0181307. [Google Scholar] [CrossRef]
- Andersson-Hall, U.K.; Järvinen, E.A.J.; Bosaeus, M.H.; Gustavsson, C.E.; Hårsmar, E.J.; Niklasson, C.A.; Albertsson-Wikland, K.G.; Holmäng, A.B. Maternal obesity and gestational diabetes mellitus affect body composition through infancy: The PONCH study. Pediatr. Res. 2018, 85, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Maple-Brown, L.; Lee, I.-L.; Longmore, D.; Barzi, F.; Connors, C.; Boyle, J.A.; Moore, E.; Whitbread, C.; Kirkwood, M.; Graham, S.; et al. Pregnancy and Neonatal Diabetes Outcomes in Remote Australia: The PANDORA study—An observational birth cohort. Int. J. Epidemiol. 2018, 48, 307–318. [Google Scholar] [CrossRef]
- Prentice, P.M.; Olga, L.; Petry, C.J.; Simmons, D.; Murphy, H.R.; Hughes, I.A.; Acerini, C.L.; Ong, K.K.; Dunger, D.B. Reduced size at birth and persisting reductions in adiposity in recent, compared with earlier, cohorts of infants born to mothers with gestational diabetes mellitus. Diabetologia 2019, 62, 1977–1987. [Google Scholar] [CrossRef]
- Samsuddin, S.; Arumugam, P.A.; Amin, S.M.; Yahya, A.; Musa, N.; Lim, L.; Paramasivam, S.S.; Ratnasingam, J.; Ibrahim, L.; Chooi, K.C.; et al. Maternal lipids are associated with newborn adiposity, independent of GDM status, obesity and insulin resistance: A prospective observational cohort study. BJOG Int. J. Obstet. Gynaecol. 2019, 127, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Dauncey, M.J.; Gandy, G.; Gairdner, D. Assessment of total body fat in infancy from skinfold thickness measurements. Arch. Dis. Child. 1977, 52, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Weststrate, J.A.; Deurenberg, P. Body composition in children: Proposal for a method for calculating body fat percentage from total body density or skinfold-thickness measurements. Am. J. Clin. Nutr. 1989, 50, 1104–1115. [Google Scholar] [CrossRef]
- Catalano, P.M.; Thomas, A.J.; Avallone, D.A.; Amini, S.B. Anthropometric estimation of neonatal body composition. Am. J. Obstet. Gynecol. 1995, 173, 1176–1181. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, E.J. Pregnancy and diabetes scenario around the world: Africa. Int. J. Gynecol. Obstet. 2009, 104, S39–S41. [Google Scholar] [CrossRef]
- Ryu, R.J.; Hays, K.E.; Hebert, M.F. Gestational diabetes mellitus management with oral hypoglycemic agents. Semin. Perinatol. 2014, 38, 508–515. [Google Scholar] [CrossRef]
- Balsells, M.; García-Patterson, A.; Solà, I.; Roqué, M.; Gich, I.; Corcoy, R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: A systematic review and meta-analysis. BMJ 2015, 350, h102. [Google Scholar] [CrossRef]
- Hiden, U.; Maier, A.; Bilban, M.; Ghaffari-Tabrizi, N.; Wadsack, C.; Lang, I.; Dohr, G.; Desoye, G. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia 2005, 49, 123–131. [Google Scholar] [CrossRef]
- Hebert, M.F.; X Obstetric-Fetal Pharmacology Research Unit Network; Naraharisetti, S.B.; Krudys, K.M.; Umans, J.G.; Hankins, G.D.V.; Caritis, S.N.; Miodovnik, M.; Mattison, D.R.; Unadkat, J.D.; et al. Are We Optimizing Gestational Diabetes Treatment with Glyburide? The Pharmacologic Basis for Better Clinical Practice. Clin. Pharmacol. Ther. 2009, 85, 607–614. [Google Scholar] [CrossRef]
- Nguyen, L.; Chan, S.-Y.; Teo, A.K.K. Metformin from mother to unborn child—Are there unwarranted effects? EBioMedicine 2018, 35, 394–404. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Aiken, C.E.; Ozanne, S.E. Comparative impact of pharmacological treatments for gestational diabetes on neonatal anthropometry independent of maternal glycaemic control: A systematic review and meta-analysis. PLoS Med. 2020, 17, e1003126. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Aiken, C.E.; Ozanne, S.E. Neonatal, infant, and childhood growth following metformin versus insulin treatment for gestational diabetes: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002848. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Kim, D.; Kim, J.S. Body Fat Distribution and the Risk of Incident Metabolic Syndrome: A Longitudinal Cohort Study. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Brumbaugh, D.E.; Friedman, J.E. Developmental origins of nonalcoholic fatty liver disease. Pediatr. Res. 2014, 75, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Determinants of body composition in breastfed infants using bioimpedance spectroscopy and ultrasound skinfolds—Methods comparison. Pediatr. Res. 2016, 81, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.M.; Emsley, R.J.; Jeffries, S.; Andrzejewska, I.; Hyde, M.J.; Gale, C.; Chappell, K.; Mandalia, S.; Santhakumaran, S.; Parkinson, J.R.; et al. Development of Early Adiposity in Infants of Mothers with Gestational Diabetes Mellitus. Diabetes Care 2016, 39, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.M.; Gale, C.; Hyde, M.J.; Santhakumaran, S.; Modi, N. Diabetes in pregnancy and infant adiposity: Systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 102, F65–F72. [Google Scholar] [CrossRef] [PubMed]

| First Author, Year, Study Design, Time of Data Collection, Location | Study Groups n (Males%) | GDM Identification/Definition | Treatment(s) | Target Blood Glucose Levels (BGLs) and Level of Glycaemic Control | Infants’ Age | Infants’ Body Composition Assessment Method/SKINFOLD Thickness Measurements | Findings |
|---|---|---|---|---|---|---|---|
| (1) Treated GDM vs. no treatment for GDM | |||||||
| Landon, 2009 Randomised trial 2002–2007 Bethesda, MD, USA [36] | Control = 473 Treatment = 485 | At 24th and 30th weeks using 4th International workshop conference criteria | Diet therapy (n = 427) and insulin (n = 36) | Targeted for fasting glucose <5.3 mmol/L or 2-h post-prandial glucose, <6.7 mmol/L Good glycaemic control achieved | Birth | FM was calculated as proposed by Catalano et al., 1995. Flank skinfold (data not given) | FM: Lower in treatment group (427 ± 198 vs. 464 ± 222, p = 0.003) |
| (2) Different treatment regimens for GDM | |||||||
| (a) Studies that measured only skinfolds | |||||||
| Simmons, 1997 1991–1992 Middlemore Hospital and National Women’s Hospital, Auckland, New Zealand [30] | All GDM Non-insulin = 11 (46%) Insulin = 9 (33%) | At 28–32 weeks gestation, using modified O’Sullivan criteria | All women received dietary therapy | Targeted fasting glucose >5.5 mmol/L and/or 2-h post-prandial glucose >7.0 mmol/L | <24 h | Subscapular | SFT: Not significantly different subscapular 5.4 (4.8–7.0) vs. 6.8 (5.0–7.9) |
| Rowan, 2008 randomised, open-label trial 10 New Zealand and Australian urban obstetrical hospitals [34] | All GDM Metformin = 363 Insulin = 370 | According to the criteria of the Australasian Diabetes in Pregnancy Society (ADIPS) | Metformin = 363 Insulin = 370 | Aimed for the capillary glucose levels recommended by the ADIPS (after an overnight fast, <5.5 mmol/L; 2-h post-prandial level, <7.0 mmol/L | <48 h | Triceps and subscapular | SFT: Metformin group not significantly different from insulin group triceps (5.2 ± 1.6 vs. 5.1 ± 1.2, p = 0.30) subscapular (5.2 ± 1.5 vs. 5.2 ± 1.3, p = 0.60) |
| (b) Studies that measured body composition | |||||||
| Catalano, 2003 Prospective cohort 1990–2000 Pregnancy Diabetes Clinic in Cleveland Ohio, USA [7] | NGT = 220 (54%) GDM = 195 (51%) | National Diabetes Data Group criteria | Diet only = 128 Diet + insulin = 67 | Targeted fasting glucose >5.5 mmol/L and/or 2-h post-prandial glucose >6.7 mmol/L; Women maintained glucose values within the target range with diet and exercise (66%), plus insulin (34%) | <72 h | TOBEC | FM: Higher in diet + insulin group (492 ± 215 vs. 407 ± 196, p = 0.006) %FM: Higher in diet + insulin group (13.6 ± 4.6 vs. 11.7 ± 4.5, p = 0.007) |
| Lain, 2009 Randomised clinical trial 2002–2005 Magee-Women’s Hospital, Pittsburgh, Pennsylvania [35] | Insulin = 41 (55.3%) Glyburide = 41 (58.5%) | Carpenter and Coustan criteria. Participants with a glucose level of >7.5 mmol/L had a 3-h 100-g OGTT | Insulin = 41 Glyburide = 41 | Targeted fasting glucose >5.5 mmol/L and/or 2-h post-prandial glucose >6.7 mmol/L. Post-prandial dinner glucose was increased in the glyburide group. | <36 h | TOBEC Triceps, subscapular, suprailiac, and anterior thigh SFT (individual and sum given) | FM: Insulin group not significantly different from glyburide group (370 ± 167 vs. 473 ± 278, p = 0.06) %FM: Insulin group not significantly different from glyburide group (11.2 ± 4.2 vs. 12.8 ± 5.7, p = 0.18) SFT: Insulin group not significantly different from glyburide group triceps (3.9 ± 0.7 vs. 3.9 ± 0.9, p = 0.89), subscapular (4.1 ± 1.0 vs. 4.5 ± 1.3, p = 0.10), suprailiac (2.1 ± 0.6 vs. 2.1 ± 0.6, p = 0.85) and thigh (5.1 ± 1.2 vs. 5.4 ± 1.7, p = 0.28) |
| (3) Treated GDM vs. NGT | ‡ IGDMtr compared to INGT | ||||||
| (a) Studies that measured only skinfolds | |||||||
| Stevenson, 1991 Cross-sectional USA [28] | AGA NGT = 20 LGA NGT = 20 AGA GDM = 13 | O’Sullivan and Mahan criteria | Dietary control | ‘Well-managed GDM’ | <72 h | Triceps | Not significantly different triceps compared to AGA NGT group (5.0 ± 1.1 vs. 4.3 ± 0.8, p > 0.05) and LGA NGT group (5.0 ± 1.1 vs. 6.2 ± 2.0, p = 0.058) |
| Vohr, 1995 Prospective longitudinal cohort 1991–1993 Women and Infants’ hospital, Rhode Island [29] | AGA NGT = 69 AGA GDM = 62 LGA GDM = 57 LGA NGT = 74 | Carpenter and Coustan criteria | Diet only = 385 Diet + insulin = 34 Diet includes 45–50% carbohydrates, 25% protein, and 25% fat. | Targeted fasting glucose >5.5 mmol/L and/or 2-h post-prandial glucose >6.7 mmol/L. The management team worked with all mothers to maintain BGL targets | 20 ± 12 h | Triceps, subscapular, abdominal, suprailiac, and medial calf SFT | AGA GDM vs. AGA NGT Not significantly different triceps (3.5 ± 0.9 vs. 3.6 ± 0.8), subscapular (3.9 ± 1.0 vs. 3.9 ± 0.9), abdominal (3.5 ± 1.0 vs. 3.7 ± 0.9), suprailiac (3.4 ± 0.9 vs. 3.6 ± 1.0) and medial calf (4.8 ± 1.1 vs. 5.1 ± 1.1) LGA GDM vs. LGA NGT Not significantly different subscapular (5.5 ± 1.5 vs. 5.3 ± 1.3), suprailiac (4.9 ± 1.1 vs. 4.5 ± 1.1) and medial calf (6.7 ± 1.3 vs. 6.3 ± 1.1) significantly higher triceps (4.7 ± 1.0 vs. 4.5 ± 1.0) and abdominal (5.3 ± 1.4 vs. 4.9 ± 1.2) LGA GDM vs. AGA GDM Significantly higher subscapular (5.5 ± 1.5 vs. 3.9 ± 1.0), Abdominal (5.3 ± 1.4 vs. 3.5 ± 1.0), suprailiac (4.9 ± 1.1 vs. 3.4 ± 0.9) and medial calf (6.7 ± 1.3 vs. 4.8 ± 1.1) Not significantly different triceps (4.7 ± 1.0 vs. 3.5 ± 0.9)” |
| Ng, 2004 Cross-sectional Prince of Wales Hospital Hong Kong [32] | NGT = 40 (50%) GDM = 42 (45.5%) | ADIPS criteria (1998) | Low-energy diet (1800 kcal/d) | Not reported | <24 h | Triceps and subscapular | SFT: Not significantly different triceps (4.8(4.2–5.1) vs. 4.7(4.1–5.5)) and subscapular (4.8(4.3–5.3) vs. 4.8(4.1–5.3), p > 0.05) |
| Westage, 2006 case-control 1999–2001 Middlemore Hospital, South Auckland New Zealand [33] | NGT = 95 GDM = 138 | Local criteria for diagnosis of GDM fasting glucose ≥5.5 mmol/Land/or a 2-h value after a 75 g glucose load ≥9.0 mmol/l | Insulin, usually as lispro insulin up to three times daily along with Humulin N if target fasting glucose exceeded two occasions or post-prandial readings were consistently high. | Target fasting glucose <5.5 mmol/L and post-prandial readings <6.5 mmol/l. | <24 h | Triceps and scapular | SFT: Significantly higher triceps (5.0 ± 1.2 vs. 4.4 ± 1.0) and scapular (5.6 ± 1.6 vs. 4.4 ± 1.0) |
| Kara, 2017 Cohort Ataturk University, Medical Hospital, Erzurum, Turkey [42] | NGT = 20 GDM = 15groups were matched for gestational age and sex | At 24–28 gestational week using World Health Organisation (WHO) criteria | All were treated with dietary intervention, physical activity recommendation, and lifestyle management. All of them (diabetic) have used insulin therapy. | While the mean HbA1c level of mothers with gestational diabetes was 5.9 ± 1.7%, that of the controls was 5.2 ± 0.33%; there was no significant difference. Therefore, mothers with gestational diabetes were well controlled. | Birth | Triceps, Biceps, subscapular | SFT: Significantly higher triceps (3.9 ± 0.7 vs. 3.3 ± 1.1, p = 0.009) and subscapular (3.8 ± 0.8 vs. 3.4 ± 1.2, p = 0.04) Not significantly different biceps (2.8 ± 0.6 vs. 2.6 ± 0.9, p = 0.32) |
| Mitanchez, 2017 prospective cohort exposure-matched cohort 2010–2013 Paris, France [43] | Lean NGT = 164 Lean GDM = 41 Obese NGT = 120 Obese GDM = 90 | Fasting blood glucose (FBG) in the first trimester for women with BMI ≥30 kg/m2, and a 75 g OGTT between 24–28 weeks regardless of maternal BMI. Women were also screened for GDM at 32 weeks by performing a 75 g OGTT, regardless of maternal BMI. International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria | The first line treatment was dietary intervention with a standard 1800 kcal daily meal plan divided into three meals and snacks. Insulin treatment after two weeks of failed dietary therapy. | Target fasting glucose <5.0 mmol/L and post-prandial level <6.7 mmol/L. | <72 h | Triceps, biceps, suprailiac and subscapular | SFT: Normal weight group Not significantly different sum of SFT (triceps, biceps, subscapular, suprailiac) (18.6 ± 3.7 vs. 17.8 ± 3.1, p > 0.05) Obese group Not significantly different sum of SFT (19.9 ± 44 vs. 19.0 ± 3.5, p > 0.05) |
| Prentice, 2019 Prospective cohort 2001–2009 and 2011–2013 Rosie Maternity Hospital, Cambridge, UK [46] (additional data provided by authors) | Earlier GDM = 98 (53%) Recent GDM = 122 (54%) Recent NGT = 876 (52%) | At around 28 weeks using IADPSG criteria | “Earlier” GDM was mostly treated with diet and lifestyle modification, with or without insulin. 19% of the ‘earlier’ GDM group were not diagnosed and did not receive any treatment. “Recent” all GDM women received standardised dietary and lifestyle advice and metformin and/or insulin if required. | Not reported | <8 days | Triceps, subscapular, flank, quadriceps | SFT: Earlier GDM Not significantly different sum of SFT (triceps, subscapular, flank, quadriceps) (26.0 ± 6.3 vs. 24.6 ± 6.0) Significantly higher skinfold SDS (0.31 ± 0.85 vs. 0.03 ± 0.86) Recent GDM Significantly lower sum of SFT (20.0 ± 3.6 vs. 24.6 ± 6.0) Significantly lower skinfold SDS (−0.41 ± 0.61 vs. 0.03 ± 0.86) |
| Buhling, 2012 Prospective cohort 2005–2006 Hamburg, Germany [38] (additional data provided by authors) | NGT = 142 GDM = 30 | GDM was defined according to the clinic’s guidelines, O’Sullivan criteria. | Treated with diet or diet + insulin. | Not reported | <72 h | Left anterior iliac spine, at the lower angle of the left scapula, at the middle of the femur, above the left quadriceps femoris and at the middle of the left triceps, midway between acromion and olecranon | SFT: Not significantly different all 4 sites triceps, 4.6 ± 0.9 vs. 4.8 ± 1.5, p = 0.67 scapular, 4.3 ± 1.41 vs. 4.1 ± 0.97, p = 0.54 iliac, 4.4 ± 1.3 vs. 4.2 ± 1.0, p = 0.45 femur, 5.2 ± 1.8 vs. 4.7 ± 1.4, p = 0.72 |
| (b) Studies that measured body composition | |||||||
| ‘Dauncy et al. equation [48]’ | |||||||
| Enzi, 1980 Cohort Italy [27] | NGT = 17 GDM = 17 | White’s classification, class A (abnormal glucose tolerance that reverted to normal postpartum) | Low-carbohydrate diet | Not reported | Birth | FM and FM% calculated by Dauncy et al. equation Sum of subscapular, subcostal, tricipital, and crural SFT | FM: Not significantly different (553 ± 49 vs. 386 ± 22) %FM: Significantly higher (17.1 ± 1.7 vs. 12.2 ± 0.5) SFT: Significantly higher sum of SFT (23.0 ± 1.4 vs. 17.8 ± 0.7) |
| Naf, 2012Prospective case-control Joan XXIII University Hospital, Tarragona, Spain [39] | NGT = 130 (46.1%) GDM = 84 (53.2%) | National Diabetes Data Group criteria were used to define GDM before 30 weeks | Diet = 48 Diet + insulin = 29 | Target fasting glucose values <5.3 mmol/L and or 1-h post-prandial values <7.8 mmol/L. GDM women had higher levels of fasting glucose4.5 ± 0.4 vs. 4.8 ± 0.6 mmol/L | <48 h | FM by Dauncy et al. equation. Triceps, biceps, subscapular, and flank skinfold thickness (data not given) | FM: Not significantly different (291 ± 131 vs. 318 ± 133, p = 0.198) |
| ‘Weststrate and Deurenberg equation [49]’ | |||||||
| Ubel, 2014 Cohort Abteilung für Geburtshilfe und Perinatalmedizin der Frauenklinik, Klinikum rechts der Isar, Technische Universität München Munich, Germany [41] | Lean NGT = 15 (46.7%) Obese NGT = 13 (61.5%) Obese GDM = 16 (81.3%) | Hyperglycaemia and Pregnancy Outcome (HAPO) criteria | Diet = 7 Insulin treated = 9 | fasting BGL at 3rd trimester did not significantly differ between the groups and was <5.1 mmol/L | 1 week | FM by the equations of Weststrate and Deurenberg Sum of Biceps, triceps, subscapular, suprailiac | FM: Significantly higher compared to lean NGT (694 ± 117, vs. 583 ± 139, p < 0.05); Not significantly different compared to obese NGT (694 ± 117, vs. 660 ± 114, p > 0.05) SFT: Significantly higher compared to lean NGT (21.6 ± 2.4 vs. 18.9 ± 3.1) Not significantly different compared to obese NGT (21.6 ± 2.4 vs. 20.3 ± 2.6) |
| ‘Catalano et al. equation [50]’ | |||||||
| Aman, 2011 Case-control Örebro University Hospital, Sweden [20] | NGT = 28 GDM = 10 | 2-h capillary whole-blood glucose concentration above 11 mmol/L, following a 75 g OGTT after 24th week of pregnancy | Dietary adjustments and multiple pre-meal insulin injections. | Daily blood glucose target, HbA1c 3.5–5.3% Glycaemic control was fairly good, with mean HbA1c values below the upper reference limit for healthy from the 24th to the 36th week of gestation. | <2 days | FM by Catalano et al., equation. Triceps, subscapular and abdomen flank SFT | FM: Significantly higher (700 ± 200 vs. 500 ± 200, p < 0.01) %FM: Significantly higher (17.0 ± 3.2 vs. 13.5 ± 3.5, p < 0.01) SFT: Significantly higher in triceps (6.6 ± 1.7 vs. 5.3 ± 1.1, p < 0.05) and subscapular (6.0 ± 2.1 vs. 4.8 ± 1.1, p < 0.05)Not significantly different in abdominal flank (5.1 ± 1.5 vs. 3.9 ± 1.0, p > 0.05) |
| Schaefer-Graf,2011 Cohort 2007–2008 Vivantes Medical Centre, Berlin, Germany [37] | NGT = 190 (48.4%) GDM = 150 (44.0%) | American Diabetes Association criteria for measurements in venous plasma. With respect to lower glucose concentrations in capillary compared with venous blood, the threshold for fasting glucose was modified into 5.0 mmol/L, while post challenge capillary glucose levels correspond with those in venous blood. | Dietary instruction and performed self-monitoring of BGL. Insulin therapy given before 36 weeks gestation based on BGL and/or foetal abdominal circumference (AC). | fasting <5.0 mmol/L or 2-h postprandial <6.7 mmol/L or when AC > 75th percentile fasting <5.0 mmol/L or 2-h postprandial <11.1 mmol/L ‘Well-controlled’ Maternal serum glucose levels did not differ between control subjects and women with GDM | <48 h | FM by Catalano et al., equation. | FM: Significantly higher (433 ± 14 vs. 381 ± 13, p < 0.01) |
| Maple-Brown, 2019 Longitudinal cohort study 2011–2017 Northern Territory, Australia [45] | Indigenous NGT = 117 Indigenous GDM/DI p = 278 Non-indigenous NGT = 118 Non-indigenous GDM/DIP* = 461 | GDM were diagnosed by either the ADIPS guidelines or a universal 75 gm OGTT and revised glucose cut points as recommended by the WHO. DIP, was defined as diabetes first identified in pregnancy, but with glucose or HbA1c values higher glucose than GDM), and identified from medical records | Diet only or Metformin only or Insulin only or Metformin and insulin | Not reported | <72 h | FM by Catalano et al. equation. | FM: Not significantly different (11.3 ± 4.2 vs. 11.5 ± 3.7, p = 0.65) Non-indigenous Significantly lower (10.2 ± 3.7 vs. 11.5 ± 3.5, p = 0.0006) |
| Samsuddin, 2020 Prospective cohort 2014–2017 Tertiary antenatal clinic, Kuala Lumpur, Malaysia [47] | Obese NGT = 94 Non-obese NGT = 268 GDM = 145 BMI categories (Asian) Normal:18.5–22.9 kg/m2; Overweight: 23–27.4 kg/m2; Obese: ≥27.5 kg/m2 | FPG ≥ 5.1 mmol/L and/or 2-h glucose ≥7.8 mmol/L after a 75 g OGTT (based on the study centre’s definition and the Malaysian 2015 Clinical Practice Guideline | Nutrition therapy. If >30% of the self-monitoring of blood glucose values is beyond target despite compliance with medical nutrition therapy, insulin therapy is initiated | The glycaemic targets for GDM in the study centre: fasting 3.5–5.1 mmol/L, pre-meals 4.0–5.8 mmol/L, 2-h post-prandial 4.0–6.7 mmol/L. Well-treated GDM mothers (pre-delivery HbA1c 5.3%) | <24 h | FM by Catalano et al. equation. Sum of flank, triceps, subscapular SFT | FM: Not significantly different compared to non-obese NGT (909 ± 113 vs. 924 ± 149, p > 0.05)Significantly lower compared to obese NGT (909 ± 113 vs. 973 ± 149, p < 0.05) SFT: Significantly lower sum of SFT (flank, triceps, subscapular) compared to obese NGT (14.2 ± 3.0 vs. 16.1 ± 5.3, p < 0.05) Not significantly different compared to non-obese NGT (14.2 ± 3.0 vs. 14.4 ± 2.8, p > 0.05) |
| ‘TOBEC’ | |||||||
| Okereke, 2001 Cohort 1998–2000 Metro Health Medical Centre, Cleveland, USA [31] | NGT = 44 (58.8%) GDM = 34 (59.1%) | Carpenter and Coustan criteria | Diet = 23 Diet + insulin = 11 | Not reported | <48 h | TOBEC paediatric model HP-2 | FM: Significantly higher (480 ± 210 vs. 360 ± 150, p = 0.01) %FM: Significantly higher (13.2 ± 4.3 vs. 10.5 ± 3.8, p = 0.01) |
| Catalano, 2003 Prospective cohort 1990–2000Pregnancy Diabetes Clinic in Cleveland Ohio, USA [7] | NGT = 220 (54%) GDM = 195 (51%) | At 26 to 28 weeks using National Diabetes Data Group criteria | Diet only = 128 Diet + insulin = 67 | Targeted fasting glucose >5.5 mmol/L and/or 2-h post-prandial glucose >6.7 mmol/L. Women maintained glucose values within the target range with diet and exercise (66%), plus insulin (34%). | <72 h | TOBEC Triceps and subscapular, flank, thigh, abdominal SFT | FM: Significantly higher (436 ± 206 vs. 362 ± 198, p = 0.0002) %FM: Significantly higher (12.4 ± 4.6 vs. 10.4 ± 4.6, p = 0.0001) SFT: Significantly higher at all 5 sites triceps (4.7 ± 1.1 vs. 4.2 ± 1.3, p = 0.0001) subscapular (5.4 ± 1.4 vs. 4.6 ± 1.2, p = 0.0001) flank (4.2 ± 1.2 vs. 3.8 ± 1.0, p = 0.0001) thigh (6.0 ± 1.4 vs. 5.4 ± 1.5, p = 0.0001) abdominal wall (3.5 ± 0.9 vs. 3.0 ± 0.8, p = 0.0001) |
| ‘ADP (Pea Pod)’ | |||||||
| Brumbaugh, 2013 Cross-sectional University of Colorado Hospital or Denver Health. Colorado, USA [40] | Normal NGT = 13 (53.8%) Obese/GDM = 12 (66.7%) Both groups matched for ethnicity | At 24–28 weeks using Carpenter and Coustan criteria | 2 were diet control, 10 were requiring insulin or glyburide. | Not reported | 1–3 weeks | ADP (Pea Pod) Sum of triceps and subscapular SFT | %FM: Not significantly different 14.7 ± 3.0 vs. 13.1 ± 5.0, p = 0.36 SFT:Significantly higher sum of SFT (11.7 ± 1.3 vs. 9.9 ± 2.0, p = 0.01 |
| Lingwood, 2011 Prospective cohort 2009–2010a Royal Brisbane and Women’s Hospital Queensland, Australia [21] (additional data provided by authors) | NGT = 77 (53%) GDM = 84 (50%) | ADIPS criteria | Dietary and physical activity advice. Insulin treatment was begun if more than two glucose measurements exceeded the target range in 1 week. | Target BGLs were set according to current ADIPS guidelines: 5.5 mmol/L or lower fasting, and 7.0 mmol/L or lower 2-h post-prandial. 80% met both current fasting and post-prandial ADIPS targets. 75% met the lower targets of the American Diabetes Association (5.3 and 6.7 mmol/L) | <6 days | ADP (Pea Pod) | FM: Significantly higher (413 ± 192 vs. 350 ± 162, p = 0.003) %FM: Significantly higher (12.1 ± 4.3 vs. 10.1 ± 4.1, p = 0.003) |
| Au, 2013 Cross-sectional September-October 2010 Royal Prince Alfred Hospital Sydney, Australia [22] | NGT = 532 (53%) GDM = 67 (42%) | ADIPS criteria. | Dietary and physical activity advice. Insulin therapy was commenced when glycaemic targets could not be met. | Good glycaemic control was achieved in 90% of women meeting both fasting and post-prandial ADIPS targets | <48 h | ADP (Pea Pod) | %FM: Not significantly different 7.9 ± 4.5 vs. 9.3 ± 4.3, p = 0.018 |
| Andersson-Hall, 2018 Longitudinal cohort 2009–2018 6 antenatal health units and Sahlgrenska University Hospital Gothenburg, Sweden [44] | Normal weight group 83 (50.6%) Obese group 26 (65.4%) GDM group 26 (38.5%) | All pregnant women had non-fasting blood glucose measured regularly throughout pregnancy, and women with an elevated non-fasting glucose (>8 mmol/L) underwent OGTT. GDM mothers were identified based on the European Association for the Study of Diabetes criteria, at 27 ± 7 gestational weeks. | All 26 received diet and lifestyle advice, 4 received insulin. | Not reported | 4–10 days | ADP (Pea Pod) | FM: Normal weight group Significantly different (640 ± 200 vs. 500 ± 230, p = 0.0034) Obese group Not significantly different sum of SFT (640 ± 200 vs. 580 ± 170, p = 0.29) %FM: Normal weight group Significantly different (16.44 ± 4.68 vs. 13.5 ± 4.6, p = 0.0036) Obese group Not significantly different sum of SFT (16.44 ± 4.68 vs. 15.23 ± 3.86, p = 0.26) |
| First Author | Year | Evidence Project Risk of Bias Tool Items | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Cohort | (2) Control or Comparison Group | (3) Pre/Post Intervention Data | (4) Random Assignment of Participants to the Intervention | (5) Random Selection of Participants for Assessment | (6) Follow-Up Rate of 80% or More a | (7) Comparison Groups Equivalent on Sociodemographic b | (8) Comparison Groups Equivalent at Baseline on Disclosure | ||||
| Judgement | Judgement | Judgement | Judgement | Judgement | Judgement | Follow-U p Rate | Judgement | Comment | Judgement | ||
| Enzi | 1980 | Yes | Yes | No | NA | No | Yes | 87.5% | NR | NA | |
| Stevenson | 1991 | No | Yes | No | NA | No | NA | NR | NA | ||
| Vohr | 1995 | Yes | Yes | No | NA | No | Yes | 100% | NR | NA | |
| Simmons | 1997 | Yes | Yes | No | NA | No | No | 57% | Yes | Ethnicity and sex not significantly different | NA |
| Okereke | 2001 | Yes | Yes | No | NA | No | Yes | 100% | Partial | Sex not significantly different, ethnicity significantly different | NA |
| Catalano | 2003 | Yes | Yes | No | NA | No | Yes | 100% | Partial | Ethnicity significantly different, sex not significantly different | NA |
| Ng | 2004 | No | Yes | No | NA | No | NA | Partial | Sex not significantly different | NA | |
| Westgate | 2006 | No | Yes | No | NA | No | NA | Yes | Sex and ethnicity not significantly different | NA | |
| Rowan | 2008 | Yes | Yes | No | Yes | No | Yes | 97.6% | Partial | Ethnicity not significantly different | NA |
| Lain | 2009 | Yes | Yes | No | Yes | No | Yes | 82.8% | Yes | NA | |
| Landon | 2009 | Yes | Yes | No | Yes | No | Yes | 93.9% | Partial | Ethnicity not significantly different | NA |
| Aman | 2011 | No | Yes | No | NA | No | NA | NR | NA | ||
| Lingwood | 2011 | Yes | Yes | No | NA | No | Yes | 100% | NR | NA | |
| Naf | 2011 | Yes | Yes | No | NA | No | Yes | 100% | Partial | Sex not significantly different | NA |
| Schaefer-Graf | 2011 | Yes | Yes | No | NA | No | Yes | 100% | Partial | Sex not significantly different | NA |
| Au | 2012 | No | Yes | No | NA | No | NA | No | Significant difference in maternal ethnicity | NA | |
| Buhling | 2012 | Yes | Yes | No | NA | No | Yes | 100% | Partial | Ethnicity not significantly different | NA |
| Brumbaugh | 2013 | No | Yes | No | NA | No | NA | Yes | Sex and ethnicity not significantly different | NA | |
| Ubel | 2014 | Yes | Yes | No | NA | No | Yes | 100% | No | Sex significantly different | NA |
| Mitanchez | 2017 | yes | Yes | No | NA | No | Yes | 90.3% | NR | NA | |
| Kara | 2017 | Yes | Yes | No | NA | No | Yes | 100% | Partial | Sex not significantly different | NA |
| Andersson-Hall | 2018 | Yes | Yes | No | NA | No | Yes | 83% | Partial | Sex not significantly different | NA |
| Maple-Brown | 2019 | Yes | Yes | No | NA | No | Yes | 100% | NR | NA | |
| Prentice | 2019 | Yes | Yes | No | NA | No | NR | Partial | Sex not significantly different | NA | |
| Samsuddin | 2020 | Yes | Yes | No | NA | No | Yes | 100% | No | Ethnicity significantly different | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herath, M.P.; Beckett, J.M.; Hills, A.P.; Byrne, N.M.; Ahuja, K.D.K. Gestational Diabetes Mellitus and Infant Adiposity at Birth: A Systematic Review and Meta-Analysis of Therapeutic Interventions. J. Clin. Med. 2021, 10, 835. https://doi.org/10.3390/jcm10040835
Herath MP, Beckett JM, Hills AP, Byrne NM, Ahuja KDK. Gestational Diabetes Mellitus and Infant Adiposity at Birth: A Systematic Review and Meta-Analysis of Therapeutic Interventions. Journal of Clinical Medicine. 2021; 10(4):835. https://doi.org/10.3390/jcm10040835
Chicago/Turabian StyleHerath, Manoja P., Jeffrey M. Beckett, Andrew P. Hills, Nuala M. Byrne, and Kiran D. K. Ahuja. 2021. "Gestational Diabetes Mellitus and Infant Adiposity at Birth: A Systematic Review and Meta-Analysis of Therapeutic Interventions" Journal of Clinical Medicine 10, no. 4: 835. https://doi.org/10.3390/jcm10040835
APA StyleHerath, M. P., Beckett, J. M., Hills, A. P., Byrne, N. M., & Ahuja, K. D. K. (2021). Gestational Diabetes Mellitus and Infant Adiposity at Birth: A Systematic Review and Meta-Analysis of Therapeutic Interventions. Journal of Clinical Medicine, 10(4), 835. https://doi.org/10.3390/jcm10040835








