Risk of Insulin Resistance and Metabolic Syndrome in Women with Hyperandrogenemia: A Comparison between PCOS Phenotypes and Beyond
Abstract
1. Introduction
2. Methods
3. Results
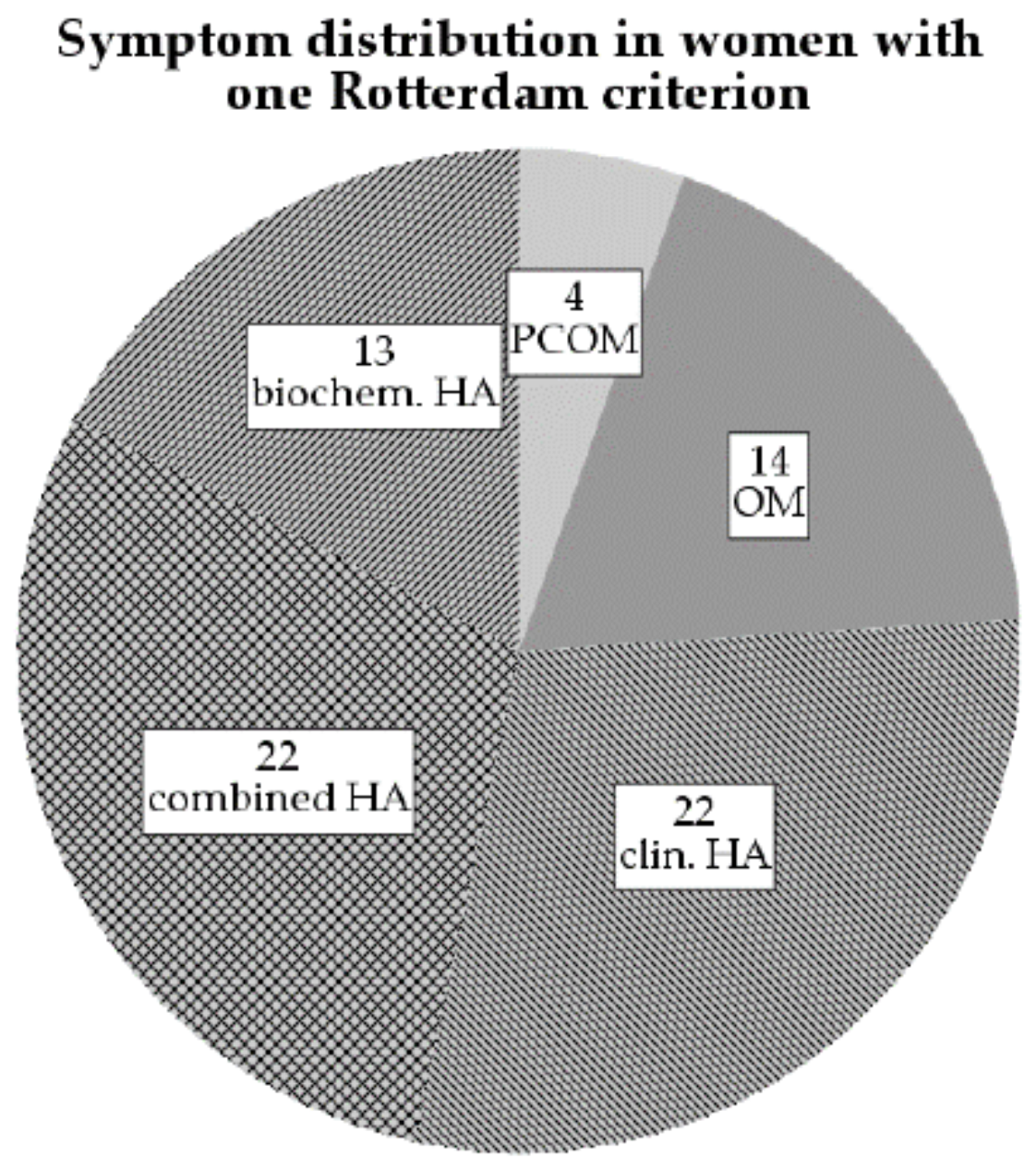
3.1. Phenotype Characteristics
3.2. Hyperandrogenemia Is Associated with Insulin Resistance in Women with One Rotterdam Criterion
3.3. Elevated Free Testosterone (fTesto) Levels Are Associated with an Increased Risk for Insulin Resistance Independently of PCOS Diagnosis and Phenotype
4. Discussion
5. Conclusions
- It is important to distinguish between the various PCOS phenotypes, as they could impact therapy decisions and potential later risk for metabolic diseases.
- Free testosterone was the most indicative androgen for the development and prevalence of insulin resistance and potential later progression to a metabolic syndrome/impaired glucose tolerance.
- Women who do not meet at least two Rotterdam criteria should still be screened for androgen excess, as they also have an increased risk for developing insulin resistance. In case IR is present, lifestyle modification and/or metformin therapy should be considered.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, Prevalence, and Phenotypes of Polycystic Ovary Syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef]
- Sirmans, S.M.; Pate, K.A. Epidemiology, Diagnosis, and Management of Polycystic Ovary Syndrome. Clin. Epidemiol. 2013. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with Polycystic Ovary Syndrome Have Intrinsic Insulin Resistance on Euglycaemic-Hyperinsulaemic Clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Papavassiliou, A.G.; Kandarakis, S.A.; Chrousos, G.P. Pathophysiology and Types of Dyslipidemia in PCOS. Trends Endocrinol. Metab. 2007, 18, 280–285. [Google Scholar] [CrossRef]
- González, F. Inflammation in Polycystic Ovary Syndrome: Underpinning of Insulin Resistance and Ovarian Dysfunction. Steroids 2012. [Google Scholar] [CrossRef]
- Polak, A.M.; Adamska, A.; Krentowska, A.; Łebkowska, A.; Hryniewicka, J.; Adamski, M.; Kowalska, I. Body Composition, Serum Concentrations of Androgens and Insulin Resistance in Different Polycystic Ovary Syndrome Phenotypes. J. Clin. Med. 2020, 9, 732. [Google Scholar] [CrossRef]
- Legro, R.S.; Bentley-Lewis, R.; Driscoll, D.; Wang, S.C.; Dunaif, A. Insulin Resistance in the Sisters of Women with Polycystic Ovary Syndrome: Association with Hyperandrogenemia Rather than Menstrual Irregularity. J. Clin. Endocrinol. Metab. 2002, 87, 2128–2133. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F.; Bonin, C.; Di Sarra, D.; Fiers, T.; Kaufman, J.M.; Giagulli, V.A.; Signori, C.; Zambotti, F.; Dall’Alda, M.; et al. Divergences in Insulin Resistance between the Different Phenotypes of the Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2013, 98. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Tarlatzis, B.C.; Kauffman, R.P. Polycystic Ovarian Syndrome (PCOS): Long-Term Metabolic Consequences. Metabolism 2018, 86, 33–43. [Google Scholar] [CrossRef]
- Teede, H.; Misso, M.; Costello, M.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R. International Evidence- Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome; Monash University: Melbourne, Australia, 2018; Volume 2. [Google Scholar]
- Wehr, E.; Gruber, H.J.; Giuliani, A.; Möller, R.; Pieber, T.R.; Obermayer-Pietsch, B. The Lipid Accumulation Product Is Associated with Impaired Glucose Tolerance in PCOS Women. J. Clin. Endocrinol. Metab. 2011, 96. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Schwetz, V.; Giuliani, A.; Obermayer-Pietsch, B. Hypertriglyceridemic Waist Is Associated with Impaired Glucose Tolerance in Polycystic Ovary Syndrome. Nutr. Metab. Cardiovasc. Dis. 2013, 23. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Schwetz, V.; Giuliani, A.; Obermayer-Pietsch, B. Assessment of Glucose Metabolism in Polycystic Ovary Syndrome: HbA1c or Fasting Glucose Compared with the Oral Glucose Tolerance Test as a Screening Method. Hum. Reprod. 2013, 28, 2537–2544. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Schwetz, V.; Giuliani, A.; Pieber, T.R.; Obermayer-Pietsch, B. Opposing Effects of Dehydroepiandrosterone Sulfate and Free Testosterone on Metabolic Phenotype in Women with Polycystic Ovary Syndrome. Fertil. Steril. 2012, 98. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Gruber, H.J.; Schwetz, V.; Giuliani, A.; Möller, R.; Pieber, T.R.; Obermayer-Pietsch, B. Fatty Liver Index in Polycystic Ovary Syndrome. Eur. J. Endocrinol. 2011, 165, 935–943. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lerchbaum, E.; Giuliani, A.; Gruber, H.J.; Pieber, T.R.; Obermayer-Pietsch, B. Adult-Type Hypolactasia and Calcium Intake in Polycystic Ovary Syndrome. Clin. Endocrinol. 2012, 77, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Schwetz, V.; Rabe, T.; Giuliani, A.; Obermayer-Pietsch, B. Hyperandrogenemia in Polycystic Ovary Syndrome: Exploration of the Role of Free Testosterone and Androstenedione in Metabolic Phenotype. PLoS ONE 2014, 9, e108263. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin Sensitivity Indices Obtained from Oral Glucose Tolerance Testing: Comparison with the Euglycemic Insulin Clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Lee, C.H.; Shih, A.Z.L.; Woo, Y.C.; Fong, C.H.Y.; Leung, O.Y.; Janus, E.; Cheung, B.M.Y.; Lam, K.S.L. Optimal Cut-Offs of Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) to Identify Dysglycemia and Type 2 Diabetes Mellitus: A15-Year Prospective Study in Chinese. PLoS ONE 2016, 11, e163424. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Büttler, R.M.; Martens, F.; Fanelli, F.; Pham, H.T.; Kushnir, M.M.; Janssen, M.J.W.; Owen, L.; Taylor, A.E.; Soeborg, T.; Blankenstein, M.A.; et al. Comparison of 7 Published LC-MS/MS Methods for the Simultaneous Measurement of Testosterone, Androstenedione, and Dehydroepiandrosterone in Serum. Clin. Chem. 2015. [Google Scholar] [CrossRef]
- Büttler, R.M.; Martens, F.; Kushnir, M.M.; Ackermans, M.T.; Blankenstein, M.A.; Heijboer, A.C. Simultaneous Measurement of Testosterone, Androstenedione and Dehydroepiandrosterone (DHEA) in Serum and Plasma Using Isotope-Dilution 2-Dimension Ultra High Performance Liquid-Chromatography Tandem Mass Spectrometry (ID-LC-MS/MS). Clin. Chim. Acta 2014, 438, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Södergard, R.; Bäckström, T.; Shanbhag, V.; Carstensen, H. Calculation of Free and Bound Fractions of Testosterone and Estradiol-17β to Human Plasma Proteins at Body Temperature. J. Steroid Biochem. 1982, 16, 801–810. [Google Scholar] [CrossRef]
- De Ronde, W.; Van Der Schouw, Y.T.; Pols, H.A.P.; Gooren, L.J.G.; Muller, M.; Grobbee, D.E.; De Jong, F.H. Calculation of Bioavailable and Free Testosterone in Men: A Comparison of 5 Published Algorithms. Clin. Chem. 2006, 52, 1777–1784. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.E.; et al. Consensus on Women’s Health Aspects of Polycystic Ovary Syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; Taylor, A.E.; Crabtree, N.J.; Hughes, B.A.; Capper, F.; Crowley, R.K.; Stewart, P.M.; Tomlinson, J.W.; Arlt, W. Hyperandrogenemia Predicts Metabolic Phenotype in Polycystic Ovary Syndrome: The Utility of Serum Androstenedione. J. Clin. Endocrinol. Metab. 2014, 99, 1027–1036. [Google Scholar] [CrossRef]
- Gupta, M.; Yadav, R.; Mahey, R.; Agrawal, A.; Upadhyay, A.; Malhotra, N.; Bhatla, N. Correlation of Body Mass Index (BMI), Anti-Mullerian Hormone (AMH), and Insulin Resistance among Different Polycystic Ovary Syndrome (PCOS) Phenotypes–a Cross-Sectional Study. Gynecol. Endocrinol. 2019, 35, 970–973. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Trakakis, E.; Chrelias, C.; Papantoniou, N.; Hatziagelaki, E. The Impact of Mild Hypercholesterolemia on Glycemic and Hormonal Profiles, Menstrual Characteristics and the Ovarian Morphology of Women with Polycystic Ovarian Syndrome. Horm. Mol. Biol. Clin. Investig. 2018, 34. [Google Scholar] [CrossRef]
- Antonio, L.; Pauwels, S.; Laurent, M.R.; Vanschoubroeck, D.; Jans, I.; Billen, J.; Claessens, F.; Decallonne, B.; De Neubourg, D.; Vermeersch, P.; et al. Free Testosterone Reflects Metabolic as Well as Ovarian Disturbances in Subfertile Oligomenorrheic Women. Int. J. Endocrinol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Polotsky, A.J.; Allshouse, A.; Crawford, S.L.; Harlow, S.D.; Khalil, N.; Santoro, N.; Legro, R.S. Relative Contributions of Oligomenorrhea and Hyperandrogenemia to the Risk of Metabolic Syndrome in Midlife Women. J. Clin. Endocrinol. Metab. 2012, 97. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Rojas, J.; Chávez, M.; Olivar, L.; Rojas, M.; Morillo, J.; Mejías, J.; Calvo, M.; Bermúdez, V. Polycystic Ovary Syndrome, Insulin Resistance, and Obesity: Navigating the Pathophysiologic Labyrinth. Int. J. Reprod. Med. 2014, 2014, 1–17. [Google Scholar] [CrossRef]
- Navarro, G.; Allard, C.; Morford, J.J.; Xu, W.; Liu, S.; Molinas, A.J.; Butcher, S.M.; Fine, N.H.; Blandino-Rosano, M.; Sure, V.N.; et al. Androgen Excess in Pancreatic β Cells and Neurons Predisposes Female Mice to Type 2 Diabetes. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Effects of Testosterone on Fat Cell Lipolysis. Species Differences and Possible Role in Polycystic Ovarian Syndrome. In Biochimie; Elsevier: Amsterdam, The Netherlands, 2005; Volume 87, pp. 39–43. [Google Scholar]
- Seow, K.M.; Tsai, Y.L.; Hwang, J.L.; Hsu, W.Y.; Ho, L.T.; Juan, C.C. Omental Adipose Tissue Overexpression of Fatty Acid Transporter CD36 and Decreased Expression of Hormone-Sensitive Lipase in Insulin-Resistant Women with Polycystic Ovary Syndrome. Hum. Reprod. 2009, 24, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Ctor, H.; Escobar-Morreale, F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; et al. The Polycystic Ovary Syndrome: A Position Statement from the European Society of Endocrinology on Behalf of the ESE PCOS Special Interest Group Position Statement G Conway and Others PCOS: An ESE Perspective. Eur. J. Endocrinol. 2014, 171, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Bendikson, K.; Butts, S.; Coutifaris, C.; Falcone, T.; Fossum, G.; Gitlin, S.; Gracia, C.; Hansen, K.; La Barbera, A.; et al. Role of Metformin for Ovulation Induction in Infertile Patients with Polycystic Ovary Syndrome (PCOS): A Guideline. Fertil. Steril. 2017, 108, 426–441. [Google Scholar] [CrossRef]
- Sharpe, A.; Morley, L.C.; Tang, T.; Norman, R.J.; Balen, A.H. Metformin for Ovulation Induction (Excluding Gonadotrophins) in Women with Polycystic Ovary Syndrome. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Kakoly, N.S.; Tan, J.W.J.; Fitzgerald, G.; Bahri Khomami, M.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; et al. Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review, Meta-Analysis and Meta-Regression. Obes. Rev. 2019, 20, 339–352. [Google Scholar] [CrossRef]

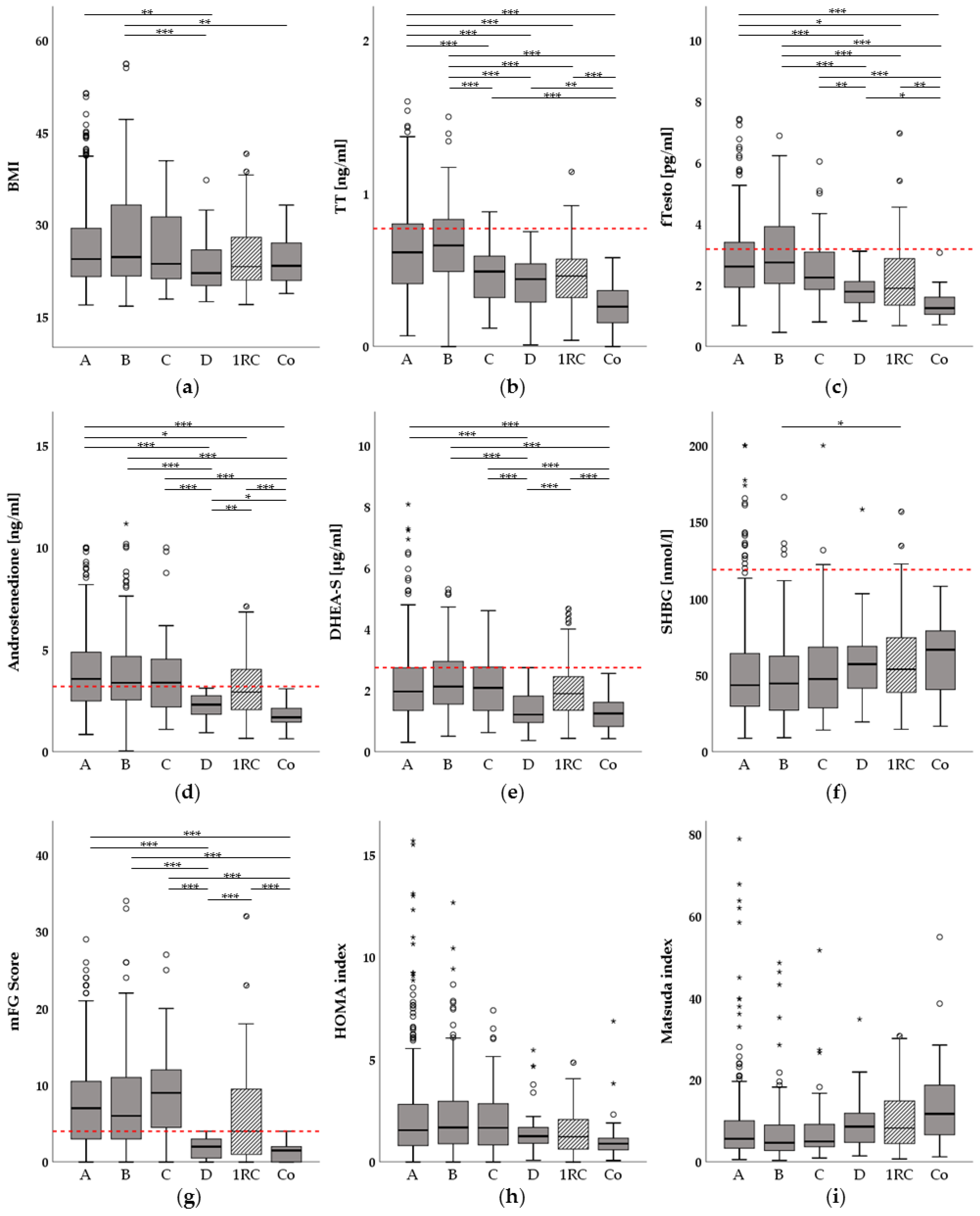
| Parameter | A | B | C | D | 1RC | Co | p |
|---|---|---|---|---|---|---|---|
| n | 392 | 170 | 52 | 38 | 75 | 23 | - |
| Age (years) | 26.6 | 27.6 | 27.3 | 27.6 | 27.6 | 36.7 | <0.001 |
| (22.8–30.2) | (23.2–31.1) | (23.9–29.5) | (23.1–30.4) | (22.5–33.9) | (31.6–40.9) | ||
| BMI (kg/m2) | 24.4 | 24.7 | 23.6 | 22.1 | 23.1 | 23.3 | <0.001 |
| (21.5–29.4) | (21.6–33.2) | (21.0–31.4) | (19.9–26.3) | (20.9–28.2) | (20.8–27.2) | ||
| TT (ng/mL) | 0.62 | 0.66 | 0.49 | 0.44 | 0.46 | 0.26 | <0.001 |
| (0.41–0.80) | (0.49–0.83) | (0.32–0.59) | (0.29–0.55) | (0.32–0.58) | (0.12–0.37) | ||
| fTesto (pg/mL) | 2.61 | 2.74 | 2.25 | 1.79 | 1.90 | 1.25 | <0.001 |
| (1.93–3.40) | (2.05–3.93) | (1.86–3.14) | (1.43–2.16) | (1.34–2.90) | (1.02–1.67) | ||
| ASD (ng/mL) | 3.57 | 3.38 | 3.39 | 2.31 | 2.93 | 1.69 | <0.001 |
| (2.49–4.89) | (2.52–4.68) | (2.20–4.54) | (1.79–2.81) | (2.05–4.08) | (1.37–2.14) | ||
| DHEA-S (µg/mL) | 1.97 | 2.13 | 2.09 | 1.22 | 1.90 | 1.25 | <0.001 |
| (1.34–2.75) | (1.55–2.96) | (1.35–2.78) | (0.94–1.83) | (1.34–2.48) | (0.78–1.67) | ||
| SHBG (nmol/L) | 43.6 | 44.6 | 47.6 | 57.3 | 53.9 | 66.7 | 0.023 |
| (29.8–64.3) | (27.1–62.5) | (28.4–68.7) | (41.4–69.7) | (38.3–77.4) | (39.5–80.4) | ||
| mFG score (1) | 7 | 6 | 9 | 2 | 4 | 1.5 | <0.001 |
| (3–11) | (3–11) | (4–12) | (0–3) | (1–10) | (0–2) | ||
| LH (mIU/mL) | 9.28 | 8.24 | 6.40 | 9.22 | 5.87 | 3.53 | 0.038 |
| (5.29–14.00) | (4.49–13.10) | (4.64–13.21) | (3.76–12.75) | (3.24–9.31) | (2.41–8.18) | ||
| FSH (mIU/mL) | 5.60 | 5.57 | 5.41 | 6.48 | 4.91 | 7.84 | 0.143 |
| (4.27–7.06) | (4.05–7.04) | (3.80–7.60) | (5.45–8.03) | (3.35–7.15) | (4.34–9.94) | ||
| LH/FSH ratio (1) | 1.64 | 1.43 | 1.51 | 1.40 | 1.12 | 0.71 | <0.001 |
| (1.07–2.46) | (0.99–2.23) | (0.84–1.96) | (0.88–1.75) | (0.76–1.85) | (0.40–1.18) | ||
| TSH (µIU/mL) | 1.92 | 1.80 | 1.92 | 1.69 | 1.77 | 1.65 | 0.38 |
| (1.41–2.61) | (1.23–2.39) | (1.46–2.87) | (1.02–2.48) | (1.23–2.42) | (1.16–2.09) | ||
| fT4 [pmol/L] | 14.2 | 14.5 | 14.2 | 14.1 | 14.6 | 14.7 | 0.868 |
| (12.8–15.8) | (13.3–15.7) | (12.9–15.2) | (12.4–16.1) | (13.0–16.2) | (13.3–16.0) | ||
| fT3 [pmol/L] | 5.0 | 5.0 | 5.0 | 4.9 | 4.8 | 4.5 | <0.001 |
| (4.6–5.4) | (4.6–5.3) | (4.7–5.4) | (4.4–5.1) | (4.4–5.2) | (4.4–5.0) | ||
| Prolactin (ng/mL) | 9.4 | 10.0 | 10.4 | 8.9 | 10.4 | 9.2 | 0.847 |
| (7.5–12.9) | (7.8–14.1) | (8.2–13.3) | (5.6–11.9) | (7.9–15.7) | (7.6–15.7) | ||
| 25OHD (ng/mL) | 26.1 | 25.3 | 23.1 | 25.9 | 24.4 | 26.3 | 0.746 |
| (18.5–33.1) | (16.8–31.8) | (19.0–32.0) | (17.9–30.7) | (17.9–33.3) | (18.9–34.9) | ||
| HbA1c (mmol/moL) | 33 | 33 | 33 | 31 | 33 | 34 | 0.324 |
| (31–35) | (31–35) | (31–36) | (30–33) | (31–34) | (31–37) | ||
| HOMA-IR (1) | 1.6 | 1.7 | 1.7 | 1.3 | 1.2 | 0.9 | 0.215 |
| (0.8–2.8) | (0.9–3.0) | (0.8–2.8) | (0.9–1.7) | (0.6–2.1) | (0.5–1.3) | ||
| Matsuda (1) | 5.7 | 4.7 | 5.0 | 8.6 | 8.3 | 11.8 | 0.198 |
| (3.3–10.1) | (2.8–9.1) | (3.7–9.9) | (4.8–12.1) | (4.5–15.1) | (6.3–21.1) | ||
| IR present (n (%)) | 157 (40.1) | 75 (44.4) | 22 (42.3) | 8 (21.1) | 20 (27.0) | 3 (13.0) | - |
| Hyperglycemia present (n (%)) | 25 (6.4) | 11 (6.5) | 2 (3.8) | 2 (5.3) | 5 (6.7) | 3 (13.0) | - |
| Total cholesterol (mg/dL) | 175 | 175 | 167 | 173 | 174 | 179 | 0.403 |
| (155–197) | (156–199) | (153–189) | (155–198) | (155–196) | (164–198) | ||
| HDL (mg/dL) | 63 | 62 | 61 | 74 | 69 | 67 | 0.082 |
| (52–75) | (52–77) | (51–74) | (61–82) | (57–83) | (57–82) | ||
| LDL (mg/dL) | 95 | 95 | 89 | 86 | 92 | 101 | 0.119 |
| (78–116) | (81–120) | (72–107) | (71–113) | (74–111) | (94–121) | ||
| Triglycerides (mg/dL) | 72 | 78 | 70 | 64 | 59 | 58 | 0.020 |
| (54–98) | (56–101) | (52–88) | (45–78) | (48–75) | (45–71) | ||
| MetS present (n (%)) | 57 (14.5) | 25 (14.7) | 5 (9.6) | 2 (5.3) | 5 (6.7) | 1 (4.3) | - |
| Group Comparison by: | Hyperandrogenemia | ||
|---|---|---|---|
| Parameter | Yes | No | p |
| n | 35 | 40 | - |
| Age (years) | 27.6 (22.5–33.9) | 36.7 (31.6–40.9) | 0.274 |
| BMI (kg/m2) | 23.1 (20.9–28.2) | 23.3 (20.8–27.2) | 0.530 |
| TT (ng/mL) | 0.46 (0.32–0.58) | 0.26 (0.12–0.37) | 0.005 |
| fTesto (pg/mL) | 1.90 (1.34–2.90) | 1.25 (1.02–1.67) | <0.001 |
| ASD (ng/mL) | 2.93 (2.05–4.08) | 1.69 (1.37–2.14) | <0.001 |
| DHEA-S (µg/mL) | 1.90 (1.34–2.48) | 1.25 (0.78–1.67) | <0.001 |
| SHBG (nmol/L) | 53.9 (38.3–77.4) | 66.7 (39.5–80.4) | 0.010 |
| mFG Score (1) | 4 (1–10) | 1.5 (0–2) | 0.656 |
| LH (mIU/mL) | 5.87 (3.24–9.31) | 3.53 (2.41–8.18) | 0.042 |
| FSH (mIU/mL) | 4.91 (3.35–7.15) | 7.84 (4.34–9.94) | 0.006 |
| HOMA-IR (1) | 1.2 (0.6–2.1) | 0.9 (0.5–1.3) | 0.021 |
| Matsuda (1) | 8.3 (4.5–15.1) | 11.8 (6.3–21.1) | 0.092 |
| Total cholesterol (mg/dL) | 174 (155–196) | 179 (164–198) | 0.231* |
| HDL (mg/dL) | 69 (57–83) | 67 (57–82) | 0.910* |
| LDL (mg/dL) | 92 (74–111) | 101 (94–121) | 0.072* |
| Triglycerides (mg/dL) | 59 (48–75) | 58 (45–71) | 0.884* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzan, V.; Lerchbaum, E.; Missbrenner, C.; Heijboer, A.C.; Goschnik, M.; Trummer, C.; Theiler-Schwetz, V.; Haudum, C.; Gumpold, R.; Schweighofer, N.; et al. Risk of Insulin Resistance and Metabolic Syndrome in Women with Hyperandrogenemia: A Comparison between PCOS Phenotypes and Beyond. J. Clin. Med. 2021, 10, 829. https://doi.org/10.3390/jcm10040829
Borzan V, Lerchbaum E, Missbrenner C, Heijboer AC, Goschnik M, Trummer C, Theiler-Schwetz V, Haudum C, Gumpold R, Schweighofer N, et al. Risk of Insulin Resistance and Metabolic Syndrome in Women with Hyperandrogenemia: A Comparison between PCOS Phenotypes and Beyond. Journal of Clinical Medicine. 2021; 10(4):829. https://doi.org/10.3390/jcm10040829
Chicago/Turabian StyleBorzan, Valentin, Elisabeth Lerchbaum, Cornelia Missbrenner, Annemieke C. Heijboer, Michaela Goschnik, Christian Trummer, Verena Theiler-Schwetz, Christoph Haudum, Roswitha Gumpold, Natascha Schweighofer, and et al. 2021. "Risk of Insulin Resistance and Metabolic Syndrome in Women with Hyperandrogenemia: A Comparison between PCOS Phenotypes and Beyond" Journal of Clinical Medicine 10, no. 4: 829. https://doi.org/10.3390/jcm10040829
APA StyleBorzan, V., Lerchbaum, E., Missbrenner, C., Heijboer, A. C., Goschnik, M., Trummer, C., Theiler-Schwetz, V., Haudum, C., Gumpold, R., Schweighofer, N., & Obermayer-Pietsch, B. (2021). Risk of Insulin Resistance and Metabolic Syndrome in Women with Hyperandrogenemia: A Comparison between PCOS Phenotypes and Beyond. Journal of Clinical Medicine, 10(4), 829. https://doi.org/10.3390/jcm10040829







