Diagnostic Errors Induced by a Wrong a Priori Diagnosis: A Prospective Randomized Simulator-Based Trial
Abstract
1. Introduction/Background
2. Methods
2.1. Study Design
2.2. Participants
2.3. Simulator and Scenario
2.4. Randomization and Intervention
2.5. Data Analysis
2.6. Statistics
3. Results
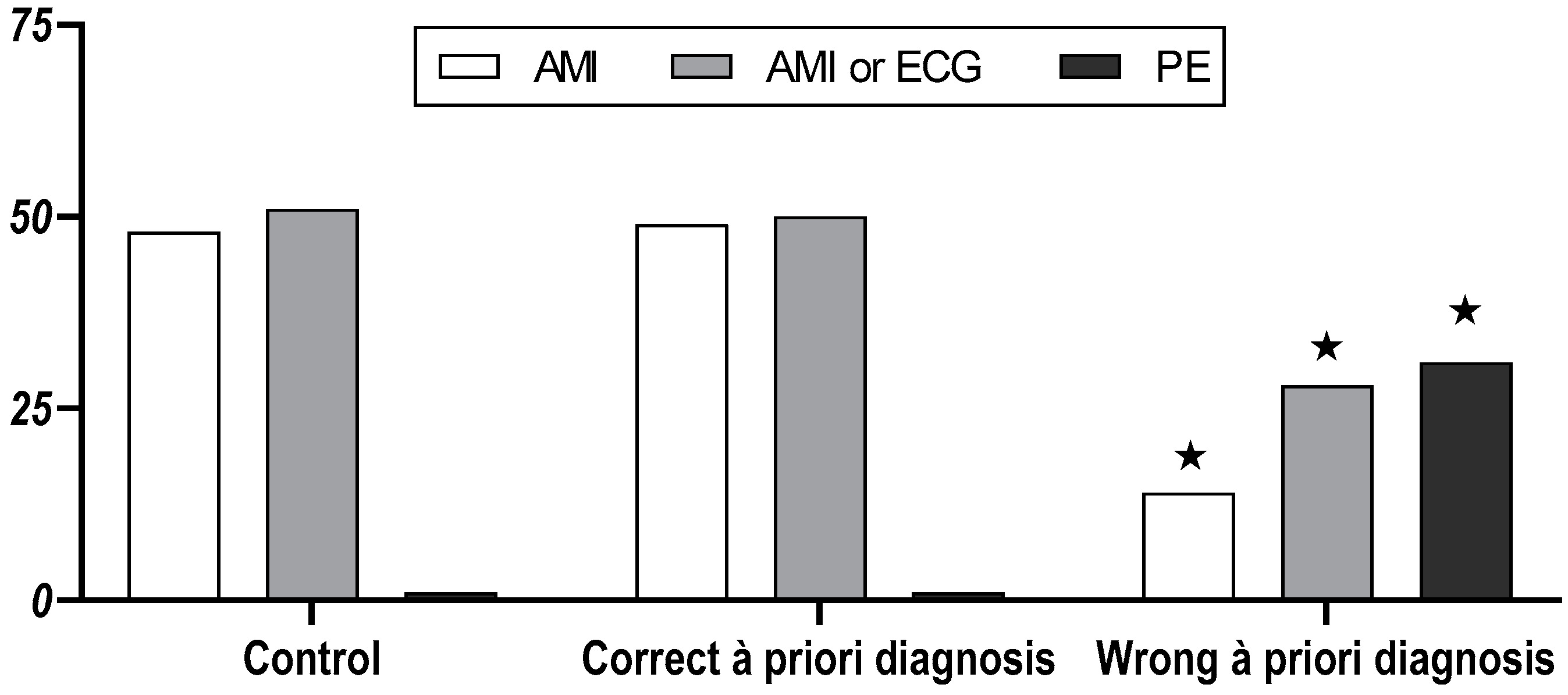
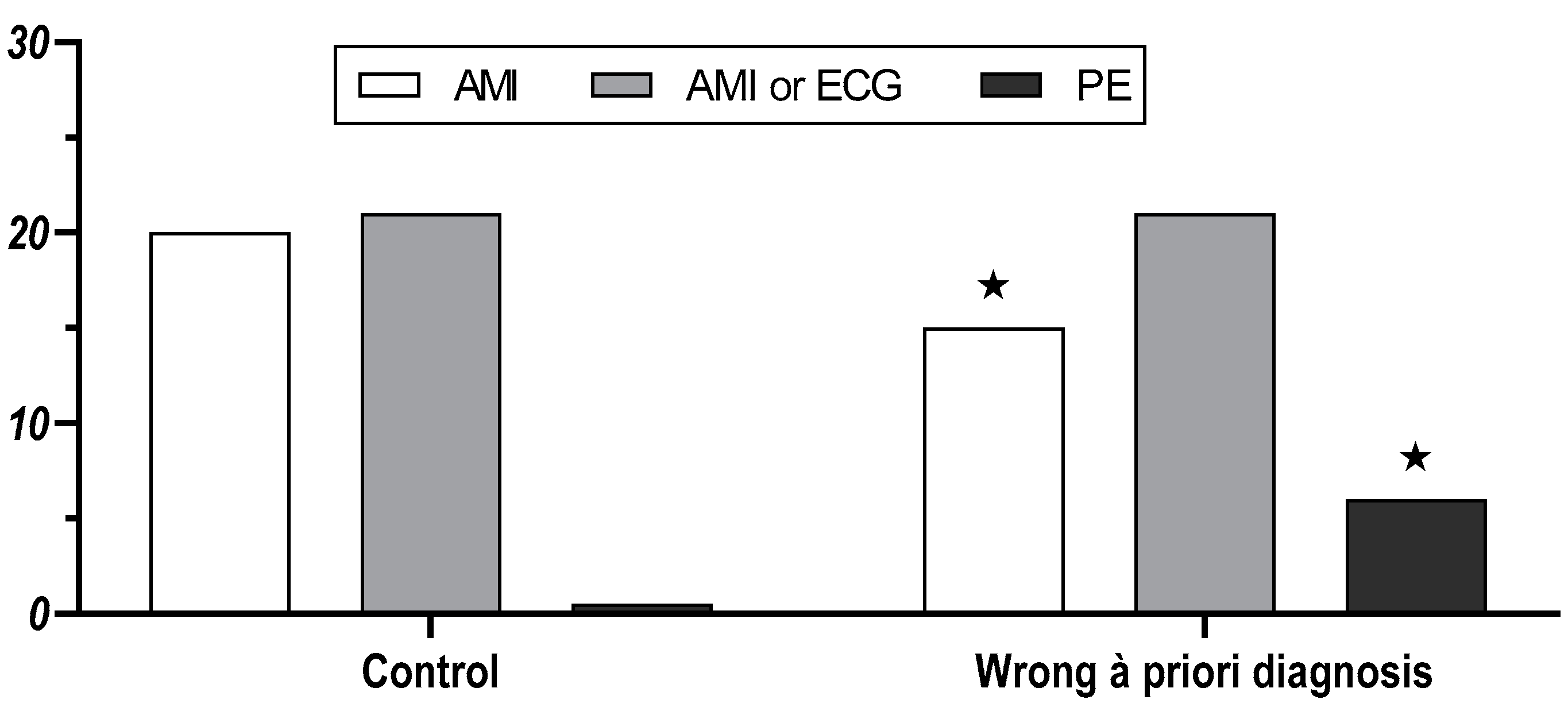
3.1. Medical Students
3.2. Physicians
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Institute of Medicine, National Academies of Sciences EaM. Improving Diagnosis in Health Care; The National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Newman-Toker, D.E.; Makary, M.A. Measuring diagnostic errors in primary care: The first step on a path forward. Comment on “Types and origins of diagnostic errors in primary care settings”. JAMA Intern. Med. 2013, 173, 425–426. [Google Scholar] [CrossRef]
- Singh, H.; Sittig, D.F. Advancing the science of measurement of diagnostic errors in healthcare: The Safer Dx framework. BMJ Qual. Saf. 2015, 24, 103. [Google Scholar] [CrossRef]
- Singh, H.; Graber, M.L. Improving Diagnosis in Health Care—The Next Imperative for Patient Safety. N. Engl. J. Med. 2015, 373, 2493–2495. [Google Scholar] [CrossRef]
- Singh, H.; Giardina, T.D.; Meyer, A.N.; Forjuoh, S.N.; Reis, M.D.; Thomas, E.J. Types and origins of diagnostic errors in primary care settings. JAMA Intern. Med. 2013, 173, 418–425. [Google Scholar] [CrossRef]
- Zwaan, L.; Thijs, A.; Wagner, C.; Wal, G.; Timmermans, D.R. Relating faults in diagnostic reasoning with diagnostic errors and patient harm. Acad. Med. 2012, 87, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, A.S.S.; Lee, H.; Mathews, S.C.; Shore, A.; Makary, M.A.; Pronovost, P.J.; Newman-Toker, D.E. 25-Year summary of US malpractice claims for diagnostic errors 1986–2010: An analysis from the National Practitioner Data Bank. BMJ Qual. Saf. 2013, 22, 672. [Google Scholar] [CrossRef]
- Khoo, E.M.; Lee, W.K.; Sararaks, S.; Abdul Samad, A.; Liew, S.M.; Cheong, A.T.; Ibrahim, M.Y.; Su, S.H.; Mohd Hanafiah, A.N.; Maskon, K. Medical errors in primary care clinics—A cross sectional study. BMC Fam. Pract. 2012, 13, 1–6. [Google Scholar] [CrossRef]
- Panagioti, M.; Khan, K.; Keers, R.N.; Abuzour, A.; Phipps, D.; Kontopantelis, E.; Bower, P.; Campbell, S.; Haneef, R.; Avery, A.J.; et al. Prevalence, severity, and nature of preventable patient harm across medical care settings: Systematic review and meta-analysis. BMJ 2019, 366, l4185. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Meyer, A.N.D.; Thomas, E.J. The frequency of diagnostic errors in outpatient care: Estimations from three large observational studies involving US adult populations. BMJ Qual. Saf. 2014, 23, 727. [Google Scholar] [CrossRef] [PubMed]
- Zwaan, L.; Monteiro, S.; Sherbino, J.; Ilgen, J.; Howey, B.; Norman, G. Is bias in the eye of the beholder? A vignette study to assess recognition of cognitive biases in clinical case workups. BMJ Qual. Saf. 2017, 26, 104. [Google Scholar] [CrossRef]
- Braun, L.T.; Zwaan, L.; Kiesewetter, J.; Fischer, M.R.; Schmidmaier, R. Diagnostic errors by medical students: Results of a prospective qualitative study. BMC Med. Educ. 2017, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, O.; Delaney, B.C.; Munro, C.W. Diagnostic difficulty and error in primary care—A systematic review. Fam. Pract. 2008, 25, 400–413. [Google Scholar] [CrossRef]
- Landrigan, C.P.; Rahman, S.A.; Sullivan, J.P.; Vittinghoff, E.; Barger, L.K.; Sanderson, A.L.; Wright, K.P.; O’Brien, C.S.; Qadri, S.; St. Hilaire, M.A.; et al. Effect on Patient Safety of a Resident Physician Schedule without 24-Hour Shifts. N. Engl. J. Med. 2020, 382, 2514–2523. [Google Scholar] [CrossRef]
- Weiner, S.J.; Schwartz, A.; Weaver, F.; Goldberg, J.; Yudkowsky, R.; Sharma, G.; Binns-Calvey, A.; Preyss, B.; Schapira, M.M.; Persell, S.D.; et al. Contextual errors and failures in individualizing patient care: A multicenter study. Ann. Intern. Med. 2010, 153, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Hautz, S.C.; Schuler, L.; Kammer, J.E.; Schauber, S.K.; Ricklin, M.E.; Sauter, T.C.; Maier, V.; Birrenbach, T.; Exadaktylos, A.; Hautz, W.E. Factors predicting a change in diagnosis in patients hospitalised through the emergency room: A prospective observational study. BMJ Open 2016, 6, e011585. [Google Scholar] [CrossRef]
- Freund, Y.; Goulet, H.; Leblanc, J.; Bokobza, J.; Ray, P.; Maignan, M.; Guinemer, S.; Truchot, J.; Feral-Pierssens, A.L.; Yordanov, Y.; et al. Effect of Systematic Physician Cross-checking on Reducing Adverse Events in the Emergency Department: The CHARMED Cluster Randomized Trial. JAMA Intern. Med. 2018, 178, 812–819. [Google Scholar] [CrossRef]
- McCrae, R.R.; John, O.P. An Introduction to the Five-Factor Model and Its Applications. J. Personal. 1992, 60, 175–215. [Google Scholar] [CrossRef]
- Von Collani, G.; Herzberg, P.Y. Eine revidierte Fassung der deutschsprachigen Skala zum Selbstwertgefuehl von Rosenberg [A revised version of the German adaptation of Rosenberg’s Self-Esteem Scale]. Z. Differ. Diagn. Psychol. 2003, 24, 3–7. [Google Scholar] [CrossRef]
- O’Brien, P.C.; Fleming, T.R. A Multiple Testing Procedure for Clinical Trials. Biometrics 1979, 35, 549–556. [Google Scholar] [CrossRef]
- Pampallona, S.; Tsiatis, A.A. Group sequential designs for one-sided and two-sided hypothesis testing with provision for early stopping in favor of the null hypothesis. J. Stat. Plan. Inference 1994, 42, 19–35. [Google Scholar] [CrossRef]
- Graber, M. Diagnostic errors in medicine: A case of neglect. Jt. Comm. J. Qual. Patient Saf. 2005, 31, 106–113. [Google Scholar] [CrossRef]
- Schiff, G.D.; Kim, S.; Abrams, R.; Cosby, K.; Lambert, B.; Elstein, A.S.; Hasler, S.; Krosnjar, N.; Odwazny, R.; Wisniewski, M.F.; et al. Diagnosing Diagnosis Errors: Lessons from a Multi-institutional Collaborative Project. In Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology); Henriksen, K., Battles, J.B., Marks, E.S., Lewin, D.I., Eds.; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2005. [Google Scholar]
- Kostopoulou, O.; Oudhoff, J.; Nath, R.; Delaney, B.C.; Munro, C.W.; Harries, C.; Holder, R. Predictors of diagnostic accuracy and safe management in difficult diagnostic problems in family medicine. Med. Decis. Mak. 2008, 28, 668–680. [Google Scholar] [CrossRef]
- De Luca, G.; Suryapranata, H.; Ottervanger, J.P.; Antman, E.M. Time Delay to Treatment and Mortality in Primary Angioplasty for Acute Myocardial Infarction: Every Minute of Delay Counts. Circulation 2004, 109, 1223–1225. [Google Scholar] [CrossRef]
- Guerchicoff, A.; Brener, S.J.; Maehara, A.; Witzenbichler, B.; Fahy, M.; Xu, K.; Gersh, B.J.; Mehran, R.; Gibson, C.M.; Stone, G.W. Impact of Delay to Reperfusion on Reperfusion Success, Infarct Size, and Clinical Outcomes in Patients With ST-Segment Elevation Myocardial Infarction: The INFUSE-AMI Trial (INFUSE-Anterior Myocardial Infarction). JACC Cardiovasc. Interv. 2014, 7, 733–740. [Google Scholar] [CrossRef]
- Coderre, S.; Mandin, H.; Harasym, P.H.; Fick, G.H. Diagnostic reasoning strategies and diagnostic success. Med. Educ. 2003, 37, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, T.K.; Kachalia, A.; Thomas, E.J.; Puopolo, A.L.; Yoon, C.; Brennan, T.A.; Studdert, D.M. Missed and delayed diagnoses in the ambulatory setting: A study of closed malpractice claims. Ann. Intern. Med. 2006, 145, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Goyder, C.R.; Jones, C.H.; Heneghan, C.J.; Thompson, M.J. Missed opportunities for diagnosis: Lessons learned from diagnostic errors in primary care. Br. J. Gen. Pract. 2015, 65, e838–e844. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Potter, D.A.; Feldman, H.A. Non-medical influences on medical decision-making. Soc. Sci. Med. 1996, 42, 769–776. [Google Scholar] [CrossRef]
- Lambe, K.A.; Reilly, G.; Kelly, B.D.; Curristan, S. Dual-process cognitive interventions to enhance diagnostic reasoning: A systematic review. BMJ Qual. Saf. 2016, 25, 808. [Google Scholar] [CrossRef]
- Norman, G.R.; Monteiro, S.D.; Sherbino, J.; Ilgen, J.S.; Schmidt, H.G.; Mamede, S. The Causes of Errors in Clinical Reasoning: Cognitive Biases, Knowledge Deficits, and Dual Process Thinking. Acad. Med. 2017, 92, 23–30. [Google Scholar] [CrossRef]
- Evans, J.S.; Stanovich, K.E. Dual-Process Theories of Higher Cognition: Advancing the Debate. Perspect. Psychol. Sci. 2013, 8, 223–241. [Google Scholar] [CrossRef]
- Crowley, R.S.; Legowski, E.; Medvedeva, O.; Reitmeyer, K.; Tseytlin, E.; Castine, M.; Jukic, D.; Mello-Thoms, C. Automated detection of heuristics and biases among pathologists in a computer-based system. Adv. Health Sci. Educ. Theory Pract. 2013, 18, 343–363. [Google Scholar] [CrossRef]
- Ely, J.W.; Graber, M.L.; Croskerry, P. Checklists to reduce diagnostic errors. Acad. Med. 2011, 86, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Graber, M.L. The incidence of diagnostic error in medicine. BMJ Qual. Saf. 2013, 22, ii21. [Google Scholar] [CrossRef]
- Ogdie, A.R.; Reilly, J.B.; Pang, W.G.; Keddem, S.; Barg, F.K.; Feldt, J.M.; Myers, J.S. Seen through their eyes: Residents’ reflections on the cognitive and contextual components of diagnostic errors in medicine. Acad. Med. 2012, 87, 1361. [Google Scholar] [CrossRef]
- Saposnik, G.; Redelmeier, D.; Ruff, C.C.; Tobler, P.N. Cognitive biases associated with medical decisions: A systematic review. BMC Med Inform. Decis. Mak. 2016, 16, 138. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, M.P.; Ruskin, K.J. Decision-making and safety in anesthesiology. Curr. Opin. Anaesthesiol. 2012, 25, 724–729. [Google Scholar] [CrossRef]
- Graber, M.L.; Franklin, N.; Gordon, R. Diagnostic error in internal medicine. Arch. Intern. Med. 2005, 165, 1493–1499. [Google Scholar] [CrossRef]
- Gruver, R.H.; Freis, E.D. A study of diagnostic errors. Ann. Intern. Med. 1957, 47, 108–120. [Google Scholar] [PubMed]
- Kuhn, G.J. Diagnostic errors. Acad. Emerg. Med. 2002, 9, 740–750. [Google Scholar] [CrossRef]
- Schiff, G.D. Finding and fixing diagnosis errors: Can triggers help? BMJ Qual. Saf. 2012, 21, 89–92. [Google Scholar] [CrossRef] [PubMed]
| Questions of the Participants | Answers of the Patient |
|---|---|
| Exploration of chest pain (chest pain bloc) | |
| Localisation | In the middle of the chest behind the breastbone |
| Quality | Pressing and constricting |
| Radiation | Yes, to the left elbow and neck |
| Severity | Severe. When a pain score is asked: 8 out of 10 |
| Aggravating or relieving factors | None |
| Previous experience | Never experienced something similar before |
| Duration | Continuous pain over the last 30 min |
| Circumstances | Sudden onset while sitting at the desk working. When asked: no preceding physical activity or unusual stress |
| Associated symptoms | |
| Nausea or vomiting | Slight Nausea, no vomiting |
| Sweating | Initially heavy sweating, that now has ceased |
| Fear | Initially strong fear of death, now frightened |
| Dyspnoea | Slight shortness of breath |
| Cardiovascular risk factors | |
| Smoking | One package cigarettes a day for the last 30 years |
| Hypertension | No. Blood pressure normal, according to GP |
| Diabetes | No |
| Blood fats | Normal, according to GP |
| Family history | Mother died due to a stroke with 70 years; father 82 years old with dementia; two healthy brothers |
| Previous illness and medication | |
| Prior diseases | None |
| Prior hospitalisations | Appendectomy when 16 years old |
| Current medication | None |
| Allergies | Not known |
| Last visit with GP | Normal “check-up” one year ago |
| Social history | |
| Age | 49 years old |
| Next of kin | Married, no children |
| Profession/occupation | Accountant in banking |
| Recreation activities/hobbies | Cycling, fitness centre, traveling |
| Exploration for pulmonary embolism | |
| Cough | None |
| Haemoptoe | No |
| Chest pain aggravated by breathing | No relation of chest pain with breathing activity |
| Painful or swollen leg(s) | No |
| Previous thrombotic event | No |
| Immobilisation | No. When specifically asked: no trauma, no operation, no recent travel |
| Risk factors for thrombotic event | When specifically asked: patient not aware of cancer, coagulopathy, coagulopathy in family |
| Control Group (n = 52) | Correct a Priori Diagnosis Group (n = 52) | Wrong a Priori Diagnosis Group (n = 52) | |
|---|---|---|---|
| Female:male | 31:21 | 34:18 | 30:22 |
| AMI knowledge % | 50 [33–67] | 50 [33–67] | 50 [50–67] |
| Emergencies knowledge % | 48 [37–60] | 42 [27–58] | 44 [37–54] |
| ECG knowledge % | 42 [25–54] | 33 [29–50] | 42 [25–58] |
| Self-esteem (Rosenberg) | 23 [20–26] | 24 [20–26] | 22 [17–24] |
| Neuroticism | 2.8 [2.2–3.3] | 2.9 [2.2–3.3] | 2.9 [2.5–3.5] |
| Extraversion | 4.3 [3.7–4.8] | 4.2 [3.8–4.8] | 4.2 [3.7–4.7] |
| Openness | 4.0 [3.7–4.6] | 4.5 [3.9–4.8] | 4.2 [3.8–4.6] |
| Agreeableness | 4.7 [4.3–5.0] | 4.7 [4.5–5.0] | 4.7 [4.3–5.0] |
| Conscientiousness | 4.7 [3.8–4.8] | 4.5 [3.9–4.8] | 4.3 [3.8–5.0] |
| Control Group (n = 52) | Correct a Priori Diagnosis Group (n = 52) | Wrong a Priori Diagnosis Group (n = 52) | |
|---|---|---|---|
| Number of measures taken (n) | 4.5 [3.0–6.0] | 5.0 [3.0–5.5] | 5.0 [4.0–5.0] |
| Supplemental oxygen (n; %) | 28/52 (54%) | 29/52 (56%) | 16/52 (31%) *¶ |
| Cue ECG monitoring (n; %) | 25/52 (48%) | 30/52 (58%) | 28/52 (54%) |
| Blood pressure and heart rate measured (n, %) | 43/52 (83%) | 39/52 (75%) | 35/52 (67%) |
| Oxygen saturation measured (n; %) | 3/52 (6%) | 1/52 (2%) | 3/52 (6%) |
| Chest auscultation (n; %) | 32/52 (62%) | 25/52 (48%) | 42/52 (81%) *¶ |
| Examination of legs (n; %) | 0/52 (0%) | 0/52 (0%) | 9/52 (17%) *¶ |
| Morphium given (n; %) | 32/52 (62%) | 29/52 (56%) | 12/52 (23%) *¶ |
| Nitro-glycerin given (n; %) | 25/52 (48%) | 22/52 (42%) | 4/52 (8%) *¶ |
| Aspirin given (n; %) | 9/52 (17%) | 13/52 (25%) | 1/52 (2%) *¶ |
| Heparin given (n; %) | 0/52 (0%) | 4/52 (8%) | 3/52 (6%) |
| Chest Radiograph ordered (n; %) | 0/52 (0%) | 0/52 (0%) | 9/52 (17%) *¶ |
| Chest CT ordered (n; %) | 0/52 (0%) | 0/52 (0%) | 9/52 (17%) *¶ |
| Blood sample taken (n; %) | 24/52 (46%) | 25/52 (48%) | 43/52 (83%) *¶ |
| Troponin requested (n; %) | 14/52 (27%) | 16/52 (31%) | 4/52 (8%) *¶ |
| D-Dimer requested (n; %) | 0/52 (0%) | 3/52 (6%) | 22/52 (42%) *¶ |
| Final Diagnosis Acute Coronary Syndrome (n = 21) | Final Diagnosis Pulmonary Embolism (n = 31) | |
|---|---|---|
| Female:male (n) | 13:8 | 17:14 |
| AMI knowledge % | 67 [50–67] | 50 [50–67] |
| Emergencies knowledge % | 45 [30–53] | 42 [39–57] |
| ECG knowledge % | 33 [25–58] | 42 [33–58] |
| Self-esteem (Rosenberg) | 22 [20–23] | 22 [16–24] |
| Neuroticism | 2.8 [2.7–3.2] | 3.0 [2.5–3.8] |
| Extraversion | 4.3 [3.8–4.7] | 4.2 [3.7–4.5] |
| Openness | 4.3 [3.8–4.7] | 4.0 [3.7–4.5] |
| Agreeableness | 4.7 [4.3–5.0] | 4.7 [4.3–5.0] |
| Conscientiousness | 4.5 [4.0–5.0] | 4.2 [3.7–5.0] |
| Number of measures taken (n; %) | 5.0 [4.0–5.0] | 5.0 [4.0–6.0] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, F.M.L.; Filipovic, M.G.; Balestra, G.M.; Tisljar, K.; Sellmann, T.; Marsch, S. Diagnostic Errors Induced by a Wrong a Priori Diagnosis: A Prospective Randomized Simulator-Based Trial. J. Clin. Med. 2021, 10, 826. https://doi.org/10.3390/jcm10040826
Meyer FML, Filipovic MG, Balestra GM, Tisljar K, Sellmann T, Marsch S. Diagnostic Errors Induced by a Wrong a Priori Diagnosis: A Prospective Randomized Simulator-Based Trial. Journal of Clinical Medicine. 2021; 10(4):826. https://doi.org/10.3390/jcm10040826
Chicago/Turabian StyleMeyer, Felix M.L., Mark G. Filipovic, Gianmarco M. Balestra, Kai Tisljar, Timur Sellmann, and Stephan Marsch. 2021. "Diagnostic Errors Induced by a Wrong a Priori Diagnosis: A Prospective Randomized Simulator-Based Trial" Journal of Clinical Medicine 10, no. 4: 826. https://doi.org/10.3390/jcm10040826
APA StyleMeyer, F. M. L., Filipovic, M. G., Balestra, G. M., Tisljar, K., Sellmann, T., & Marsch, S. (2021). Diagnostic Errors Induced by a Wrong a Priori Diagnosis: A Prospective Randomized Simulator-Based Trial. Journal of Clinical Medicine, 10(4), 826. https://doi.org/10.3390/jcm10040826






