The Effect of Antiglaucoma Procedures (Trabeculectomy vs. Ex-PRESS Glaucoma Drainage Implant) on the Corneal Biomechanical Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Setting
2.2. Participants
2.3. Surgical Technique-Postoperative Management
2.4. Data Collection
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Leske, M.C.; Connell, A.M.; Wu, S.Y.; Hyman, L.G.; Schachat, A.P. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch. Ophthalmol. 1995, 113, 918–924. [Google Scholar] [CrossRef]
- Cairns, J.E. Trabeculectomy. Preliminary report of a new method. Am. J. Ophthalmol. 1968, 66, 673–679. [Google Scholar] [CrossRef]
- Jampel, H.D.; Solus, J.F.; Tracey, P.A.; Gilbert, D.L.; Loyd, T.L.; Jefferys, J.L.; Quigley, H.A. Outcomes and bleb-related complications of trabeculectomy. Ophthalmology 2012, 119, 712–722. [Google Scholar] [CrossRef]
- Fontana, H.; Nouri-Mahdavi, K.; Lumba, J.; Ralli, M.; Caprioli, J. Trabeculectomy with mitomycin C: Outcomes and risk factors for failure in phakic open-angle glaucoma. Ophthalmology 2006, 113, 930–936. [Google Scholar] [CrossRef]
- Edmunds, B.; Thompson, J.R.; Salmon, J.F.; Wormald, R.P. The National Survey of Trabeculectomy. III. Early and late complications. Eye 2002, 16, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kohlhaas, M.; Boehm, A.G.; Spoerl, E.; Pürsten, A.; Grein, H.J.; Pillunat, L.E. Effect of central corneal thickness, corneal curvature, and axial length on applanation tonometry. Arch. Ophthalmol. 2006, 124, 471–476. [Google Scholar] [CrossRef]
- Soergel, F.; Jean, B.; Seiler, T.; Bende, T.; Mücke, S.; Pechhold, W.; Pels, L. Dynamic mechanical spectroscopy of the cornea for measurement of its viscoelastic properties in vitro. Ger. J. Ophthalmol. 1995, 4, 151–156. [Google Scholar]
- Liu, J.; Roberts, C.J. Influence of corneal biomechanical properties on intraocular pressure measurement: Quantitative analysis. J. Cataract. Refract. Surg. 2005, 31, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Weinreb, R.N. Evaluation of the influence of corneal biomechanical properties on intraocular pressure measurements using the ocular response analyzer. J. Glaucoma 2006, 15, 364–370. [Google Scholar] [CrossRef]
- Kotecha, A.; Elsheikh, A.; Roberts, C.R.; Zhu, H.; Garway-Heath, D.F. Corneal thickness- and age-related biomechanical properties of the cornea measured with the ocular response analyzer. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5337–5347. [Google Scholar] [CrossRef]
- Dahan, E.; Ben Simon, G.J.; Lafuma, A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: A prospective, randomised study. Eye 2012, 26, 703–710. [Google Scholar] [CrossRef]
- Marzette, L.; Herndon, L.W. Comparison of the Ex-PRESS™ mini glaucoma shunt with standard trabeculectomy in the surgical treatment of glaucoma. Ophthalmic. Surg. Lasers Imaging 2011, 42, 453–459. [Google Scholar] [CrossRef]
- Terai, N.; Raiskup, F.; Haustein, M.; Pillunat, L.E.; Spoerl, E. Identification of biomechanical properties of the cornea: The ocular response analyzer. Curr. Eye Res. 2012, 37, 553–562. [Google Scholar] [CrossRef]
- Carbonaro, F.; Andrew, T.; Mackey, D.A.; Spector, T.D.; Hammond, C.J. The heritability of corneal hysteresis and ocular pulse amplitude: A twin study. Ophthalmology 2008, 115, 1545–1549. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, R.; He, L.-Y. Corneal hysteresis and glaucoma. Int. Ophthalmol. 2019, 39, 1909–1916. [Google Scholar] [CrossRef]
- Congdon, N.G.; Broman, A.T.; Bandeen-Roche, K.; Grover, D.; Quigley, H.A. Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am. J. Ophthalmol. 2006, 141, 868–875. [Google Scholar] [CrossRef]
- Zhang, C.; Tatham, A.J.; Abe, R.Y.; Diniz-Filho, A.; Zangwill, L.M.; Weinreb, R.N.; Medeiros, F.A. Corneal hysteresis and progressive retinal nerve fiber layer loss in glaucoma. Am. J. Ophthalmol. 2016, 166, 29–36. [Google Scholar] [CrossRef]
- Zimprich, L.; Diedrich, J.; Bleeker, A.; Schweitzer, J.A. Corneal Hysteresis as a Biomarker of Glaucoma: Current Insights. Clin. Ophthalmol. 2020, 14, 2255–2264. [Google Scholar] [CrossRef]
- Touboul, D.; Roberts, C.; Kérautret, J.; Garra, C.; Maurice-Tison, S.; Saubusse, E.; Colin, J. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J. Cataract Refract. Surg. 2015, 30, 335–339. [Google Scholar] [CrossRef]
- Kaushik, S.; Pandav, S.S.; Banger, A.; Aggarwal, K.; Gupta, A. Relationship between corneal biomechanical properties, central corneal thickness, and intraocular pressure across the spectrum of glaucoma. Am. J. Ophthalmol. 2012, 153, 840–849. [Google Scholar] [CrossRef]
- Pillunat, K.R.; Spoerl, E.; Terai, N.; Pillunat, L.E. Corneal Biomechanical Changes After Trabeculectomy and the Impact on Intraocular Pressure Measurement. J. Glaucoma 2017, 26, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Ayala, M.; Chen, E. Measuring corneal hysteresis: Threshold estimation of the waveform score from the Ocular Response Analyzer. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1803–1806. [Google Scholar] [CrossRef]
- de Freitas Valbon, B.; Ventura, M.P.; da Silva, R.S.; Canedo, A.L.; Velarde, G.C.; Ambrósio, R., Jr. Central corneal thickness and biomechanical changes after clear corneal phacoemulsification. J. Refract. Surg. 2012, 28, 215–219. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, H.; Dong, H.; Wang, L.; Jia, Y.D.; Zhang, S.H. Corneal biomechanical properties changes after coaxial 2.2-mm microincision and standard 3.0-mm phacoemulsification. Int. J. Ophthalmol. 2016, 9, 230–234. [Google Scholar] [CrossRef]
- Sun, L.; Shen, M.; Wang, J.; Fang, A.; Xu, A.; Fang, H.; Lu, F. Recovery of corneal hysteresis after reduction of intraocular pressure in chronic primary angle-closure glaucoma. Am. J. Ophthalmol. 2009, 147, 1061–1066. [Google Scholar] [CrossRef]
- Pakravan, M.; Afroozifar, M.; Yazdani, S. Corneal Biomechanical Changes Following Trabeculectomy, Phaco-trabeculectomy, Ahmed Glaucoma Valve Implantation and Phacoemulsification. J. Ophthalmic. Vis. Res. 2014, 9, 7–13. [Google Scholar]
- Bolívar, G.; Sánchez-Barahona, C.; Teus, M.; Castejón, M.A.; Paz-Moreno-Arrones, J.; Gutiérrez-Ortiz, C.; Mikropoulos, D.G. Effect of topical prostaglandin analogues on corneal hysteresis. Acta Ophthalmol. 2015, 93, e495–e498. [Google Scholar] [CrossRef]
- Tsikripis, P.; Papaconstantinou, D.; Koutsandrea, C.; Apostolopoulos, M.; Georgalas, I. The effect of prostaglandin analogs on the biomechanical properties and central thickness of the cornea of patients with open-angle glaucoma: A 3-year study on 108 eyes. Drug Des. Devel. Ther. 2013, 7, 1149–1156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pillunat, K.R.; Spoerl, E.; Terai, N.; Pillunat, L.E. Effect of selective laser trabeculoplasty on corneal biomechanics. Acta. Ophthalmol. 2016, 94, e501–e504. [Google Scholar] [CrossRef]
- Sahin, A.; Bayer, A. Corneal hysteresis changes in diabetic eyes. J. Cataract. Refract. Surg. 2010, 36, 361–362. [Google Scholar] [CrossRef]
- Sharifipour, F.; Panahi-Bazaz, M.; Bidar, R.; Idani, A.; Cheraghian, B. Age-related variations in corneal biomechanical properties. J. Curr. Ophthalmol. 2016, 28, 117–122. [Google Scholar] [CrossRef]
| Demographics and Ocular Parameters | Ex-PRESS | Trabeculectomy | p Value |
|---|---|---|---|
| Sex (M/F) | 10/9 | 6/5 | |
| Age: range (mean) | 16–81(62.4) | 60–78 (67.2) | 0.18 |
Diagnosis
| 11 8 | 7 4 | |
| Pre-op IOP (mean ± SD) (mmHg) | 29.4 ± 7.39 | 33.2 ± 8.61 | 0.2 |
| Pre-op antiglaucoma agents (mean ± SD) | 2.2 ± 0.7 | 2.3 ± 0.6 | |
| Pre-op CH (mean ± SD) | 7.31 ± 1.13 | 7.82 ± 2.55 | 0.24 |
| Pre-op CRF (mean ± SD) | 10.25 ± 2.76 | 11.11 ± 1.99 | 0.43 |
| CH (Mean) | CH | Mean Difference | SE | p Value a (Repeated Measures ANOVA) |
|---|---|---|---|---|
| Preop: 7.31 | Postop—1 month | 0.606 | 0.308 | 0.394 |
| —6 months | 1.011 | 0.316 | 0.0318 | |
| —12 months | 1.056 | 0.313 | 0.0218 |
| CRF (Mean) | CRF | Mean Difference | SE | p Value a (Repeated Measures ANOVA) |
|---|---|---|---|---|
| Preop: 10.26 | Postop—1 month | −2.178 | 0.540 | 0.0052 |
| —6 months | −2.133 | 0.482 | 0.0022 | |
| —12 months | −1.9 | 0.479 | 0.0060 |
| CH | Median | Minimum–Maximum | CH Preop—Time Points | p Value a (Friedman Test) |
|---|---|---|---|---|
| preop | 7.85 | 3.9–13.9 | Preop | 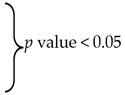 |
| 1 month | 8.9 | 6.2–14.7 | —1 month | |
| 6 months | 8.8 | 6.4–14 | —6 months | |
| 12 months | 8.6 | 6.6–14.8 | —12 months |
| CRF (Mean) | CRF | Mean Difference | SE | p Value a (Repeated Measures ANOVA) |
|---|---|---|---|---|
| Preop: 11.11 | Preop—1 month | 2.9 | 0.548 | 0.0015 |
| —6 months | 2.783 | 0.438 | 0.0003 | |
| —12 months | 2.825 | 0.422 | 0.0002 |
| CH (Preop-postop Time Points) | 1 Month Mean ± SD | p Value (Welch Test) | 6 Months Mean ± SD | p Value (Welch Test) | 12 Months Mean ± SD | p Value (Welch Test) |
|---|---|---|---|---|---|---|
| Ex-PRESS group | 0.75 ± 2.23 | 0.44 | 1.59 ± 2.82 | 0.56 | 1.64 ± 2.82 | 0.47 |
| Trab group | 1.22 ± 1.08 | 1.15 ± 1.11 | 1.1 ± 1.06 |
| CRF (Preop-Postop Time Points) | 1 Month Mean ± SD | p Value (Unpaired t-Test) | 6 Months Mean ± SD | p Value (Unpaired t-Test) | 12 Months Mean ± SD | p Value (Unpaired t-Test) |
|---|---|---|---|---|---|---|
| Ex-PRESS group | –1.67 ± 2.7 | 0.23 | –2.13 ± 2.04 | 0.62 | –1.9 ± 2.03 | 0.27 |
| Trab group | –2.9 ± 1.89 | –1.7 ± 2.7 | –2.81 ± 1.47 |
| Mean IOP | Trabeculectomy (Mean ± SD) | ExPRESS (Mean ± SD) | p Value (Unpaired t-Test) | ||
|---|---|---|---|---|---|
| Preop | 33.2 ± 8.61 | 29.4 ± 7.39 | 0.2 | ||
| —1 month | 10.4 ± 4.86 | 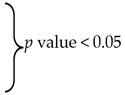 | 12.9 ± 4.57 | 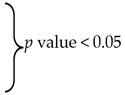 | 0.16 |
| —6 months | 13.6 ± 4.1 | 15.9 ± 3.23 | 0.10 | ||
| —12 months | 15.9 ± 3.07 | 16.1 ± 3.77 | 0.87 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantinidis, A.; Panagiotopoulou, E.-K.; Panos, G.D.; Sideroudi, H.; Mehmet, A.; Labiris, G. The Effect of Antiglaucoma Procedures (Trabeculectomy vs. Ex-PRESS Glaucoma Drainage Implant) on the Corneal Biomechanical Properties. J. Clin. Med. 2021, 10, 802. https://doi.org/10.3390/jcm10040802
Konstantinidis A, Panagiotopoulou E-K, Panos GD, Sideroudi H, Mehmet A, Labiris G. The Effect of Antiglaucoma Procedures (Trabeculectomy vs. Ex-PRESS Glaucoma Drainage Implant) on the Corneal Biomechanical Properties. Journal of Clinical Medicine. 2021; 10(4):802. https://doi.org/10.3390/jcm10040802
Chicago/Turabian StyleKonstantinidis, Aristeidis, Eirini-Kanella Panagiotopoulou, Georgios D. Panos, Haris Sideroudi, Aysel Mehmet, and Georgios Labiris. 2021. "The Effect of Antiglaucoma Procedures (Trabeculectomy vs. Ex-PRESS Glaucoma Drainage Implant) on the Corneal Biomechanical Properties" Journal of Clinical Medicine 10, no. 4: 802. https://doi.org/10.3390/jcm10040802
APA StyleKonstantinidis, A., Panagiotopoulou, E.-K., Panos, G. D., Sideroudi, H., Mehmet, A., & Labiris, G. (2021). The Effect of Antiglaucoma Procedures (Trabeculectomy vs. Ex-PRESS Glaucoma Drainage Implant) on the Corneal Biomechanical Properties. Journal of Clinical Medicine, 10(4), 802. https://doi.org/10.3390/jcm10040802










