Triple Monitoring May Avoid Intraneural Injection during Interscalene Brachial Plexus Block for Arthroscopic Shoulder Surgery: A Prospective Preliminary Study
Abstract
1. Introduction
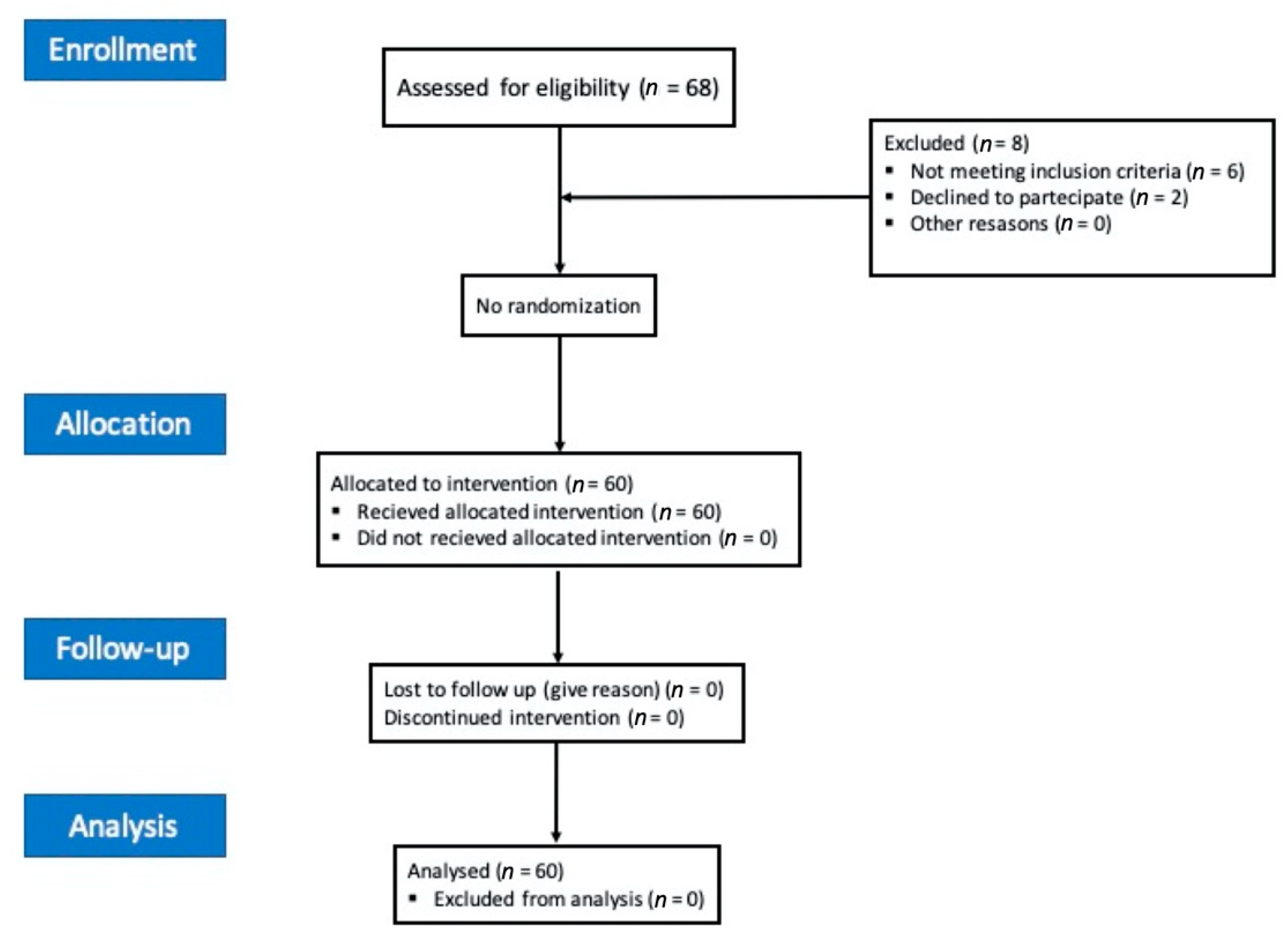
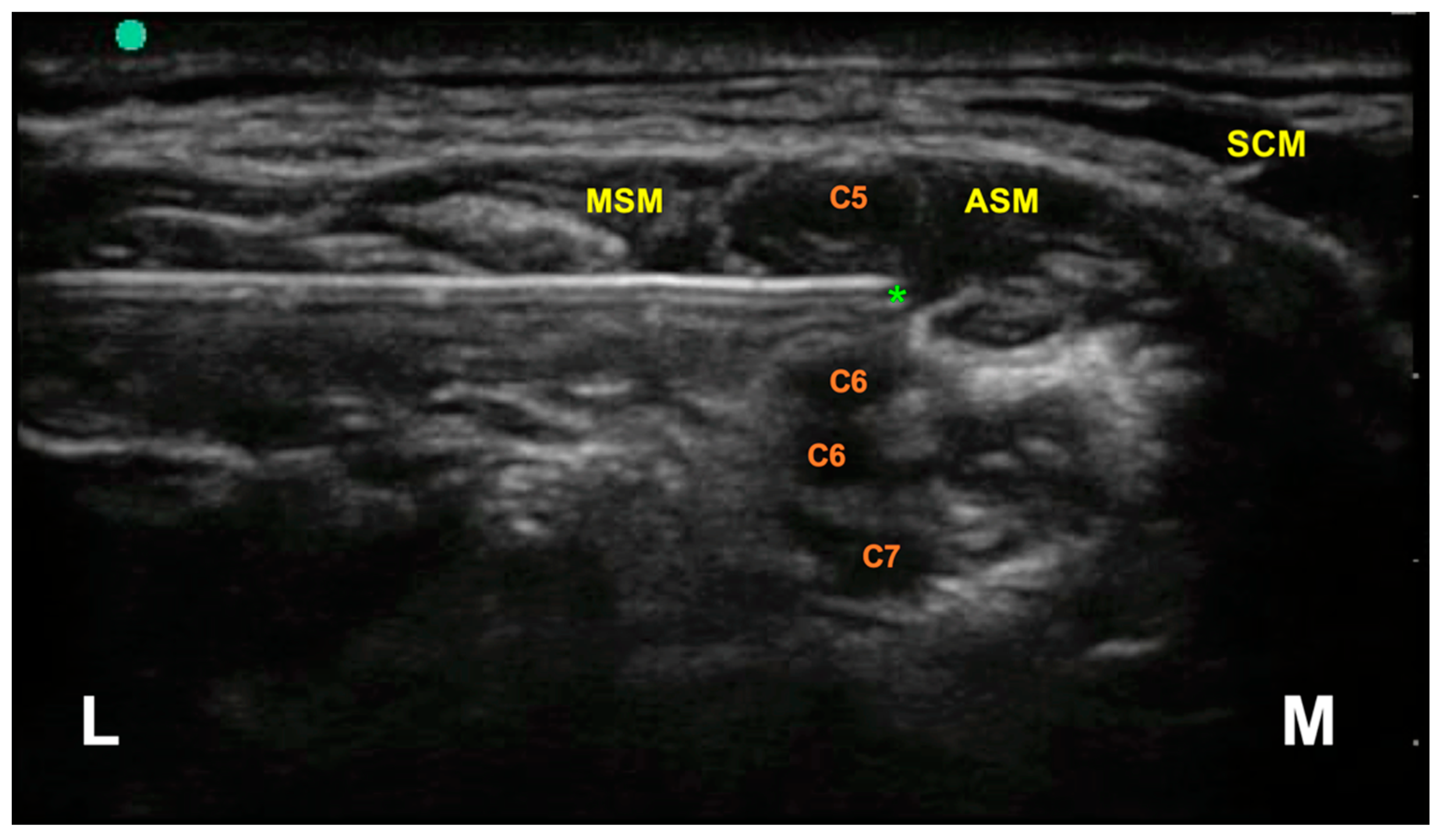
2. Methods
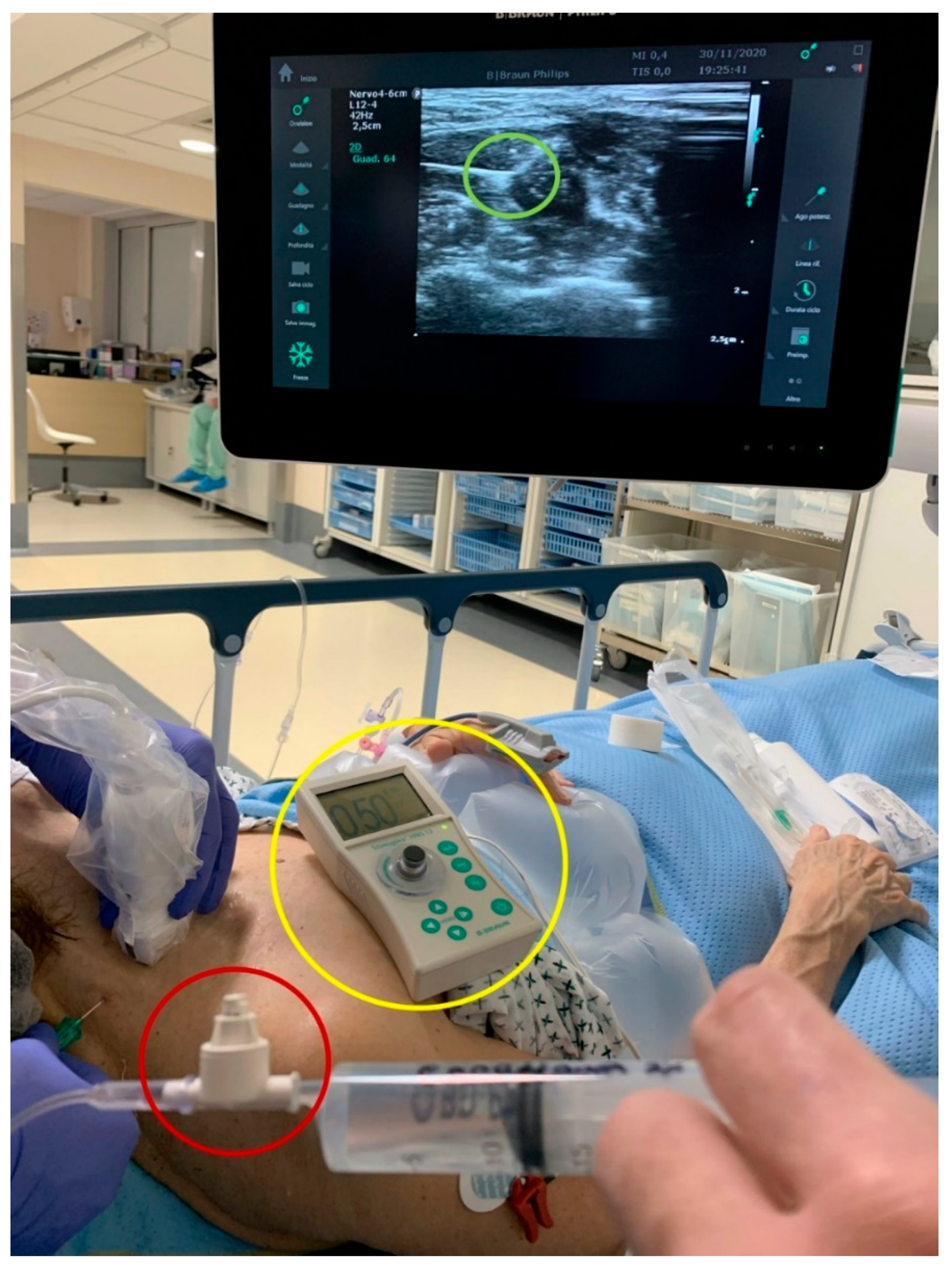
- An ultrasound machine (Sonosite M-Turbo, Sononite, Bothell, WA, USA) with multifrequency linear probe (8–12 MHz);
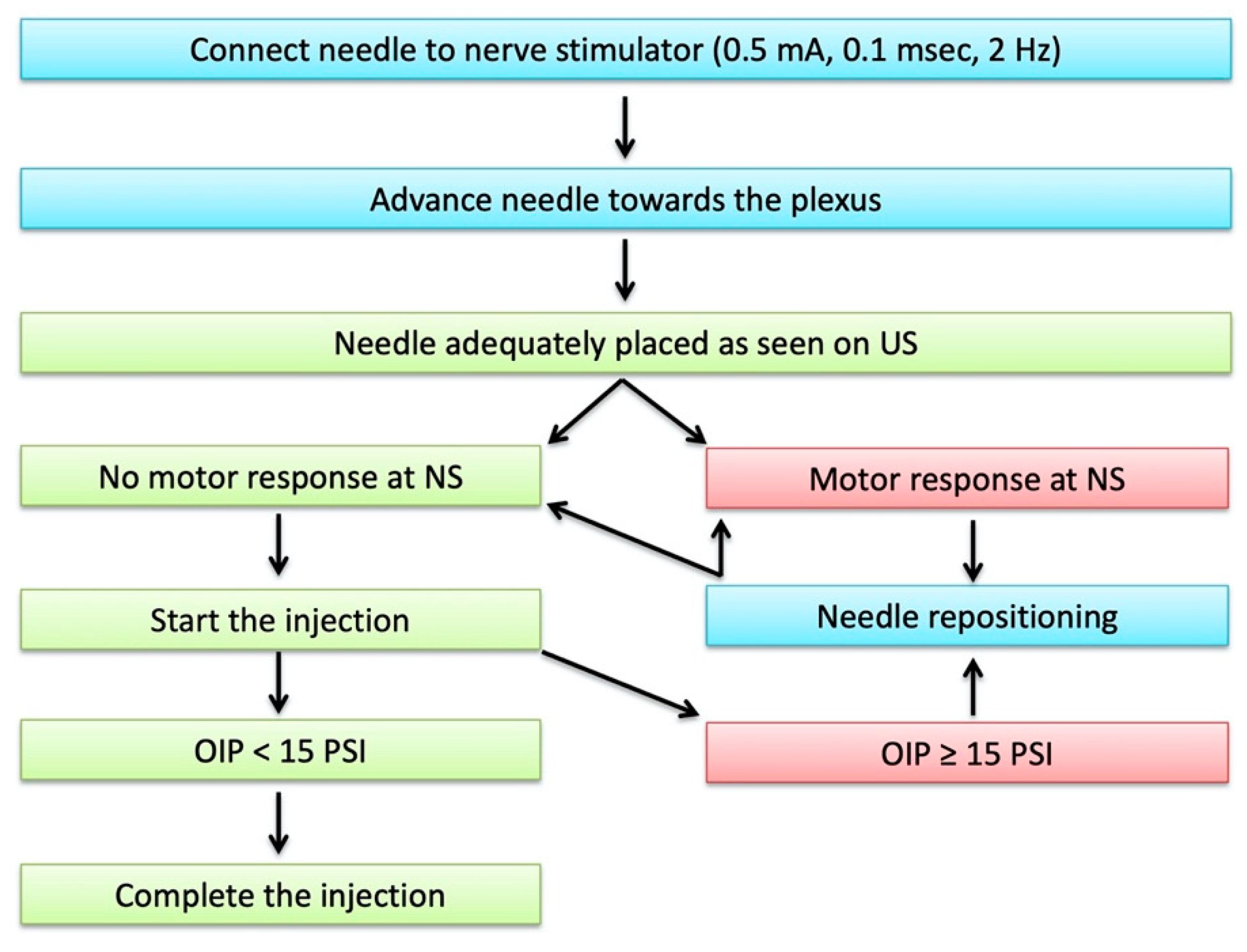
- A nerve stimulator (Stimuplex TM HNS 12, B. Braun Medical, Melsungen, Germany) set at 0.5 mA (2 Hz, 0.1 ms);
- BSmart® (B. Braun Medical, Melsungen, Germany), an injection pressure monitoring system designed to avoid accidental intraneural injection. This device must be placed between the syringe and the needle extension tubing. It consists of a piston with three different coloured bands indicating three different levels of injection pressure (white: <15 psi; yellow: 15–20 psi; red: >20 psi). An OIP ≥ 15 PSI is suggestive of intraneural injection.
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, N.; Goldar, G.; Ragina, N.; Banfield, L.; Laffey, J.G.; Abdallah, F.W. Suprascapular and Interscalene Nerve Block for Shoulder Surgery. Anesthesiology 2017, 127, 998–1013. [Google Scholar] [CrossRef]
- Kwofie, K.; Shastri, U.; Vandepitte, C. Standard approaches for upper extremity nerve blocks with an emphasis on outpatient surgery. Curr. Opin. Anaesthesiol. 2013, 26, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kelly, C.; O’Brien, T.; Wilson, J.; Warner, J.J. Ultrasound-Guided Interscalene Block Anesthesia for Shoulder Arthroscopy. J. Bone Jt. Surg. -Am. Vol. 2012, 94, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Hewson, D.W.; Bedforth, N.M.; Hardman, J.G. Peripheral nerve injury arising in anaesthesia practice. Anaesthesia 2018, 73, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Neal, J.M.; Barrington, M.J.; Brull, R.; Hadzic, A.; Hebl, J.R.; Horlocker, T.T.; Huntoon, M.A.; Kopp, S.L.; Rathmell, J.P.; Watson, J.C. The Second ASRA Practice Advisory on Neurologic Complications Associated with Regional Anesthesia and Pain Medicine. Reg. Anesth. Pain Med. 2015, 40, 401–430. [Google Scholar] [CrossRef]
- Brull, R.; Hadzic, A.; Reina, M.A.; Barrington, M.J. Pathophysiology and Etiology of Nerve Injury Following Peripheral Nerve Blockade. Reg. Anesth. Pain Med. 2015, 40, 479–490. [Google Scholar] [CrossRef]
- Hogan, Q.H. Pathophysiology of Peripheral Nerve Injury during Regional Anesthesia. Reg. Anesth. Pain Med. 2008, 33, 435–441. [Google Scholar] [CrossRef]
- Pascarella, G.; Costa, F.; Rizzo, S.; Del Buono, R.; Agrò, F.E.; Carassiti, M. Electrical needle stimulation for ultrasound training. Minerva Anestesiol. 2020, 86, 998–1000. [Google Scholar] [CrossRef]
- Barrington, M.J.; Lirk, P. Reducing the risk of neurological complications after peripheral nerve block: What is the role of pressure monitoring? Anaesthesia 2019, 74, 9–12. [Google Scholar] [CrossRef]
- Claudio, R.; Hadzic, A.; Shih, H.; Vloka, J.D.; Castro, J.; Koscielniak-Nielsen, Z.; Thys, D.M.; Santos, A.C. Injection Pressures by Anesthesiologists during Simulated Peripheral Nerve Block. Reg. Anesth. Pain Med. 2004, 29, 201–205. [Google Scholar] [CrossRef]
- Gadsden, J.C.; Choi, J.J.; Lin, E.; Robinson, A. Opening Injection Pressure Consistently Detects Needle–Nerve Contact during Ultrasound-guided Interscalene Brachial Plexus Block. Anesthesiology 2014, 120, 1246–1253. [Google Scholar] [CrossRef]
- Weisman, R.S.; Bhavsar, N.P.; Schuster, K.A.; Gebhard, R.E. Evaluation of the B-Smart manometer and the CompuFlo computerized injection pump technology for accurate needle-tip injection pressure measurement during peripheral nerve blockade. Reg. Anesth. Pain Med. 2019, 44, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.S.; Shim, J.C.; Han, K.H.; Shim, J.H. A comparison of motor stimulation threshold in ultrasound-guided interscalene brachial plexus block for arthroscopic shoulder surgery: A randomized trial. Can. J. Anesth. /J. Can. d’anesthésie 2015, 63, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Bigeleisen, P.E.; Moayeri, N.; Groen, G.J. Extraneural versus Intraneural Stimulation Thresholds during Ultrasound-guided Supraclavicular Block. Anesthesiology 2009, 110, 1235–1243. [Google Scholar] [CrossRef]
- Koscielniak-Nielsen, Z.J.; Dahl, J.B. Ultrasound-guided peripheral nerve blockade of the upper extremity. Curr. Opin. Anaesthesiol. 2012, 25, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Strumia, A.; Costa, F.; Pascarella, G.; Del Buono, R.; Agrò, F.E. U smart: Ultrasound in your pocket. J. Clin. Monit. 2020, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Vermeylen, K.; Hermans, M.; Soetens, F.; Vereecke, E.; Steinfeldt, T.; Groen, G.; Hadzic, A.; Van De Velde, M. Opening Injection Pressure Is Higher in Intraneural Compared With Perineural Injections During Simulated Nerve Blocks of the Lower Limb in Fresh Human Cadavers. Reg. Anesth. Pain Med. 2017, 42, 362–367. [Google Scholar] [CrossRef]
- Gadsden, J.; Latmore, M.; Levine, D.M.; Robinson, A. High Opening Injection Pressure Is Associated With Needle-Nerve and Needle-Fascia Contact During Femoral Nerve Block. Reg. Anesth. Pain Med. 2016, 41, 50–55. [Google Scholar] [CrossRef]
- Tsai, T.; Vuckovic, I.; Dilberovic, F.; Obhodzas, M.; Kapur, E.; Divanovic, K.; Hadzic, A. Intensity of the Stimulating Current May Not Be a Reliable Indicator of Intraneural Needle Placement. Reg. Anesth. Pain Med. 2008, 33, 207–210. [Google Scholar] [CrossRef]
- Park, S.K.; Sung, M.H.; Suh, H.J.; Choi, Y.S. Ultrasound Guided Low Approach Interscalene Brachial Plexus Block for Upper Limb Surgery. Korean J. Pain 2016, 29, 18–22. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cho, S.-H.; Kim, S.-H.; Chae, W.-S.; Jin, H.-C.; Lee, J.-S.; Kim, Y.-I. Ropivacaine for ultrasound-guided interscalene block: 5 mL provides similar analgesia but less phrenic nerve paralysis than 10 mL. Can. J. Anesth. /J. Can. d’anesthésie 2011, 58, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Gautier, P.; Vandepitte, C.; Ramquet, C.; Decoopman, M.; Xu, D.; Hadzic, A. The Minimum Effective Anesthetic Volume of 0.75% Ropivacaine in Ultrasound-Guided Interscalene Brachial Plexus Block. Anesth. Analg. 2011, 113, 951–955. [Google Scholar] [CrossRef] [PubMed]
| Patients’ Characteristics and Main Outcomes | |
| Number of patients | 60 |
| Sex (M/F) | 24/36 |
| Age (Mean ± SD), years | 59.9 ± 5.3 |
| BMI (Mean ± SD), kg/m2 | 26.4 ± 5.9 |
| Time for nerve block execution (Mean ± SD), s | 67.2 ± 5.3 |
| Pain during block execution | none |
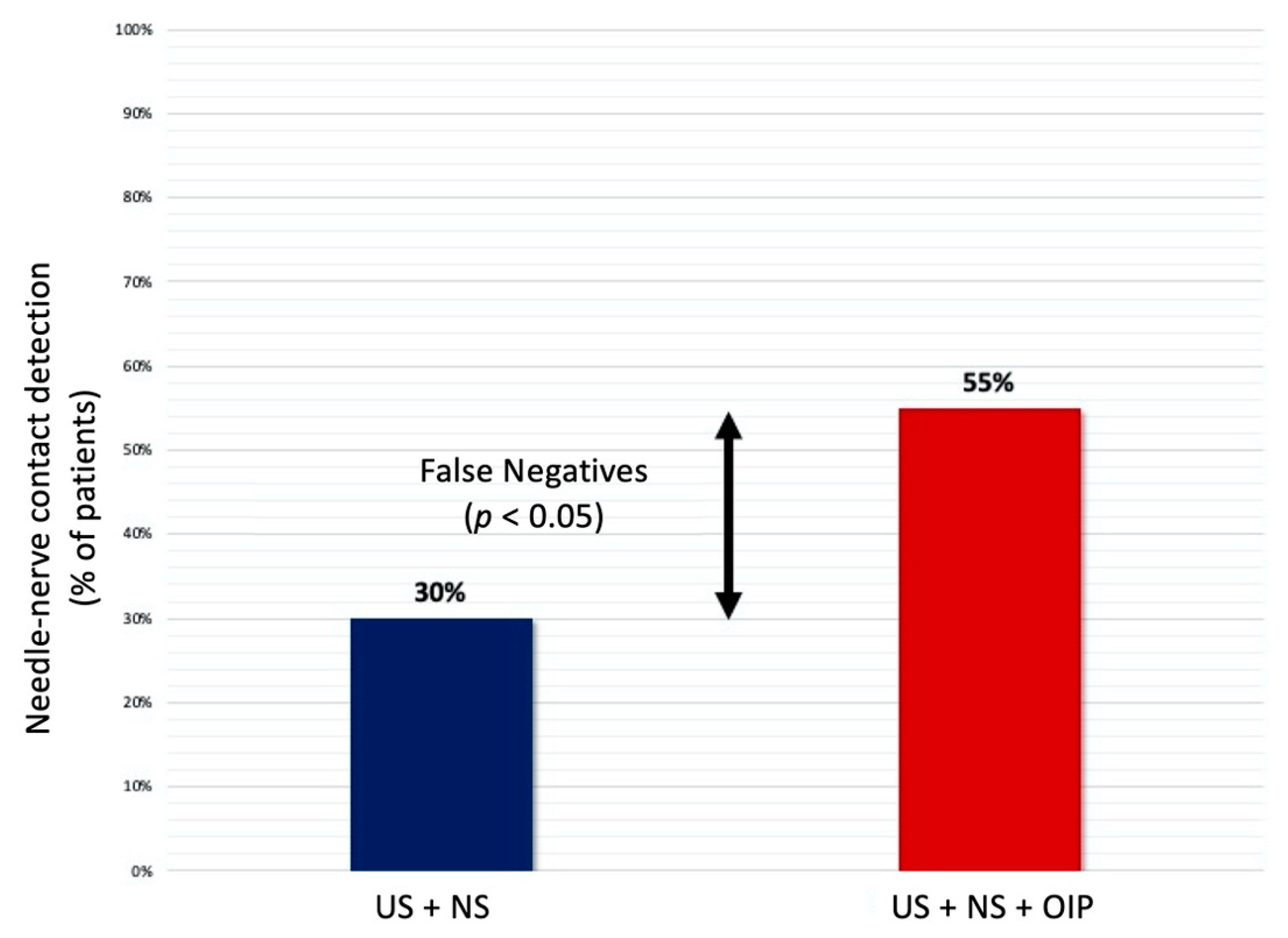
Needle–nerve contact detection, n (%)
| 18 (30%) 33 (55%) |
| Block onset time (Mean ± SD), min | 13 ± 5.2 |
| Motor block duration (Mean ± SD), hrs | 8.5 ± 1 |
| Sensitive block duration (Mean ± SD), hrs | 10.2 ± 2 |
| Postoperative neurological complications | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascarella, G.; Strumia, A.; Costa, F.; Rizzo, S.; Del Buono, R.; Remore, L.M.; Bruno, F.; Agrò, F.E. Triple Monitoring May Avoid Intraneural Injection during Interscalene Brachial Plexus Block for Arthroscopic Shoulder Surgery: A Prospective Preliminary Study. J. Clin. Med. 2021, 10, 781. https://doi.org/10.3390/jcm10040781
Pascarella G, Strumia A, Costa F, Rizzo S, Del Buono R, Remore LM, Bruno F, Agrò FE. Triple Monitoring May Avoid Intraneural Injection during Interscalene Brachial Plexus Block for Arthroscopic Shoulder Surgery: A Prospective Preliminary Study. Journal of Clinical Medicine. 2021; 10(4):781. https://doi.org/10.3390/jcm10040781
Chicago/Turabian StylePascarella, Giuseppe, Alessandro Strumia, Fabio Costa, Stefano Rizzo, Romualdo Del Buono, Luigi Maria Remore, Federica Bruno, and Felice Eugenio Agrò. 2021. "Triple Monitoring May Avoid Intraneural Injection during Interscalene Brachial Plexus Block for Arthroscopic Shoulder Surgery: A Prospective Preliminary Study" Journal of Clinical Medicine 10, no. 4: 781. https://doi.org/10.3390/jcm10040781
APA StylePascarella, G., Strumia, A., Costa, F., Rizzo, S., Del Buono, R., Remore, L. M., Bruno, F., & Agrò, F. E. (2021). Triple Monitoring May Avoid Intraneural Injection during Interscalene Brachial Plexus Block for Arthroscopic Shoulder Surgery: A Prospective Preliminary Study. Journal of Clinical Medicine, 10(4), 781. https://doi.org/10.3390/jcm10040781






