Clinical Efficacy and Safety of Low-Level Laser Therapy in Patients with Perennial Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Sample Size Calculation
2.3. Participants
2.4. Randomization and Blinding
2.5. Intervention
2.6. Primary Outcome
2.7. Secondary Outcome
2.8. Adverse Events
2.9. Statistical Analysis
3. Results
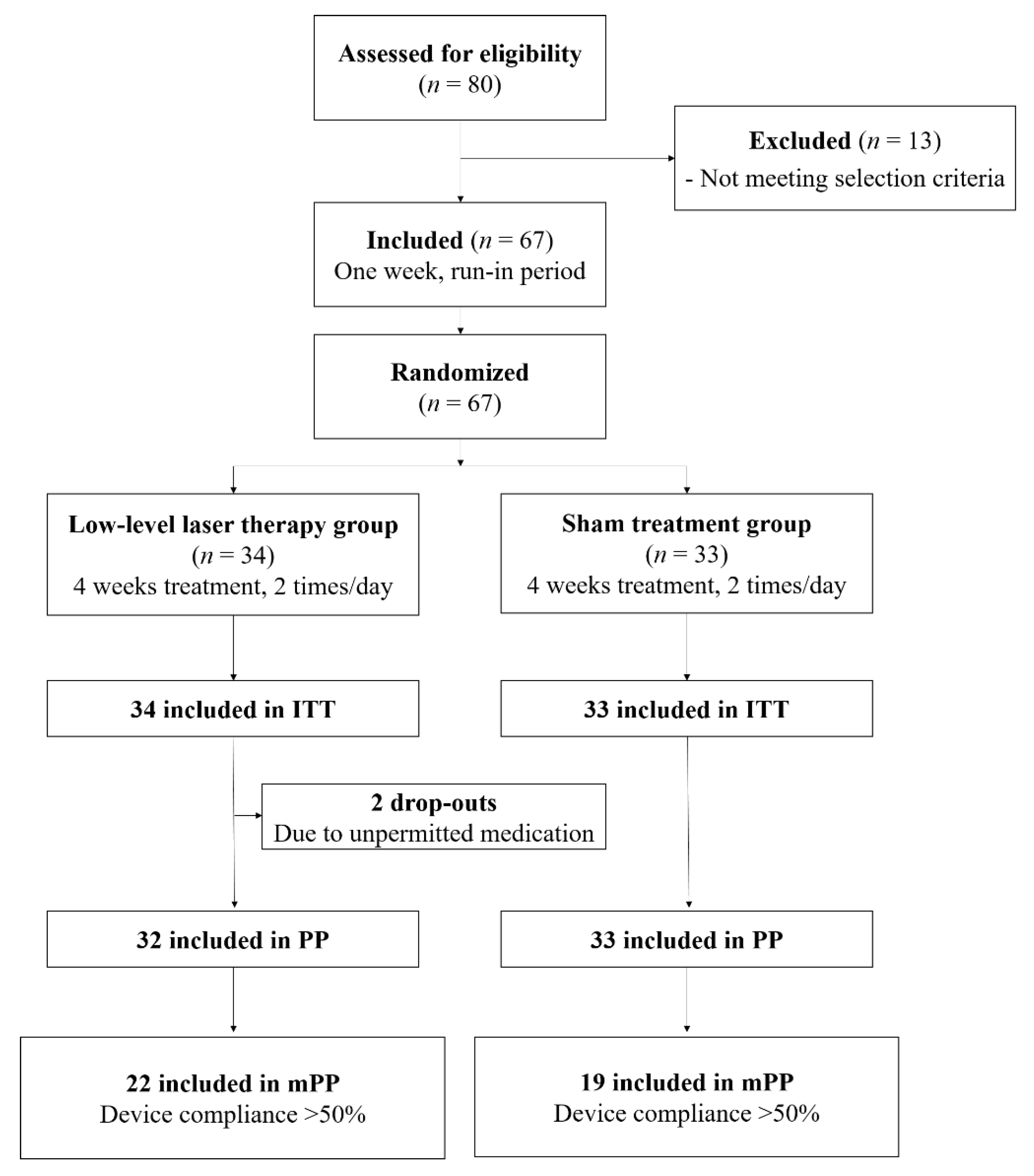
3.1. Participants and Baseline Characteristics
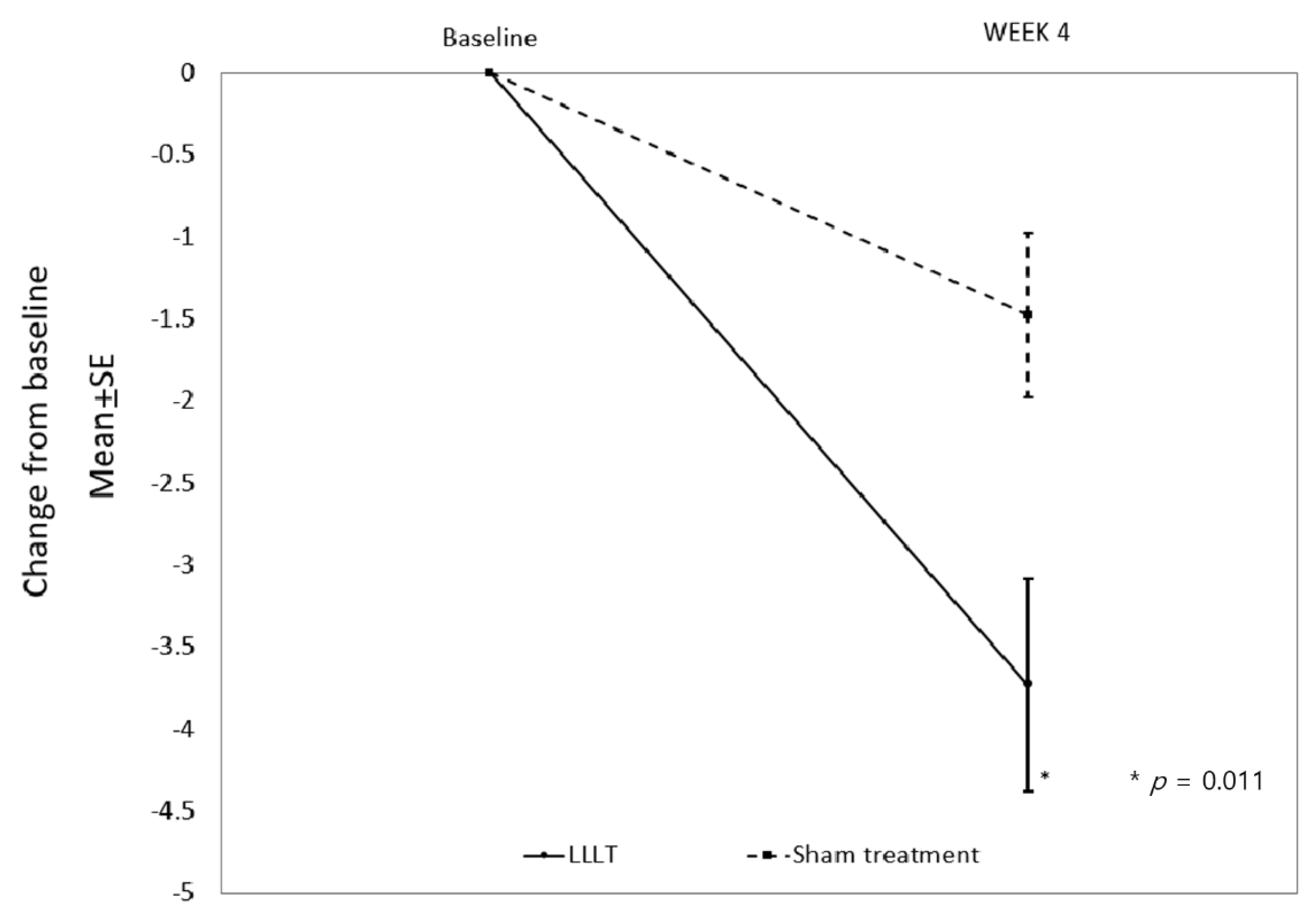
3.2. Primary Outcome
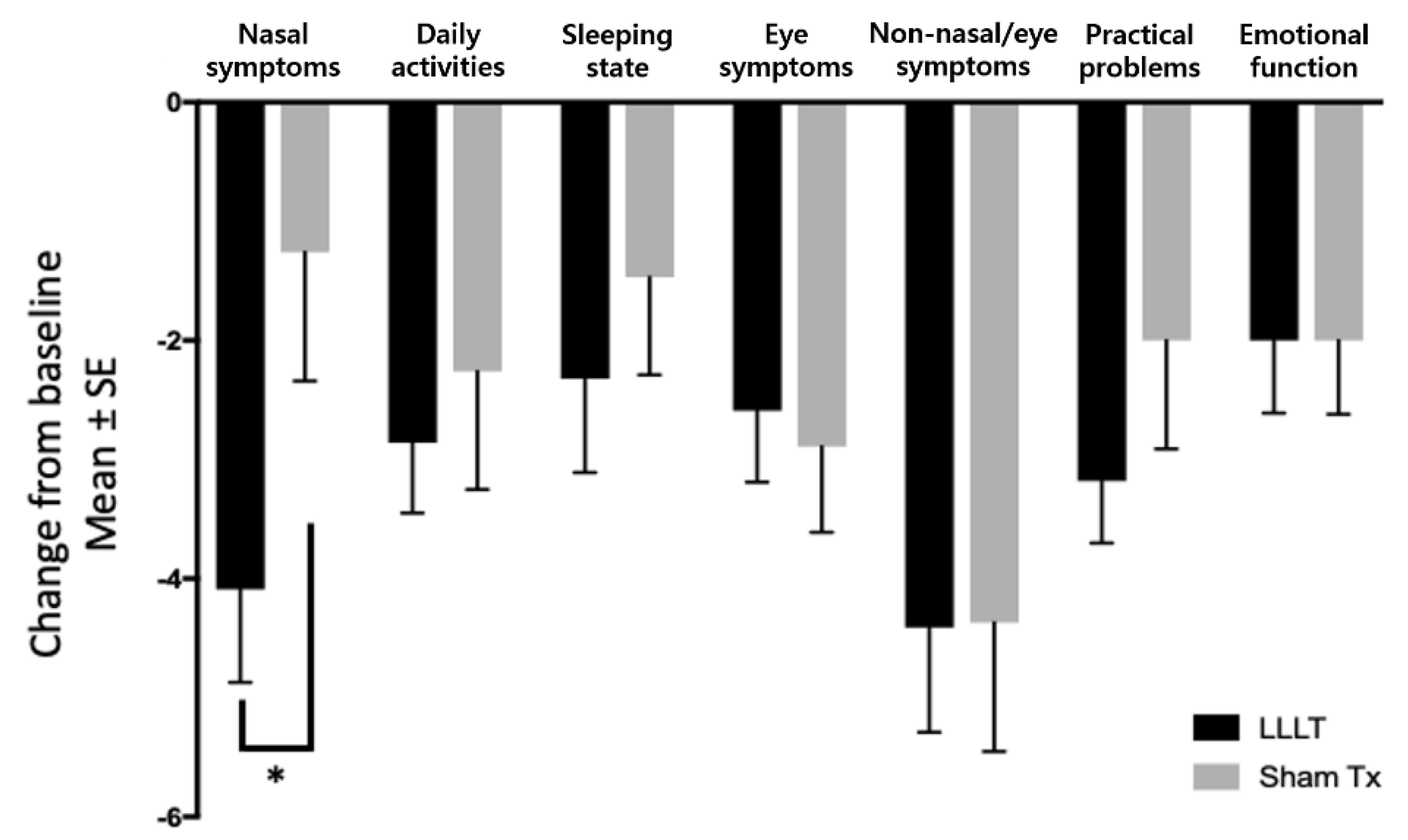
3.3. Secondary Outcome
3.4. Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Blaiss, M.S.; Naclerio, R.M.; Stoloff, S.W.; Derebery, M.J.; Nelson, H.S.; Boyle, J.M.; Wingertzahn, M.A. Burden of allergic rhinitis: Allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc. 2012, 33, 113–141. [Google Scholar] [CrossRef]
- Caballer, B.D.L.H.; Rodríguez, M.; Fraj, J.; Cerecedo, I.; Antolín-Amérigo, D.; Colas, C. Allergic Rhinitis and Its Impact on Work Productivity in Primary Care Practice and a Comparison with Other Common Diseases: The Cross-sectional Study to Evaluate Work Productivity in Allergic Rhinitis Compared with Other Common Diseases (CAPRI) Study. Am. J. Rhinol. Allergy 2012, 26, 390–394. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95. [Google Scholar] [CrossRef]
- Meltzer, E.O.; Nathan, R.; Derebery, J.; Stang, P.E.; Campbell, U.B.; Yeh, W.-S.; Corrao, M.; Stanford, R. Sleep, quality of life, and productivity impact of nasal symptoms in the United States: Findings from the Burden of Rhinitis in America survey. Allergy Asthma Proc. 2009, 30, 244–254. [Google Scholar] [CrossRef]
- Felix, M.; Paz, C.V.; Mata, V.L.; Vanegas, E.; Larenas-Linnemann, D.; Rosario, N.A.; Letort, J.; Cherrez-Ojeda, I. Perceptions and Management of Allergic Rhinitis Among Ecuadorian Otorhinolaryngologists: A Survey-Based Study. J. Multidiscip. Healthc. 2020, 13, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.A. Allergic and mixed rhinitis: Epidemiology and natural history. Allergy Asthma Proc. 2010, 31, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.A. Management of Patients with Allergic Rhinitis and Asthma: Literature Review. South. Med. J. 2009, 102, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Csoma, Z.; Koreck, A.; Ignacz, F.; Bor, Z.; Szabo, G.; Bodai, L.; Dobozy, A.; Kemeny, L. PUVA treatment of the nasal cavity improves the clinical symptoms of allergic rhinitis and inhibits the immediate-type hypersensitivity reaction in the skin. J. Photochem. Photobiol. B Biol. 2006, 83, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Koreck, A.I.; Csoma, Z.; Bodai, L.; Ignacz, F.; Kenderessy, A.S.; Kadocsa, E.; Szabo, G.; Bor, Z.; Erdei, A.; Szony, B. Rhinophototherapy: A new therapeutic tool for the management of allergic rhinitis. J. Allergy Clin. Immunol. 2005, 115, 541–547. [Google Scholar] [CrossRef]
- Hu, K.-H.; Li, W.-T. Clinical Effects of Far-Infrared Therapy in Patients with Allergic Rhinitis. In Proceedings of the 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; Volume 2007, pp. 1479–1482. [Google Scholar]
- Lee, H.M.; Park, M.S.; Park, I.H.; Lee, S.H.; Lee, S.K.; Kim, K.S.; Choi, H. A comparative pilot study of symptom improvement before and after phototherapy in Korean patients with perennial allergic rhinitis. Photochem. Photobiol. 2013, 89, 751–757. [Google Scholar] [CrossRef]
- Cingi, C.; Cakli, H.; Yaz, A.; Songu, M.; Bal, C. Phototherapy for allergic rhinitis: A prospective, randomized, single-blind, placebo-controlled study. Ther. Adv. Respir. Dis. 2010, 4, 209–213. [Google Scholar] [CrossRef]
- Kemény, L.; Koreck, A. Ultraviolet light phototherapy for allergic rhinitis. J. Photochem. Photobiol. B Biol. 2007, 87, 58–65. [Google Scholar] [CrossRef]
- Koreck, A.; Szechenyi, A.; Morocz, M.; Cimpean, A.; Bella, Z.; Garaczi, E.; Raica, M.; Olariu, T.; Rasko, I.; Kemeny, L. Effects of intranasal phototherapy on nasal mucosa in patients with allergic rhinitis. J. Photochem. Photobiol. B Biol. 2007, 89, 163–169. [Google Scholar] [CrossRef]
- Aimbire, F.; Albertini, R.; Pacheco, M.T.; Castro-Faria-Neto, H.C.; Leonardo, P.S.; Iversen, V.V.; Lopes-Martins, R.A.; Bjordal, J.M. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed. Laser Surg. 2006, 24, 33–37. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.G.; Aarestrup, F.M.; Miranda, C.; Vieira, B.J.; Ferreira, A.P.; Andrade, L.C. Low-Level Laser Therapy Reduces Delayed Hypersensitivity Reaction to Ovalbumin in Balb/C Mice. Photomed. Laser Surg. 2010, 28, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Chang, M.S.; Kim, H.Y.; Park, J.-W.; Ryu, B.; Kim, J. Effects of Low Level Laser Therapy on Ovalbumin-Induced Mouse Model of Allergic Rhinitis. Evidence-Based Complement. Altern. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hampel, F.C.; Ratner, P.H.; Van Bavel, J.; Amar, N.; Daftary, P.; Wheeler, W.; Sacks, H. Double-blind, placebo-controlled study of azelastine and fluticasone in a single nasal spray delivery device. Ann. Allergy Asthma Immunol. 2010, 105, 168–173. [Google Scholar] [CrossRef]
- Juniper, E.F.; Thompson, A.K.; Ferrie, P.J.; Roberts, J.N. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J. Allergy Clin. Immunol. 1999, 104, 364–369. [Google Scholar] [CrossRef]
- Krespi, Y.P.; Kizhner, V. Phototherapy for chronic rhinosinusitis. Lasers Surg. Med. 2011, 43, 187–191. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Lopes-Martins, R.Á.B.; Joensen, J.; Iversen, V.V. The anti-inflammatory mechanism of low level laser therapy and its relevance for clinical use in physiotherapy. Phys. Ther. Rev. 2010, 15, 286–293. [Google Scholar] [CrossRef]
- De Lima, F.M.; Villaverde, A.B.; Albertini, R.; Correa, J.C.; Carvalho, R.L.P.; Munin, E.; Araújo, T.; Silva, J.A.; Aimbire, F. Dual Effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: Action on anti- and pro-inflammatory cytokines. Lasers Surg. Med. 2011, 43, 410–420. [Google Scholar] [CrossRef]
- Gao, X.; Xing, D. Molecular mechanisms of cell proliferation induced by low power laser irradiation. J. Biomed. Sci. 2009, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Csoma, Z.; Ignacz, F.; Bor, Z.; Szabo, G.; Bodai, L.; Dobozy, A.; Kemény, L. Intranasal irradiation with the xenon chloride ultraviolet B laser improves allergic rhinitis. J. Photochem. Photobiol. B Biol. 2004, 75, 137–144. [Google Scholar] [CrossRef]
- Garaczi, E.; Koreck, A.; Boros-Gyevi, M.; Bella, Z.; Csoma, Z.; Kemény, L. Intranasal Phototherapy Is More Effective Than Fexofenadine Hydrochloride in the Treatment of Seasonal Allergic Rhinitis: Results of a Pilot Study. Photochem. Photobiol. 2011, 87, 474–477. [Google Scholar] [CrossRef]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. Mol. Mech. Mutagen. 2005, 571, 3–17. [Google Scholar] [CrossRef]
- Kuluncsics, Z.; Perdiz, D.; Brulay, E.; Muel, B.; Sage, E. Wavelength dependence of ultraviolet-induced DNA damage distri-bution: Involvement of direct or indirect mechanisms and possible artefacts. J. Photochem. Photobiol. B 1999, 49, 71–80. [Google Scholar] [CrossRef]
- Mitchell, D.; Paniker, L.; Sánchez, G.; Bella, Z.; Garaczi, E.; Szell, M.; Hamid, Q.; Kemény, L.; Koreck, A. Molecular response of nasal mucosa to therapeutic exposure to broad-band ultraviolet radiation. J. Cell. Mol. Med. 2008, 14, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.C. Rhinophototherapy: Gimmick or an emerging treatment option for allergic rhinitis? Rhinol. J. 2011, 49, 499–506. [Google Scholar]
- Okubo, K.; Ichimura, M.; Koyama, T.; Susuta, Y.; Izaki, H. Double-blind placebo-controlled study of bepotastine besilate in pediatric patients with perennial allergic rhinitis. Expert Opin. Pharmacother. 2015, 16, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.H.; Jeong, J.H.; Kim, T.H.; Kim, S.T.; Kim, S.W.; Lee, K.H.; Hong, S.N.; Kim, H.Y.; Kim, D.W.; Kim, D.Y.; et al. Double-Blind Placebo-Controlled Trial of Bepotastine Salicylate in Patients With Allergic Rhinitis. Laryngoscope 2021, 131, E702–E709. [Google Scholar] [CrossRef] [PubMed]
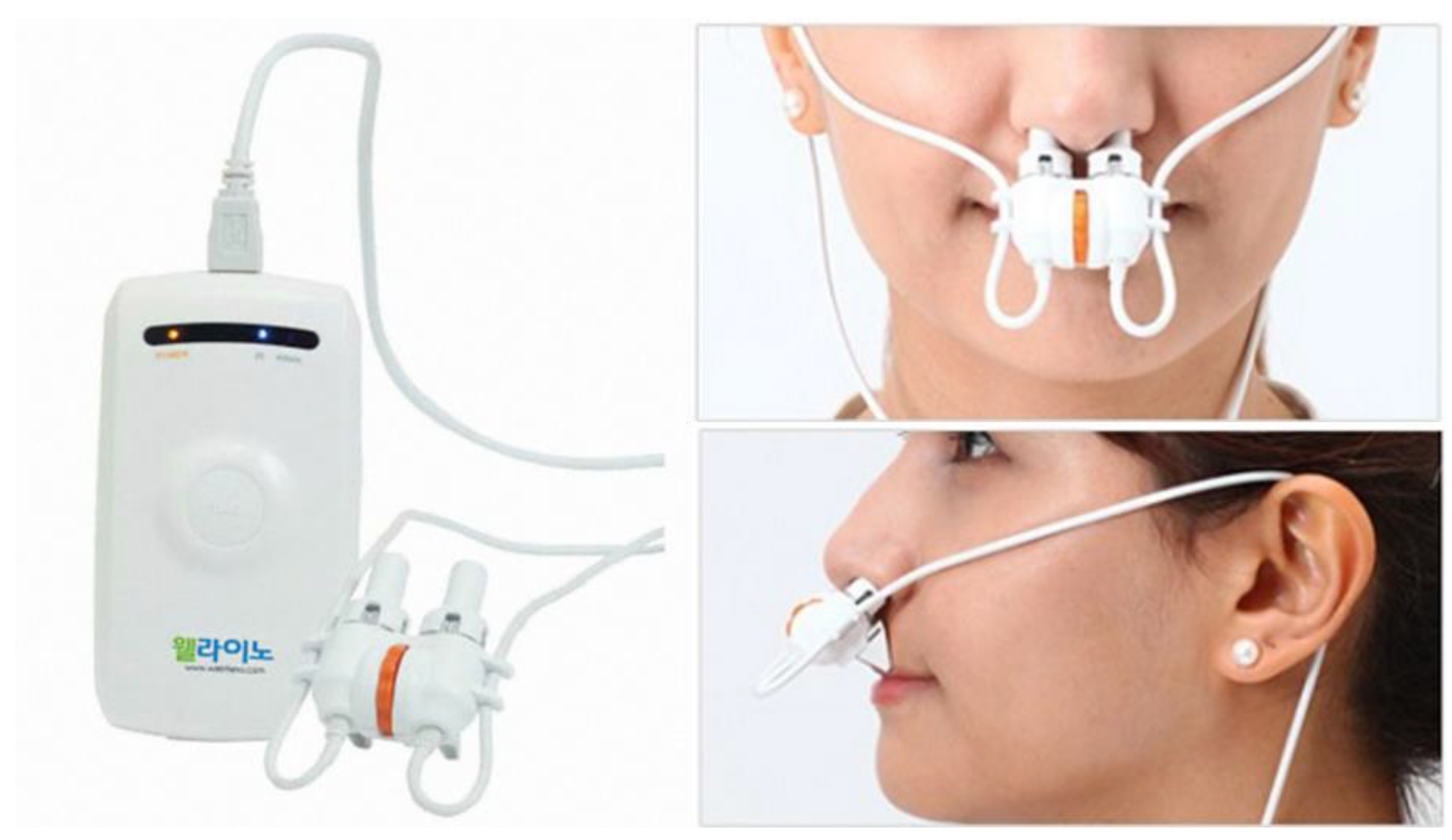
| Laser Wavelength | Output Power | Gain Medium |
|---|---|---|
| 670 nm (visible ray) | 670 nm (visible ray): 3 mW (1 mW, 3 each) | 670 nm: AlGaInP |
| 830 nm (infrared ray) | 830 nm (infrared ray): 20 mW | 830 nm: GaAs |
| Product | Wellrhino |
|---|---|
| Light source | VCSEL |
| Wavelength | 670 and 830 nm |
| Laser power | 670 nm, 3 mW; 830 nm, 20 mW |
| Total power | 23 mW |
| Using Time | 20 and 40 min |
| Variable | LLLT (n = 34) | Sham Treatment (n = 33) | p-Value |
|---|---|---|---|
| Age (years) | 27.8 ± 6.7 | 33.8 ± 12.3 | 0.018 |
| Gender (male:female) | 17:17 | 13:20 | 0.383 |
| TNSS | 5.94 ± 3.29 | 6.64 ± 2.06 | 0.243 |
| RQLQ | 58.29 ± 26.06 | 61.39 ± 22.91 | 0.433 |
| Daily activities | 7.00 ± 2.76 | 7.97 ± 3.12 | 0.066 |
| Sleeping state | 6.53 ± 4.42 | 6.00 ± 3.30 | 0.935 |
| Eye symptoms | 7.18 ± 4.98 | 7.55 ± 5.16 | 0.748 |
| Non-nasal/eye symptoms | 13.65 ± 7.55 | 13.79 ± 6.27 | 0.665 |
| Practical problems | 7.76 ± 3.64 | 8.73 ± 3.77 | 0.304 |
| Emotional function | 5.53 ± 4.30 | 6.55 ± 3.76 | 0.174 |
| Nasal symptoms | 10.65 ± 4.66 | 10.82 ± 3.90 | 0.823 |
| Group | Baseline | End of Treatment | Difference | Difference between Groups: p-Value |
|---|---|---|---|---|
| LLLT (n = 22) | 7.05 ± 3.11 | 3.32 ± 2.85 | −3.73 ± 3.03 | 0.011 |
| Sham treatment (n = 19) | 6.21 ± 2.15 | 4.74 ± 2.35 | −1.47 ± 2.20 |
| Compliance | Group | Baseline | End of Treatment | Difference | Comparison of Difference between Groups: p-Value |
|---|---|---|---|---|---|
| (Modified Per-Protocol) | LLLT (n = 22) | 60.50 ± 27.43 | 39.05 ± 27.01 | −21.45 ± 14.79 | 0.383 |
| Sham treatment (n = 19) | 57.63 ± 25.07 | 41.37 ± 26.92 | −16.26 ± 22.58 |
| Domains | Group | Baseline | End of Treatment | Difference | p-Value |
|---|---|---|---|---|---|
| Nasal symptoms | LLLT (n = 22) | 11.27 ± 4.41 | 7.18 ± 4.24 | −4.09 ± 3.65 | 0.036 |
| Sham treatment (n = 19) | 9.63 ± 3.35 | 8.37 ± 4.65 | −1.26 ± 4.69 | ||
| Daily activities | LLLT (n = 22) | 7.32 ± 2.71 | 4.45 ± 2.94 | −2.86 ± 2.78 | 0.596 |
| Sham treatment (n = 19) | 7.74 ± 3.36 | 5.47 ± 3.88 | −2.26 ± 4.33 | ||
| Sleeping state | LLLT (n = 22) | 7.09 ± 4.47 | 4.77 ± 3.78 | −2.32 ± 3.68 | 0.464 |
| Sham treatment (n = 19) | 5.84 ± 3.73 | 4.37 ± 3.68 | −1.47 ± 3.60 | ||
| Eye symptoms | LLLT (n = 22) | 6.95 ± 5.72 | 4.36 ± 4.48 | −2.59 ± 2.81 | 0.745 |
| Sham treatment (n = 19) | 6.89 ± 5.30 | 4.00 ± 3.57 | −2.89 ± 3.14 | ||
| Non-nasal/eye symptoms | LLLT (n = 22) | 14.18 ± 7.76 | 9.77 ± 7.37 | −4.41 ± 4.15 | 0.977 |
| Sham treatment (n = 19) | 13.32 ± 6.38 | 8.95 ± 6.39 | −4.37 ± 4.73 | ||
| Practical problems | LLLT (n = 22) | 7.82 ± 3.25 | 4.64 ± 3.65 | −3.18 ± 2.46 | 0.272 |
| Sham treatment (n = 19) | 8.05 ± 3.26 | 6.05 ± 4.14 | −2.00 ± 3.99 | ||
| Emotional function | LLLT (n = 22) | 5.86 ± 4.55 | 3.86 ± 3.72 | −2.00 ± 2.88 | 1.000 |
| Sham treatment (n = 19) | 6.16 ± 4.30 | 4.16 ± 4.11 | −2.00 ± 2.71 |
| Group | Adverse Events (n) |
|---|---|
| LLLT group | Definitely unrelated: idiopathic sudden hearing loss (1), injury of unrelated body part (1), pain of unrelated body part (1) |
| Sham treatment group | Definitely unrelated: dyspepsia (1), pain of unrelated body part (1), incidental finding of unrelated body part (1) Possibly unrelated: acne (1), upper respiratory infection (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.J.; Chung, Y.-J.; Choi, Y.-S.; Chung, P.S.; Mo, J.-H. Clinical Efficacy and Safety of Low-Level Laser Therapy in Patients with Perennial Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Med. 2021, 10, 772. https://doi.org/10.3390/jcm10040772
Jung HJ, Chung Y-J, Choi Y-S, Chung PS, Mo J-H. Clinical Efficacy and Safety of Low-Level Laser Therapy in Patients with Perennial Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Trial. Journal of Clinical Medicine. 2021; 10(4):772. https://doi.org/10.3390/jcm10040772
Chicago/Turabian StyleJung, Hahn Jin, Young-Jun Chung, Young-Seok Choi, Phil Sang Chung, and Ji-Hun Mo. 2021. "Clinical Efficacy and Safety of Low-Level Laser Therapy in Patients with Perennial Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Trial" Journal of Clinical Medicine 10, no. 4: 772. https://doi.org/10.3390/jcm10040772
APA StyleJung, H. J., Chung, Y.-J., Choi, Y.-S., Chung, P. S., & Mo, J.-H. (2021). Clinical Efficacy and Safety of Low-Level Laser Therapy in Patients with Perennial Allergic Rhinitis: A Randomized, Double-Blind, Placebo-Controlled Trial. Journal of Clinical Medicine, 10(4), 772. https://doi.org/10.3390/jcm10040772






