Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Definition of Cancer
2.3. Assessment of Antihypertensive Drug Use
2.4. Other Covariates
2.5. Data Source
2.6. Statistical Analysis
3. Results
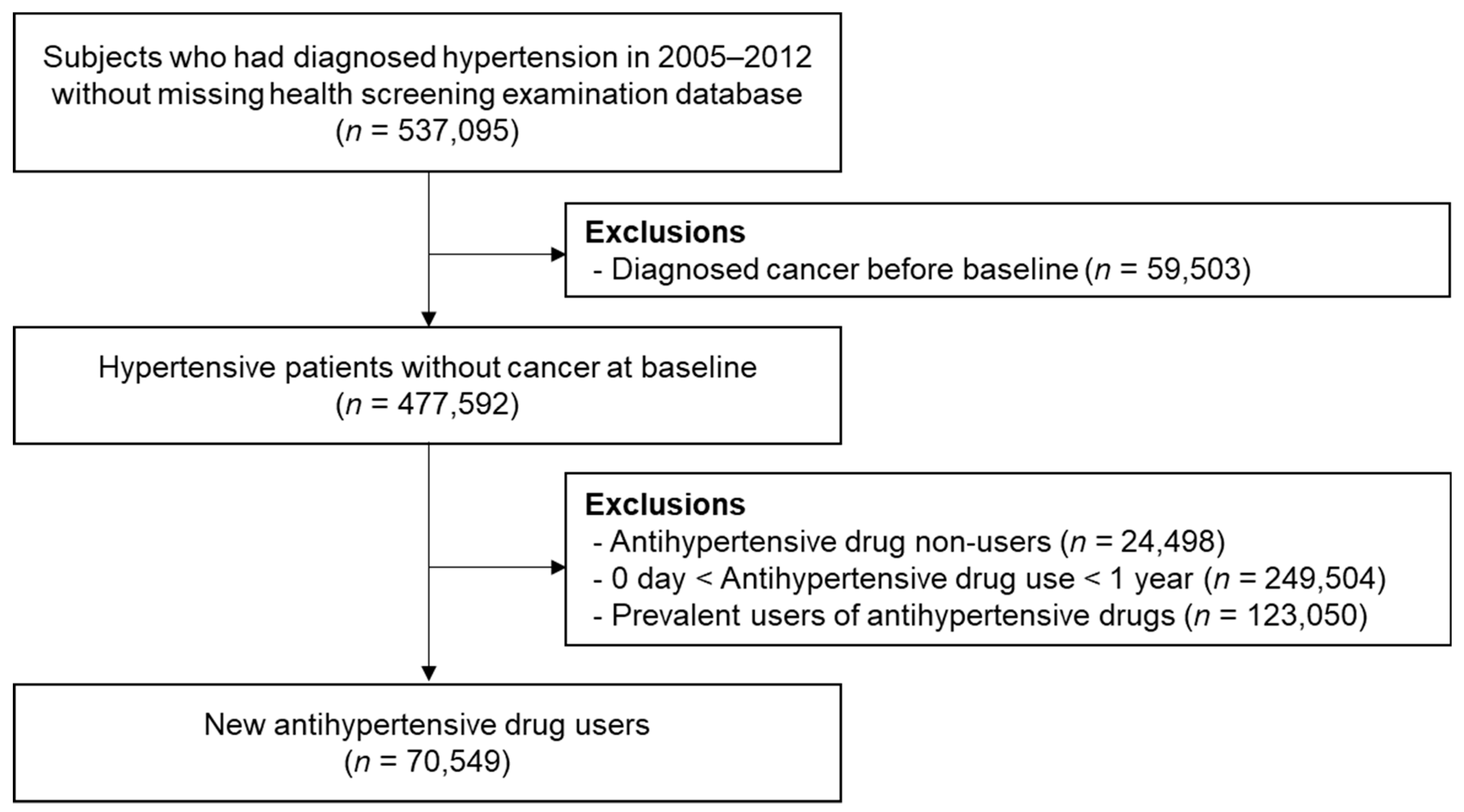
3.1. Study Population
3.2. Cancer Risk by Antihypertensive Medication
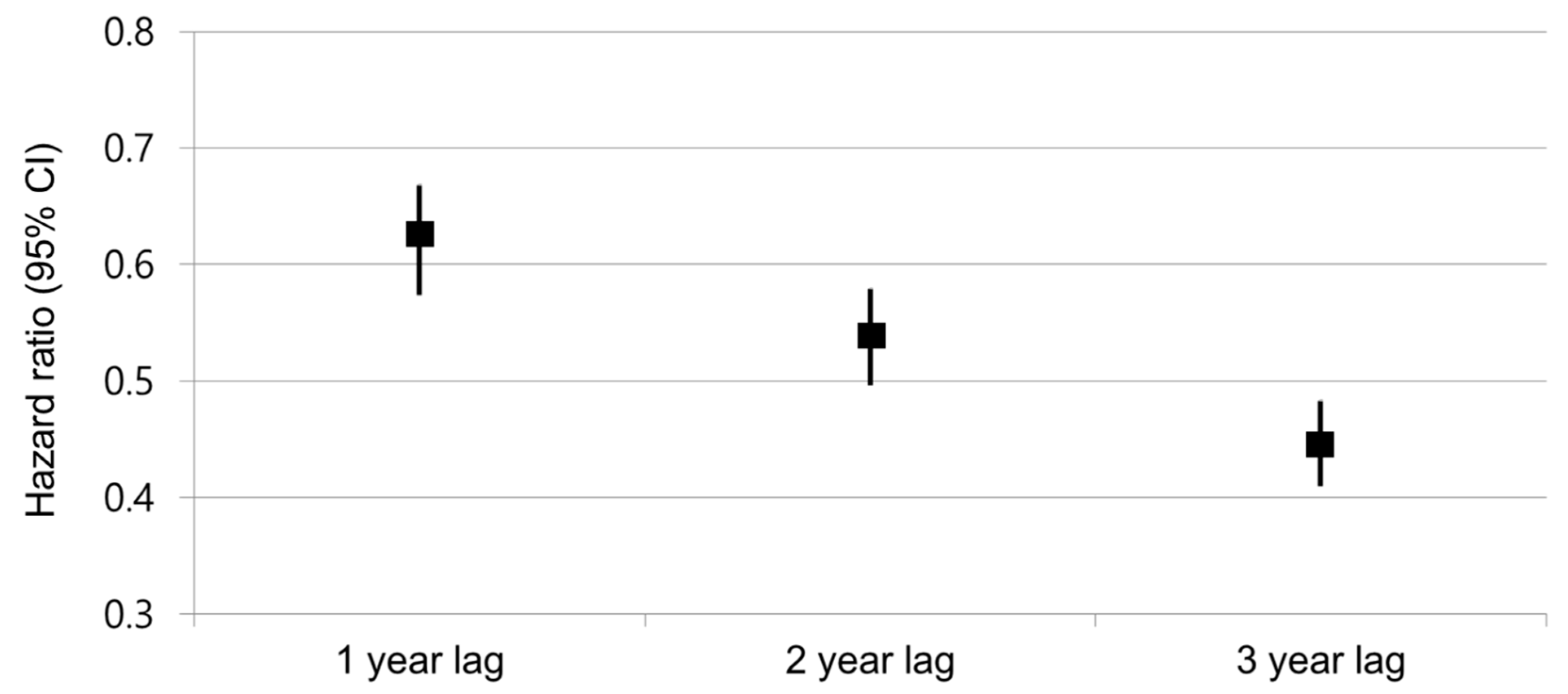
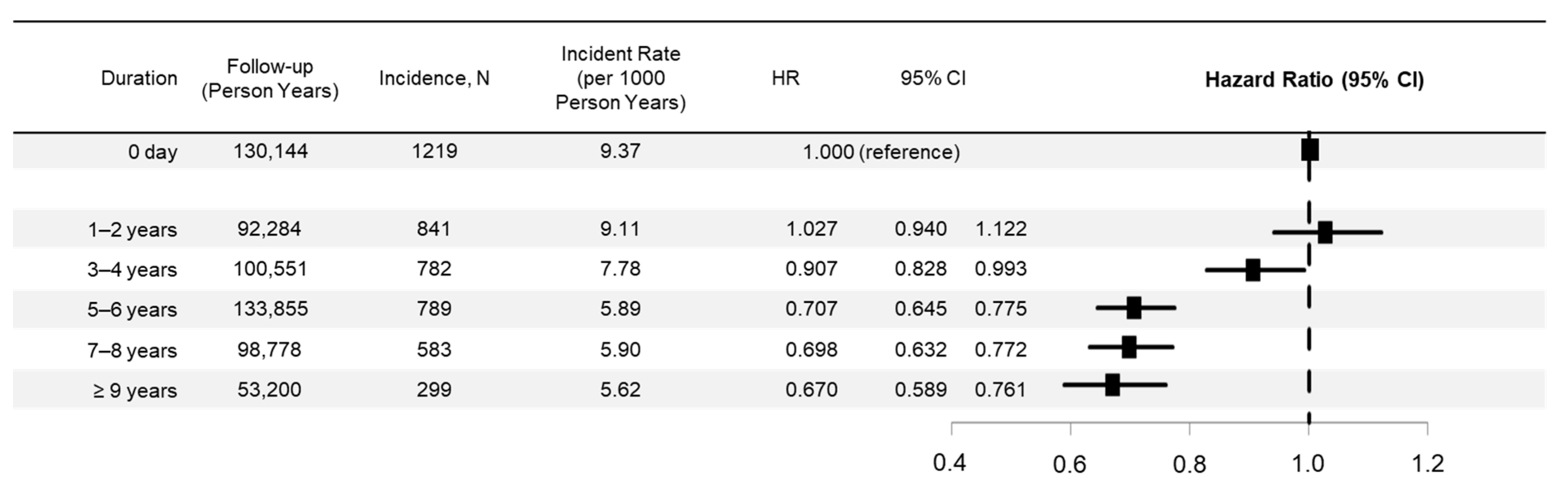
3.3. Risk Trends by Duration of ARB Use and Subgroups
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.F.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, N.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Grossman, E.; Messerli, F.H. Long-term safety of antihypertensive therapy. Prog. Cardiovasc. Dis. 2006, 49, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Kumar, S.E.; Kjeldsen, S.; Makani, H.; Grossman, E.; Wetterslev, J.; Gupta, A.K.; Sever, P.S.; Gluud, C.; Messerli, F.H. Antihypertensive drugs and risk of cancer: Network meta-analyses and trial sequential analyses of 324 168 participants from randomised trials. Lancet Oncol. 2011, 12, 65–82. [Google Scholar] [CrossRef]
- Grossman, E.; Messerli, F.; Goldbourt, U. Antihypertensive therapy and the risk of malignancies. Eur. Heart J. 2001, 22, 1343–1352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Felmeden, D.C.; Lip, G.Y. Antihypertensive therapy and cancer risk. Drug Saf. 2001, 24, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Assimes, T.L.; Elstein, E.; Langleben, A.; Suissa, S. Long-term use of antihypertensive drugs and risk of cancer. Pharmacoepidemiol. Drug Saf. 2008, 17, 1039–1049. [Google Scholar] [CrossRef]
- Rosenthal, T.; Gavras, I. Renin-Angiotensin Inhibition in Combating Malignancy: A Review. Anticancer Res. 2019, 39, 4597–4602. [Google Scholar] [CrossRef]
- Chow, W.-H.; Dong, L.M.; Devesa, S.S. Epidemiology and risk factors for kidney cancer. Nat. Rev. Urol. 2010, 7, 245–257. [Google Scholar] [CrossRef]
- Kim, C.S.; Han, K.-D.; Choi, H.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Association of Hypertension and Blood Pressure With Kidney Cancer Risk: A Nationwide Population-Based Cohort Study. Hypertension 2020, 75, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Guo, W.; Shi, W.; Yu, Y.; Zhang, Y.; Ye, X.; He, J. Hypertension and breast cancer risk: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 44877. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Chiodini, P.; Capuano, A.; Bellastella, G.; Maiorino, M.I.; Rafaniello, C.; Panagiotakos, D.B.; Giugliano, D. Colorectal cancer association with metabolic syndrome and its components: A systematic review with meta-analysis. Endocrine 2013, 44, 634–647. [Google Scholar] [CrossRef]
- Kirk, J.K. Angiotensin-II receptor antagonists: Their place in therapy. Am. Fam. Physician 1999, 59, 3140–3148. [Google Scholar] [PubMed]
- Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; McMurray, J.J.V.; Michelson, E.L.; Olofsson, B.; Östergren, J.; Yusuf, S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-Overall programme. Lancet 2003, 362, 759–766. [Google Scholar] [CrossRef]
- Sipahi, I.; Debanne, S.M.; Rowland, D.Y.; Simon, D.I.; Fang, J.C. Angiotensin-receptor blockade and risk of cancer: Meta-analysis of randomised controlled trials. Lancet Oncol. 2010, 11, 627–636. [Google Scholar] [CrossRef]
- ARB Trialists Collaboration. Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J. Hypertens. 2011, 29, 623–635. [Google Scholar] [CrossRef]
- Pasternak, B.; Svanström, H.; Callréus, T.; Melbye, M.; Hviid, A. Use of angiotensin receptor blockers and the risk of cancer. Circulation 2011, 123, 1729–1736. [Google Scholar] [CrossRef][Green Version]
- Bhaskaran, K.; Douglas, I.; Evans, S.; Van Staa, T.; Smeeth, L. Angiotensin receptor blockers and risk of cancer: Cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ 2012, 344, e2697. [Google Scholar] [CrossRef] [PubMed]
- Pickel, L.; Matsuzuka, T.; Doi, C.; Ayuzawa, R.; Maurya, D.K.; Xie, S.; Berkland, C.; Tamura, M. Over-expression of angiotensin II type 2 receptor gene induces cell death in lung adenocarcinoma cells. J. Cancer Biol. 2010, 9, 277–285. [Google Scholar] [CrossRef][Green Version]
- Benndorf, R.; Böger, R.H.; Ergün, S.; Steenpass, A.; Wieland, T. Angiotensin II type 2 receptor inhibits vascular endothelial growth factor–induced migration and in vitro tube formation of human endothelial cells. J. Circ. Res. 2003, 93, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Doi, C.; Egashira, N.; Kawabata, A.; Maurya, D.K.; Ohta, N.; Uppalapati, D.; Ayuzawa, R.; Pickel, L.; Isayama, Y.; Troyer, D.; et al. Angiotensin II type 2 receptor signaling significantly attenuates growth of murine pancreatic carcinoma grafts in syngeneic mice. BMC Cancer 2010, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.P.; Gray, R. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- (NHIS) NHIS. National Health Screening Statistical Yearbook; National Health Insurance Service: Seoul, Korea, 2010.
- Taylor, A.A.; Siragy, H.; Nesbitt, S. Angiotensin receptor blockers: Pharmacology, efficacy, and safety. J. Clin. Hypertens. 2011, 13, 677–686. [Google Scholar] [CrossRef]
- Makar, G.A.; Holmes, J.H.; Yang, Y.-X. Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Lin, J.-W.; Wu, L.-C.; Lai, M.S. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: A nationwide case-control study. J. Clin. Oncol. 2011, 29, 3001–3007. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chan, W.-L.; Chen, Y.-C.; Chen, T.-J.; Lin, S.-J.; Chen, J.-W.; Leu, H.-B. Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am. J. Cardiol. 2011, 107, 1028–1033. [Google Scholar] [CrossRef]
- George, A.J.; Thomas, W.G.; Hannan, R.D. The renin–angiotensin system and cancer: Old dog, new tricks. Nat. Rev. Cancer 2010, 10, 745. [Google Scholar] [CrossRef]
- Barone, M.; Viggiani, M.T.; Losurdo, G.; Principi, M.; Di Leo, A. Systematic review: Renin-angiotensin system inhibitors in chemoprevention of hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2524–2538. [Google Scholar] [CrossRef]
- Huang, W.; Wu, Y.-L.; Zhong, J.; Jiang, F.-X.; Tian, X.-L.; Yu, L.-F. Angiotensin II type 1 receptor antagonist suppress angiogenesis and growth of gastric cancer xenografts. Dig. Dis. Sci. 2008, 53, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.R.; Missailidis, S. Cancer, inflammation and the AT1 and AT2 receptors. J. Inflamm. 2004, 1, 1–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Variable | All Users | ACEI | ARB | BB | CCB | Diuretics |
|---|---|---|---|---|---|---|
| Number, N | 70,549 | 4210 | 55,645 | 13,158 | 51,036 | 32,990 |
| Number of overall cancers, N (%) | 4513 (6.4) | 362 (8.6) | 3294 (5.9) | 982 (7.5) | 3443 (6.8) | 2155 (6.5) |
| Follow-up, years | 8.6 (6.7–10.8) | 10.7 (8.4–12.0) | 8.5 (6.7–10.7) | 10.0 (7.7–11.8) | 8.8 (6.9–11.0) | 9.1 (7.2–11.3) |
| Drug exposure duration, years | - | 3.3 (1.8–5.6) | 5.5 (3.5–7.2) | 3.8 (2.1–6.4) | 5.3 (3.0–7.6) | 4.1 (2.2–6.2) |
| Deaths, N (%) | 3025 (4.3) | 353 (8.4) | 2050 (3.7) | 741 (5.6) | 2333 (4.6) | 1542 (4.7) |
| Age, years | 55.2 ± 9.2 | 57.0 ± 9.5 | 54.5 ± 9.0 | 55.4 ± 9.2 | 55.4 ± 9.2 | 55.4 ± 9.3 |
| Male sex, N (%) | 42,990 (60.9) | 3001 (71.3) | 35,113 (63.1) | 8270 (62.9) | 31,668 (62.1) | 19,747 (59.9) |
| BMI, kg/m2 | 24.9 ± 3.0 | 24.7 ± 3.0 | 25.1 ± 3.0 | 25.0 ± 3.1 | 25.0 ± 3.0 | 25.2 ± 3.1 |
| SBP, mmHg | 139.7 ± 18.3 | 138.2 ± 19.6 | 140.5 ± 18.4 | 141.5 ± 21.1 | 141.8 ± 18.5 | 142.3 ± 19.0 |
| Smoking, N (%) | ||||||
| Current | 16,214 (23.0) | 1053 (25.0) | 13,525 (24.3) | 3249 (24.7) | 12,277 (24.1) | 7882 (23.9) |
| Past smoker | 12,155 (17.2) | 712 (16.9) | 9872 (17.7) | 1979 (15.0) | 8509 (16.7) | 5050 (15.3) |
| Never-smoker | 42,180 (59.8) | 2445 (58.1) | 32,248 (58.0) | 7930 (60.3) | 30,250 (59.3) | 20,058 (60.8) |
| Alcohol, N (%) | ||||||
| None | 35,575 (50.4) | 2181 (51.8) | 26,871 (48.3) | 6759 (51.4) | 24,965 (48.9) | 16,387 (49.7) |
| 1–2/week | 22,265 (31.6) | 1323 (31.4) | 18,146 (32.6) | 4109 (31.2) | 16,322 (32.0) | 10,370 (31.4) |
| 3–4/week | 8302 (11.8) | 431 (10.2) | 6969 (12.5) | 1484 (11.3) | 6308 (12.4) | 3978 (12.1) |
| ≥5/week | 4407 (6.3) | 275 (6.5) | 3659 (6.6) | 806 (6.1) | 3441 (6.7) | 2255 (6.8) |
| Income, N (%) | ||||||
| Low (1st-3rd deciles) | 17,221 (24.4) | 1000 (23.8) | 13,456 (24.2) | 3374 (25.6) | 12,635 (24.8) | 8444 (25.6) |
| Middle (4th-7th deciles) | 23,982 (34.0) | 1458 (34.6) | 19,006 (34.2) | 4596 (34.9) | 17,676 (34.6) | 11,720 (35.5) |
| High (8th-10th deciles) | 29,346 (41.6) | 1752 (41.6) | 23,183 (41.7) | 5188 (39.4) | 20,725 (40.6) | 12,826 (38.9) |
| Comorbidities | ||||||
| Diabetes, N (%) | 13,382 (19.0) | 1156 (27.5) | 10,854 (19.5) | 1996 (15.2) | 8172 (16.0) | 5573 (16.9) |
| Heart failure, N (%) | 216 (0.3) | 29 (0.7) | 154 (0.3) | 57 (0.4) | 113 (0.2) | 86 (0.3) |
| COPD, N (%) | 1704 (2.4) | 102 (2.4) | 1227 (2.2) | 270 (2.1) | 1127 (2.2) | 752 (2.3) |
| Variable | Follow-Up (Person Years) | Cancer Incidence, N | Incident Rate (per 1000 Person Years) | Unadjusted | Age- and Sex-Adjusted | Fully Adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| ACEI | No | 566,817 | 4151 | 7.32 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 41,995 | 362 | 8.62 | 1.148 | 1.031–1.277 | 1.022 | 0.918–1.138 | 1.016 | 0.912–1.132 | |
| ARB | No | 130,144 | 1219 | 9.37 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 478,668 | 3294 | 6.88 | 0.744 | 0.696–0.794 | 0.822 | 0.769–0.878 | 0.833 | 0.775–0.896 | |
| BB | No | 483,462 | 3531 | 7.30 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 125,350 | 982 | 7.83 | 1.055 | 0.983–1.132 | 1.033 | 0.962–1.109 | 1.03 | 0.958–1.107 | |
| CCB | No | 158,876 | 1070 | 6.73 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 449,937 | 3443 | 7.65 | 1.124 | 1.049–1.204 | 1.081 | 1.009–1.158 | 1.053 | 0.981–1.13 | |
| Diuretics | No | 309,741 | 2358 | 7.61 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 299,071 | 2155 | 7.21 | 0.936 | 0.883–0.992 | 0.92 | 0.868–0.976 | 0.957 | 0.898–1.019 | |
| Variable | Follow-Up (Person Years) | Cancer Incidence, N | Incident Rate (per 1000 Person Years) | Unadjusted | Age- and Sex-Adjusted | Fully Adjusted | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Lung cancer | ||||||||||
| ACEI | No | 581,613 | 509 | 0.88 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,359 | 54 | 1.25 | 1.335 | 1.009–1.768 | 1.019 | 0.768–1.351 | 0.998 | 0.752–1.326 | |
| ARB | No | 134,482 | 159 | 1.18 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 490,490 | 404 | 0.82 | 0.716 | 0.595–0.861 | 0.843 | 0.699–1.015 | 0.86 | 0.705–1.049 | |
| BB | No | 495,930 | 433 | 0.87 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 129,042 | 130 | 1.01 | 1.1 | 0.904–1.339 | 1.046 | 0.859–1.274 | 1.053 | 0.861–1.288 | |
| CCB | No | 162,677 | 117 | 0.72 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 462,295 | 446 | 0.96 | 1.298 | 1.06–1.59 | 1.186 | 0.968–1.453 | 1.186 | 0.965–1.458 | |
| Diuretics | No | 318,319 | 283 | 0.89 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 306,652 | 280 | 0.91 | 0.993 | 0.843–1.171 | 0.964 | 0.817–1.137 | 1.019 | 0.853–1.216 | |
| Colorectal cancer | ||||||||||
| ACEI | No | 580,493 | 589 | 1.01 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,172 | 61 | 1.41 | 1.332 | 1.021–1.737 | 1.105 | 0.847–1.442 | 1.118 | 0.858–1.456 | |
| ARB | No | 134,149 | 176 | 1.31 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 489,516 | 474 | 0.97 | 0.755 | 0.635–0.898 | 0.868 | 0.728–1.036 | 0.857 | 0.711–1.031 | |
| BB | No | 494,933 | 502 | 1.01 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 128,732 | 148 | 1.15 | 1.098 | 0.914–1.2318 | 1.062 | 0.884–1.276 | 1.029 | 0.856–1.236 | |
| CCB | No | 162,441 | 133 | 0.82 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 461,224 | 517 | 1.12 | 1.342 | 1.108–1.624 | 1.258 | 1.039–1.524 | 1.178 | 0.968–1.432 | |
| Diuretics | No | 317,700 | 319 | 1.00 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 305,965 | 331 | 1.08 | 1.056 | 0.906–1.23 | 1.033 | 0.886–1.204 | 1.031 | 0.877–1.213 | |
| Hepatic cancer | ||||||||||
| ACEI | No | 581,886 | 346 | 0.59 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,403 | 28 | 0.65 | 1.029 | 0.704–1.504 | 0.833 | 0.568–1.223 | 0.795 | 0.542–1.605 | |
| ARB | No | 134,553 | 108 | 0.80 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 490,736 | 266 | 0.54 | 0.69 | 0.551–0.864 | 0.692 | 0.552–0.868 | 0.665 | 0.519–0.851 | |
| BB | No | 496,177 | 292 | 0.59 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 129,111 | 82 | 0.64 | 1.042 | 0.817–1.329 | 0.995 | 0.78–1.269 | 0.992 | 0.776–1.269 | |
| CCB | No | 162,754 | 79 | 0.49 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 462,535 | 295 | 0.64 | 1.286 | 1.002–1.65 | 1.205 | 0.939–1.547 | 1.184 | 0.918–1.528 | |
| Diuretics | No | 318,441 | 197 | 0.62 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 306,848 | 177 | 0.58 | 0.911 | 0.745–1.114 | 0.916 | 0.748–1.121 | 1.02 | 0.818–1.271 | |
| Gastric cancer | ||||||||||
| ACEI | No | 580,103 | 681 | 1.17 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,157 | 74 | 1.71 | 1.427 | 1.123–1.812 | 1.151 | 0.904–1.466 | 1.16 | 0.91–1.479 | |
| ARB | No | 133,969 | 217 | 1.62 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 489,291 | 538 | 1.10 | 0.691 | 0.59–0.81 | 0.763 | 0.65–1.895 | 0.759 | 0.636–0.906 | |
| BB | No | 494,612 | 597 | 1.21 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 128,648 | 158 | 1.23 | 1 | 0.839–1.192 | 0.96 | 0.805–1.143 | 0.937 | 0.784–1.119 | |
| CCB | No | 162,142 | 200 | 1.23 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 461,118 | 555 | 1.20 | 0.963 | 0.819–1.132 | 0.897 | 0.763–1.055 | 0.823 | 0.694–0.976 | |
| Diuretics | No | 317,323 | 407 | 1.28 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 305,937 | 348 | 1.14 | 0.877 | 0.76–1.011 | 0.865 | 0.75–0.998 | 0.904 | 0.772–1.059 | |
| Bladder cancer | ||||||||||
| ACEI | No | 582,117 | 155 | 0.27 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,386 | 18 | 0.41 | 1.49 | 0.914–2.429 | 1.096 | 0.671–1.79 | 1.078 | 0.65–1.788 | |
| ARB | No | 134,664 | 46 | 0.34 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 490,839 | 127 | 0.26 | 0.774 | 0.553–1.084 | 0.884 | 0.629–1.244 | 0.996 | 0.693–1.433 | |
| BB | No | 496,417 | 122 | 0.25 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 129,087 | 51 | 0.40 | 1.564 | 1.123–2.179 | 1.474 | 1.057–2.054 | 1.505 | 1.074–2.108 | |
| CCB | No | 162,790 | 37 | 0.23 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 462,714 | 136 | 0.29 | 1.269 | 0.882–1.826 | 1.151 | 0.8–1.657 | 1.135 | 0.78–1.651 | |
| Diuretics | No | 318,566 | 95 | 0.30 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 306,937 | 78 | 0.25 | 0.836 | 0.618–1.13 | 0.819 | 0.606–1.109 | 0.781 | 0.562–1.085 | |
| Breast cancer | ||||||||||
| ACEI | No | 581,459 | 267 | 0.46 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,407 | 8 | 0.18 | 0.413 | 0.204–0.836 | 0.599 | 0.296–1.212 | 0.609 | 0.299–1.24 | |
| ARB | No | 134,451 | 71 | 0.53 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 490,416 | 204 | 0.42 | 0.792 | 0.604–1.038 | 0.936 | 0.713–1.229 | 1.05 | 0.777–1.418 | |
| BB | No | 495,860 | 216 | 0.44 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 129,007 | 59 | 0.46 | 1.08 | 0.809–1.442 | 1.154 | 0.865–1.541 | 1.203 | 0.895–1.617 | |
| CCB | No | 162,611 | 67 | 0.41 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 462,256 | 208 | 0.45 | 1.114 | 0.884–1.468 | 1.263 | 0.957–1.667 | 1.194 | 0.896–1.593 | |
| Diuretics | No | 318,193 | 154 | 0.48 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 306,674 | 121 | 0.39 | 0.834 | 0.657–1.058 | 0.799 | 0.63–1.014 | 0.747 | 0.575–0.972 | |
| Prostate cancer | ||||||||||
| ACEI | No | 581,622 | 298 | 0.51 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,328 | 28 | 0.65 | 1.165 | 0.789–1.719 | 0.791 | 0.534–1.71 | 0.764 | 0.513–1.137 | |
| ARB | No | 134,514 | 82 | 0.61 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 490,436 | 244 | 0.50 | 0.84 | 0.654–1.08 | 0.954 | 0.739–1.232 | 0.974 | 0.741–1.279 | |
| BB | No | 495,889 | 248 | 0.50 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 129,060 | 78 | 0.60 | 1.144 | 0.888–1.474 | 1.057 | 0.82–1.362 | 1.099 | 0.848–1.425 | |
| CCB | No | 162,608 | 88 | 0.54 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 462,342 | 238 | 0.51 | 0.917 | 0.717–1.172 | 0.813 | 0.635–1.041 | 0.85 | 0.657–1.1 | |
| Diuretics | No | 318,213 | 173 | 0.54 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 306,737 | 153 | 0.50 | 0.883 | 0.712–1.096 | 0.865 | 0.697–1.074 | 0.892 | 0.707–1.125 | |
| Pancreatic cancer | ||||||||||
| ACEI | No | 582,672 | 90 | 0.15 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,445 | 7 | 0.16 | 0.959 | 0.446–2.064 | 0.827 | 0.383–1.787 | 0.893 | 0.421–1.897 | |
| ARB | No | 134,810 | 26 | 0.19 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 491,306 | 71 | 0.14 | 0.774 | 0.493–1.226 | 0.865 | 0.55–1.361 | 0.771 | 0.474–1.254 | |
| BB | No | 496,794 | 86 | 0.17 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 129,323 | 11 | 0.09 | 0.459 | 0.246–0.857 | 0.448 | 0.24–0.837 | 0.447 | 0.243–0.822 | |
| CCB | No | 162,924 | 19 | 0.12 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 463,193 | 78 | 0.17 | 1.383 | 0.84–2.277 | 1.316 | 0.799–2.167 | 1.326 | 0.797–2.206 | |
| Diuretics | No | 318,887 | 47 | 0.15 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 307,230 | 50 | 0.16 | 1.057 | 0.712–1.571 | 1.039 | 0.699–1.545 | 1.189 | 0.774–1.827 | |
| Kidney cancer | ||||||||||
| ACEI | No | 582,302 | 110 | 0.19 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 43,414 | 10 | 0.23 | 1.241 | 0.656–2.347 | 1.123 | 0.594–2.124 | 1.102 | 0.577–2.105 | |
| ARB | No | 134,721 | 28 | 0.21 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 490,995 | 92 | 0.19 | 0.909 | 0.595–1.389 | 0.881 | 0.576–1.347 | 0.846 | 0.534–1.339 | |
| BB | No | 496,511 | 93 | 0.19 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 129,205 | 27 | 0.21 | 1.135 | 0.737–1.749 | 1.111 | 0.721–1.712 | 1.084 | 0.692–1.698 | |
| CCB | No | 162,840 | 25 | 0.15 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 462,876 | 95 | 0.21 | 1.353 | 0.867–2.11 | 1.309 | 0.838–2.045 | 1.328 | 0.845–2.088 | |
| Diuretics | No | 318,716 | 59 | 0.19 | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) | |||
| Yes | 307,000 | 61 | 0.20 | 1.089 | 0.762–1.556 | 1.099 | 0.769–1.57 | 1.127 | 0.766–1.658 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, I.-J.; Shin, J.-H.; Jung, M.-H.; Kang, C.Y.; Hwang, J.; Kwon, C.H.; Kim, W.; Kim, D.-H.; Lee, C.J.; Kang, S.-H.; et al. Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort Study. J. Clin. Med. 2021, 10, 771. https://doi.org/10.3390/jcm10040771
Cho I-J, Shin J-H, Jung M-H, Kang CY, Hwang J, Kwon CH, Kim W, Kim D-H, Lee CJ, Kang S-H, et al. Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort Study. Journal of Clinical Medicine. 2021; 10(4):771. https://doi.org/10.3390/jcm10040771
Chicago/Turabian StyleCho, In-Jeong, Jeong-Hun Shin, Mi-Hyang Jung, Chae Young Kang, Jinseub Hwang, Chang Hee Kwon, Woohyeun Kim, Dae-Hee Kim, Chan Joo Lee, Si-Hyuck Kang, and et al. 2021. "Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort Study" Journal of Clinical Medicine 10, no. 4: 771. https://doi.org/10.3390/jcm10040771
APA StyleCho, I.-J., Shin, J.-H., Jung, M.-H., Kang, C. Y., Hwang, J., Kwon, C. H., Kim, W., Kim, D.-H., Lee, C. J., Kang, S.-H., Lee, J.-H., Kim, H.-L., Kim, H. M., Cho, I., Lee, H.-Y., Chung, W.-J., Ihm, S.-H., Kim, K. I., Cho, E. J., ... Sung, K.-C. (2021). Antihypertensive Drugs and the Risk of Cancer: A Nationwide Cohort Study. Journal of Clinical Medicine, 10(4), 771. https://doi.org/10.3390/jcm10040771






