Skeletal Changes in Growing Cleft Patients with Class III Malocclusion Treated with Bone Anchored Maxillary Protraction—A 3.5-Year Follow-Up
Abstract
1. Introduction
2. Subjects and Methods
2.1. Trial Registration and Ethical Approval
2.2. Treatment and Control Subjects
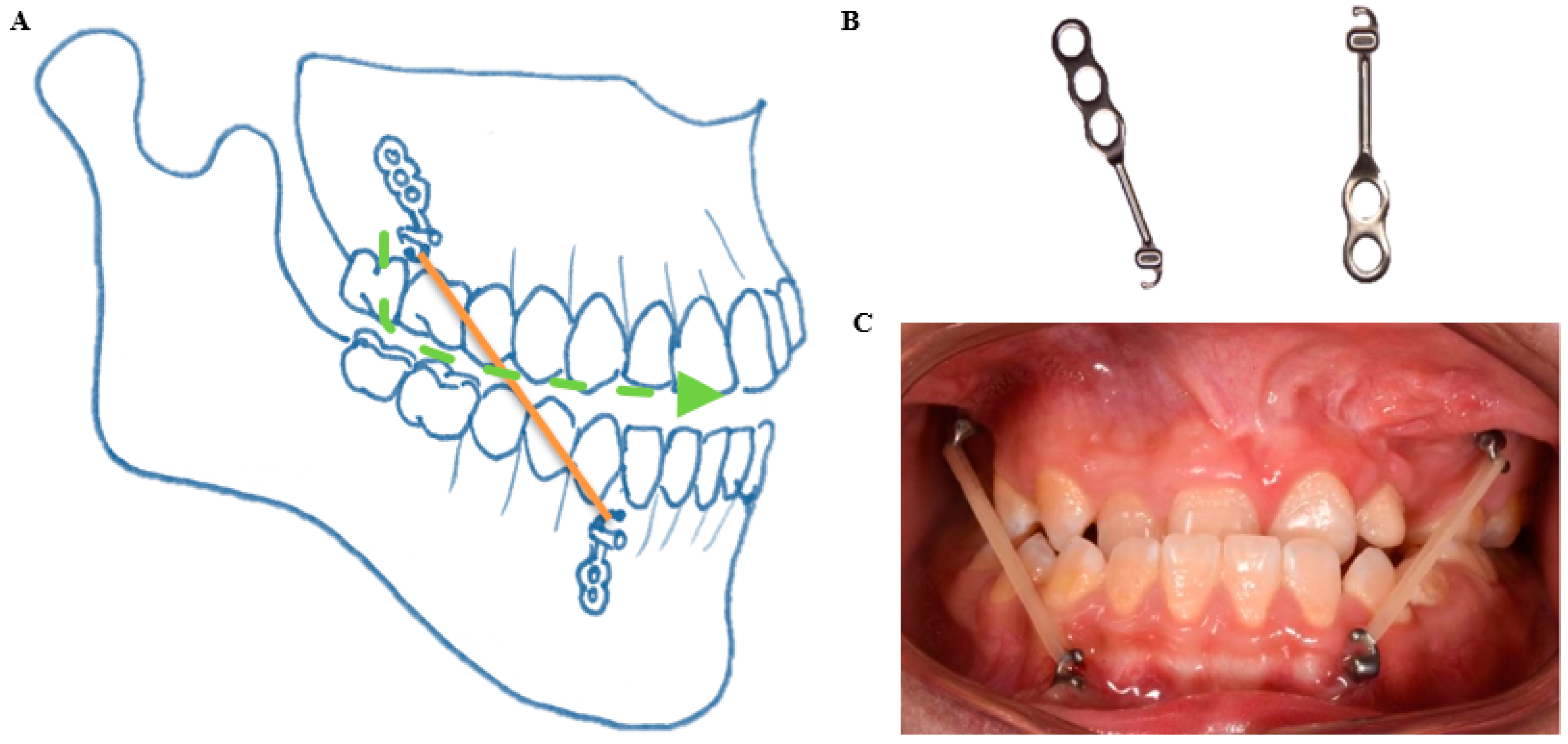
2.3. Bone Anchored Maxillary Protraction
2.4. CBCT Scans
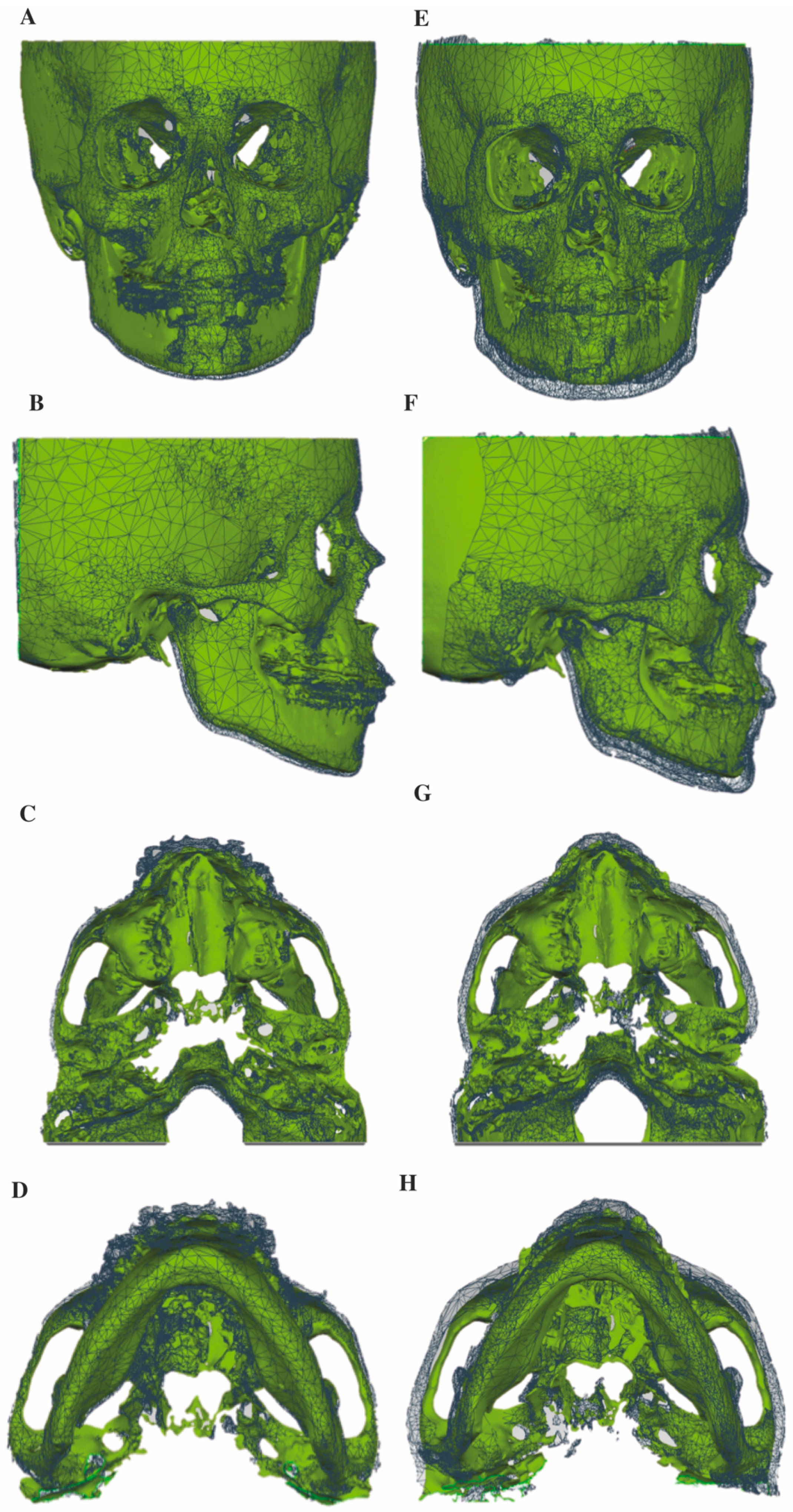
2.5. Superimposition of the CBCT 3D Surface Models
2.6. Tracing on Lateral Cephalograms
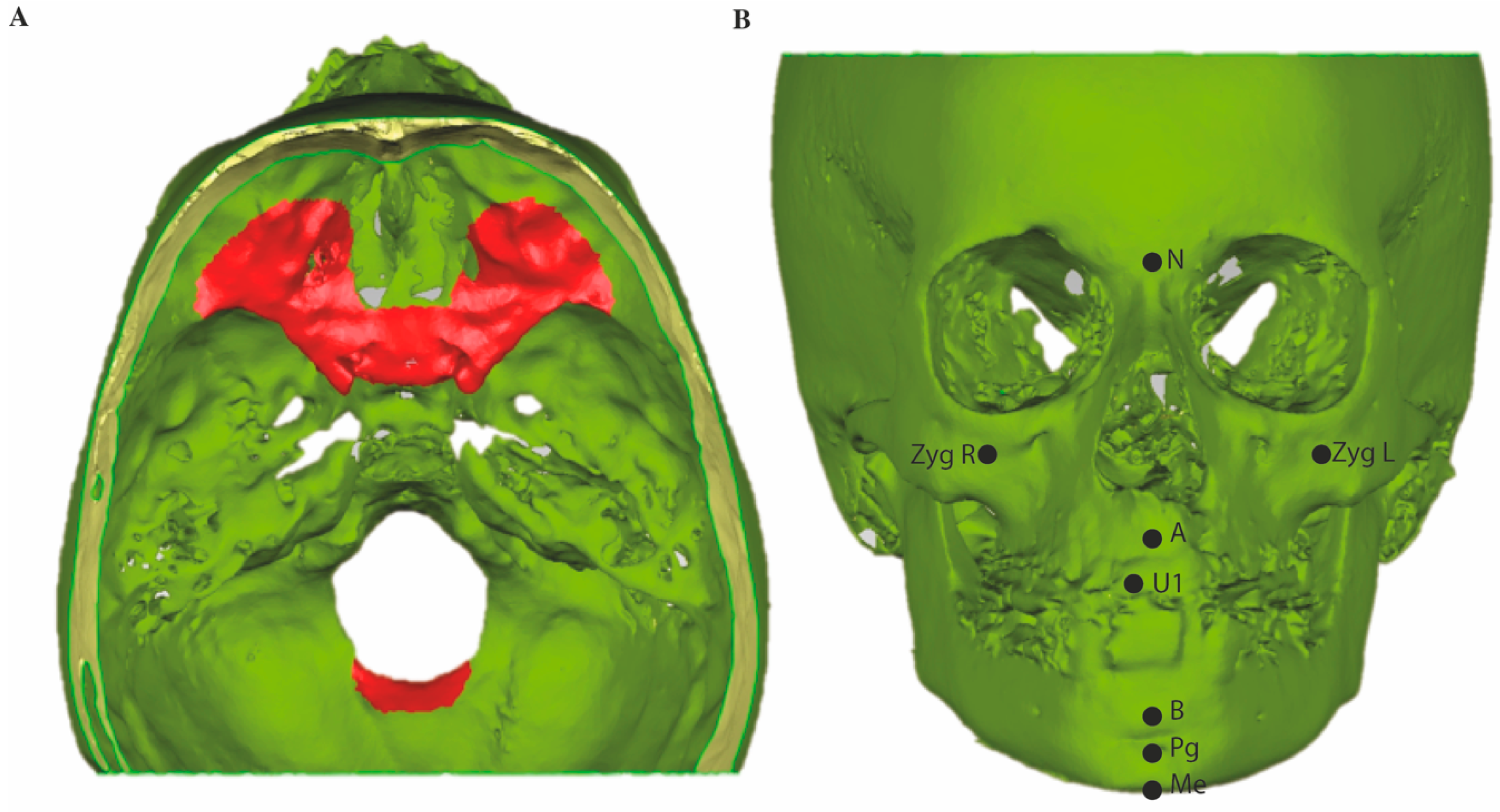
2.7. Quantitative Measurements
2.8. Statistics
3. Results
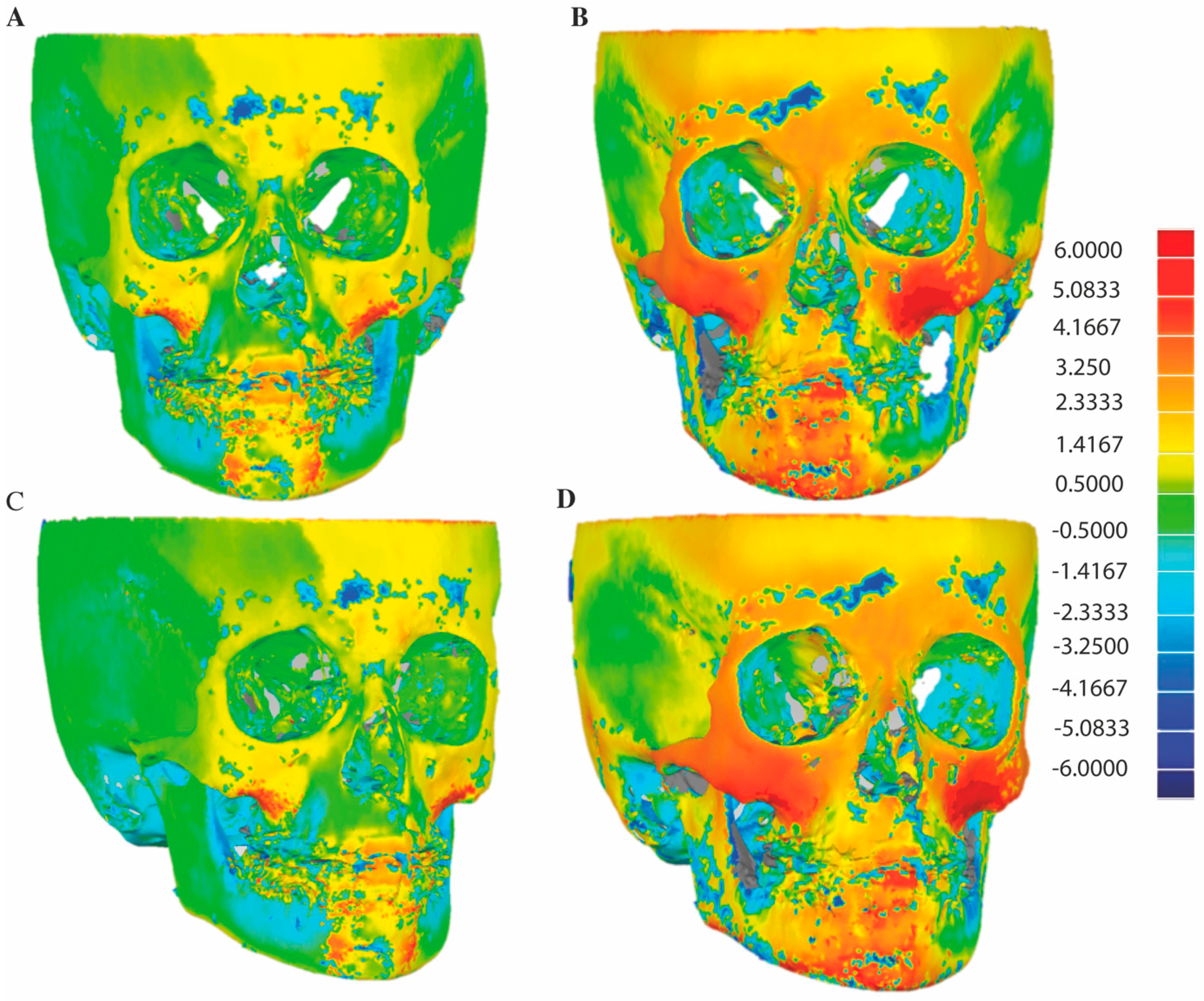
3.1. D Skeletal Changes
3.2. 2D Cephalometric Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ertaş, Ü.; Ataol, M. Evaluation of Nasal Airway Volume of Operated Unilateral Cleft Lip and Palate Patients Compared with Skeletal Class III Individuals. Cleft Palate-Craniofacial J. 2019, 56, 15–20. [Google Scholar] [CrossRef]
- Hunt, O.; Burden, D.; Hepper, P.; Stevenson, M.; Johnston, C. Self-Reports of Psychosocial Functioning among Children and Young Adults with Cleft Lip and Palate. Cleft Palate-Craniofacial J. 2006, 43, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Muntz, H.; Wilson, M.; Park, A.; Smith, M.; Grimmer, J.F. Sleep Disordered Breathing and Obstructive Sleep Apnea in the Cleft Population. Laryngoscope 2008, 118, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, J.; Tahiri, Y.; Paliga, J.T.; Taylor, J.A. Screening for obstructive sleep apnea in children with syndromic cleft lip and/or palate. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, R.T.; Fernbach, S.K.; Hunt, C.E. Obstructive sleep apnea in infants and children. J. Pediatr. 1982, 100, 31–40. [Google Scholar] [CrossRef]
- Gislason, T.; Benediktsdóttir, B. Snoring, Apneic Episodes, and Nocturnal Hypoxemia Among Children 6 Months to 6 Years Old. Chest 1995, 107, 963–966. [Google Scholar] [CrossRef]
- Havakeshian, G.; Koretsi, V.; Eliades, T.; Papageorgiou, S.N. Effect of Orthopedic Treatment for Class III Malocclusion on Upper Airways: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 3015. [Google Scholar] [CrossRef]
- Alrejaye, N.; Gao, J.; Hatcher, D.; Oberoi, S. Effect of maxillary expansion and protraction on the oropharyngeal airway in individuals with non-syndromic cleft palate with or without cleft lip. PLoS ONE 2019, 14, e0213328. [Google Scholar] [CrossRef]
- Jesani, A.; DiBiase, A.T.; Cobourne, M.T.; Newton, T. Perceived Changes by Peer Group of Social Impact Associated with Combined Orthodontic-Surgical Correction of Class III Malocclusion. J. Dent. 2014, 42, 1135–1142. [Google Scholar] [CrossRef]
- Javed, O.; Bernabe, E. Oral Impacts on Quality of Life in Adult Patients with Class I, II and III Malocclusion. Oral Health Prev. Dent. 2016, 14, 27–32. [Google Scholar] [CrossRef]
- Watkinson, S.; Harrison, J.E.; Furness, S.; Worthington, H.V. Orthodontic Treatment for Prominent Lower Front Teeth (Class III Malocclusion) in Children. Cochrane Database Syst. Rev. 2013, CD003451. [Google Scholar] [CrossRef] [PubMed]
- Buschang, P.H.; Porter, C.; Genecov, E.; Genecov, D.; Sayler, K.E. Face Mask Therapy of Preadolescents with Unilateral Cleft Lip and Palate. Angle Orthod. 1994, 64, 145–150. [Google Scholar] [CrossRef]
- Van Hevele, J.; Nout, E.; Claeys, T.; Meyns, J.; Scheerlinck, J.; Politis, C. Bone-Anchored Maxillary Protraction to Correct a Class III Skeletal Relationship: A Multicenter Retrospective Analysis of 218 Patients. J. Craniomaxillofac. Surg. 2018, 46, 1800–1806. [Google Scholar] [CrossRef]
- Hino, C.T.; Cevidanes, L.H.; Nguyen, T.T.; De Clerck, H.J.; Franchi, L.; McNamara, J.A. Three-Dimensional Analysis of Maxillary Changes Associated with Facemask and Rapid Maxillary Expansion Compared with Bone Anchored Maxillary Protraction. Am. J. Orthod. Dentofacial Orthop. 2013, 144, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Angelieri, F.; Franchi, L.; Cevidanes, L.H.S.; Hino, C.T.; Nguyen, T.; McNamara, J.A. Zygomaticomaxillary Suture Maturation: A Predictor of Maxillary Protraction? Part I—A Classification Method. Orthod. Craniofac Res. 2017, 20, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Steegman, R.; Dieters, A.; Jansma, J.; Stamatakis, H. Bone-Anchored Maxillary Protraction in Patients with Unilateral Complete Cleft Lip and Palate and Class III Malocclusion. Clin. Oral. Investig. 2019, 23, 2429–2441. [Google Scholar] [CrossRef]
- Yatabe, M.; Garib, D.G.; Faco, R.A.S.; de Clerck, H.; Janson, G.; Nguyen, T.; Cevidanes, L.H.S.; Ruellas, A.C. Bone-Anchored Maxillary Protraction Therapy in Patients with Unilateral Complete Cleft Lip and Palate: 3-Dimensional Assessment of Maxillary Effects. Am. J. Orthod. Dentofacial Orthop. 2017, 152, 327–335. [Google Scholar] [CrossRef]
- Nguyen, T.; Cevidanes, L.; Cornelis, M.A.; Heymann, G.; de Paula, L.K.; De Clerck, H. Three-Dimensional Assessment of Maxillary Changes Associated with Bone Anchored Maxillary Protraction. Am. J. Orthod. Dentofacial Orthop. 2011, 140, 790–798. [Google Scholar] [CrossRef]
- Gkantidis, N.; Schauseil, M.; Pazera, P.; Zorkun, B.; Katsaros, C.; Ludwig, B. Evaluation of 3-Dimensional Superimposition Techniques on Various Skeletal Structures of the Head Using Surface Models. PLoS ONE 2015, 10, e0118810. [Google Scholar] [CrossRef]
- Navarro Rde, L.; Oltramari-Navarro, P.V.; Fernandes, T.M.; Oliveira, G.F.; Conti, A.C.; Almeida, M.R.; Almeida, R.R. Comparison of Manual, Digital and Lateral CBCT Cephalometric Analyses. J. Appl. Oral. Sci. 2013, 21, 167–176. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, S.; Baek, S. Miniplate-anchored Maxillary Protraction in Adolescent Patients with Cleft Lip and Palate: A Literature Review of Study Design, Type and Protocol, and Treatment Outcomes. Orthod. Craniofac Res. 2020, ocr.12446. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, H.; Cevidanes, L.; Baccetti, T. Dentofacial Effects of Bone-Anchored Maxillary Protraction: A Controlled Study of Consecutively Treated Class III Patients. Am. J. Orthod. Dentofacial Orthop. 2010, 138, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Cordasco, G.; Matarese, G.; Rustico, L.; Fastuca, S.; Caprioglio, A.; Lindauer, S.J.; Nucera, R. Efficacy of Orthopedic Treatment with Protraction Facemask on Skeletal Class III Malocclusion: A Systematic Review and Meta-Analysis. Orthod. Craniofac Res. 2014, 17, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J. Mechanobiology of Craniofacial Sutures. J. Dent. Res. 2002, 81, 810–816. [Google Scholar] [CrossRef]
- Angelieri, F.; Ruellas, A.C.; Yatabe, M.S.; Cevidanes, L.H.S.; Franchi, L.; Toyama-Hino, C.; De Clerck, H.J.; Nguyen, T.; McNamara, J.A. Zygomaticomaxillary Suture Maturation: Part II-The Influence of Sutural Maturation on the Response to Maxillary Protraction. Orthod. Craniofac Res. 2017, 20, 152–163. [Google Scholar] [CrossRef] [PubMed]
| N = 19 | T1-T0 | T2-T1 | T2-T0 | |
|---|---|---|---|---|
| 1.5-years | 2-years | 3.5-years | ||
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| A | overall | 1.5 ± 1.5 | 0.9 ± 1.7 | 2.7 ± 0.9 * |
| horizontal | 1.5 ± 1.4 | 0.8 ± 1.5 | 2.6 ± 0.8 * | |
| vertical | −0.2 ± 0.5 | 0.1 ± 0.4 | −0.1 ± 0.4 | |
| B | overall | 1.2 ± 1.9 | 1.1 ± 1.9 | 2.4 ± 1.2 |
| horizontal | 1.2 ± 1.9 | 1.0 ± 1.9 | 2.3 ± 1.2 | |
| vertical | 0.1 ± 0.4 | 0.0 ± 0.4 | 0.1 ± 0.4 | |
| Zygoma left | overall | 1.7 ± 0.9 | 2.2 ± 1.3 | 3.9 ± 1.3 * |
| horizontal | 1.3 ± 0.7 | 1.7 ± 1.0 | 3.1 ± 1.0 * | |
| vertical | 0.4 ± 0.4 | 1.0 ± 0.7 # | 1.4 ± 0.7 * | |
| transversal | 1.0 ± 0.7 | 0.8 ± 0.8 | 1.6 ± 0.9 * | |
| Zygoma right | overall | 1.6 ± 1.2 | 2.0 ± 1.4 | 3.7 ± 1.1 * |
| horizontal | 1.2 ± 1.1 | 1.6 ± 1.3 | 3.0 ± 1.2 * | |
| vertical | 0.4 ± 0.5 | 1.1 ± 0.7 # | 1.5 ± 0.6 * | |
| transversal | 0.6 ± 0.6 | 0.6 ± 0.5 | 1.2 ± 0.4 * | |
| Zygoma overall | overall | 1.7 ± 1.0 | 2.1 ± 1.2 | 3.8 ± 1.2 * |
| horizontal | 1.3 ± 0.9 | 1.6 ± 1.1 | 3.0 ± 0.9 * | |
| vertical | 0.4 ± 0.4 | 1.0 ± 0.6 # | 1.4 ± 0.7 * | |
| transversal | 0.8 ± 0.6 | 0.7 ± 0.5 | 1.4 ± 0.7 * | |
| Nasion | overall | 0.7 ± 1.2 | 1.0 ± 1.1 | 1.7 ± 1.1 * |
| horizontal | 0.6 ± 1.1 | 1.0 ± 1.1 | 1.6 ± 1.1 * | |
| vertical | 0.0 ± 0.3 | 0.1 ± 0.5 | 0.1 ± 0.3 | |
| Pogonion | overall | 1.0 ± 2.0 | 1.3 ± 1.5 | 2.3 ± 1.5 * |
| horizontal | 1.0 ± 1.9 | 1.1 ± 1.4 | 2.0 ± 1.3 | |
| vertical | 0.2 ± 0.3 | 0.5 ± 0.7 | 0.7 ± 0.6 * | |
| Menton | overall | 1.6 ± 1.7 | 1.4 ± 2.0 | 3.1 ± 1.5 * |
| horizontal | 1.0 ± 1.1 | 0.6 ± 1.2 | 1.5 ± 0.8 | |
| vertical | 1.2 ± 1.4 | 1.3 ± 1.5 | 2.5 ± 1.3 * | |
| U1 | overall | 2.3 ± 1.4 | 0.5 ± 1.6 # | 2.8 ± 1.2 |
| horizontal | 2.1 ± 1.4 | 0.4 ± 1.5 # | 2.5 ± 1.1 | |
| vertical | 0.1 ± 0.6 | −0.3 ± 0.7 | −0.3 ± 1.0 | |
| Control Group (N = 17) | Bone Anchored Maxillary Protraction Group (N = 19) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T2 | ΔT2-T0 | T0 | T1 | T2 | ΔT1-T0 | ΔT2-T1 | ΔT2-T0 | |
| Age | 10.7 ± 1.2 | 14.3 ± 1.4 | 3.7 ± 0.7 | 11.4 ± 0.7 | 12.9 ± 0.8 | 15.0 ± 0.7 | 1.5 ± 0.4 | 2.1 ± 0.7 | 3.6 ± 0.7 |
| SN-FH (°) | 10.5 ± 4.0 | 8.5 ± 5.8 | −2.0 ± 5.0 | 10.4 ± 4.2 | 10.4 ± 4.2 | 10.6 ± 3.8 | 0.0 ± 1.8 | 0.2 ± 2.7 | 0.2 ± 2.6 |
| SNA (°) | 75.5 ± 5.5 | 75.2 ± 5.1 | −0.3 ± 3.5 | 77.1 ± 5.3 | 78.6 ± 5.2 | 78.6 ± 5.8 | 1.6 ± 1.5 | 0.0 ± 2.5 | 1.6 ± 2.7 |
| SNB (°) | 75.9 ± 4.5 | 77.8 ± 5.7 | 1.9 ± 3.8 | 78.1 ± 4.1 | 78.5 ± 4.1 | 78.5 ± 4.9 | 0.4 ± 2.2 | 0.1 ± 2.8 | 0.5 ± 2.2 |
| ANB (°) | −0.4 ± 3.5 | −2.6 ± 2.7 | −2.2 ± 2.3 | −1.0 ± 3.1 | 0.1 ± 3.1 | 0.1 ± 3.2 | 1.1 ± 1.7 | 0.1 ± 1.9 | 1.1 ± 2.1 @ |
| Wits (mm) | −2.9 ± 2.8 | −4.3 ± 4.7 | −1.4 ± 4.9 | −1.8 ± 3.4 | −0.2 ± 3.4 | −0.4 ± 4.0 @ | 1.6 ± 2.2 | −0.2 ± 2.9 | 1.4 ± 2.9 |
| ANS-PNS/Go-Gn (°) | 27.1 ± 6.1 | 26.9 ± 5.1 | −0.2 ± 4.4 | 23.9 ± 5.6 | 24.0 ± 6.0 | 23.2 ± 5.3 | 0.1 ± 2.0 | −0.7 ± 2.9 | −0.6 ± 3.1 |
| SN/GoGn(°) | 36.4 ± 7.6 | 35.7 ± 7.7 | −0.8 ± 3.1 | 33.6 ± 6.0 | 33.6 ± 5.0 | 32.5 ± 5.0 | −0.1 ± 2.8 | −1.1 ± 3.2 | −1.0 ± 4.1 |
| Occlusal plane (°) | 18.1 ± 6.2 | 14.7 ± 7.6 | −3.3 ± 4.1 | 14.1 ± 5.1 | 12.6 ± 4.5 | 12.5 ± 4.6 | −1.5 ± 2.6 | −0.1 ± 3.2 | −1.6 ± 3.0 |
| Gonial angle (°) | 133.2 ± 5.9 | 133.1 ± 7.3 | −0.1 ± 3.7 | 130.0 ± 6.9 | 129.0 ± 6.5 | 126.6 ± 7.0 @ | −1.0 ± 4.0 | −2.4 ± 2.5 | −3.4 ± 5.4 |
| UI to Pal (°) | 96.3 ± 6.9 | 107.9 ± 8.0 # | 11.6 ± 8.9 | 108.1 ± 11.0 @ | 111.8 ± 7.5 | 114.5 ± 5.7 | 3.7 ± 8.6 | 2.3 ± 7.3 | 6.1 ± 11.2 @ |
| UI to NA (°) | 11.4 ± 8.3 | 23.9 ± 7.2 # | 12.5 ± 7.5 | 19.7 ± 11.7 | 23.0 ± 8.7 | 26.8 ± 6.8 | 3.2 ± 7.7 | 3.9 ± 7.9 | 7.1 ± 9.9 @ |
| LI to GoGn (°) | 87.6 ± 7.0 | 86.6 ± 6.3 | −1.0 ± 6.7 | 91.4 ± 8.3 | 89.5 ± 6.7 | 90.6 ± 7.3 | −1.8 ± 3.8 | −0.8 ± 5.3 | −1.1 ± 4.2 |
| LI to NB (°) | 19.2 ± 7.6 | 20.2 ± 7.5 | 1.0 ± 7.0 | 22.6 ± 5.8 | 21.6 ± 6.3 | 21.5 ± 6.2 | −1.0 ± 3.7 | −0.1 ± 5.1 | −1.1 ± 4.2 |
| InterI angle (°) | 149.0 ± 9.6 | 138.5 ± 12.2 # | −10.5 ± 10.6 | 136.2 ± 11.4 @ | 133.3 ± 9.6 | 131.6 ± 6.3 | −2.8 ± 10.2 | −4.7 ± 13.0 | −7.5 ± 13.8 |
| Overjet (mm) | −4.4 ± 3.1 | −2.6 ± 4.1 | 1.8 ± 3.7 | −1.3 ± 2.7 | 1.0 ± 3.2 | 2.2 ± 2.8 *@ | 2.3 ± 3.3 | 1.2 ± 2.4 | 3.5 ± 3.3 |
| Overbite (mm) | 3.1 ± 3.0 | 2.1 ± 2.8 | −1.0 ± 2.7 | 1.3 ± 2.0 | 1.4 ± 1.5 | 1.2 ± 1.9 | 0.1 ± 1.6 | −0.2 ± 1.7 | −0.1 ± 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steegman, R.M.; Klein Meulekamp, A.F.; Dieters, A.; Jansma, J.; van der Meer, W.J.; Ren, Y. Skeletal Changes in Growing Cleft Patients with Class III Malocclusion Treated with Bone Anchored Maxillary Protraction—A 3.5-Year Follow-Up. J. Clin. Med. 2021, 10, 750. https://doi.org/10.3390/jcm10040750
Steegman RM, Klein Meulekamp AF, Dieters A, Jansma J, van der Meer WJ, Ren Y. Skeletal Changes in Growing Cleft Patients with Class III Malocclusion Treated with Bone Anchored Maxillary Protraction—A 3.5-Year Follow-Up. Journal of Clinical Medicine. 2021; 10(4):750. https://doi.org/10.3390/jcm10040750
Chicago/Turabian StyleSteegman, Ralph M., Annemarlien Faye Klein Meulekamp, Arjan Dieters, Johan Jansma, Wicher J. van der Meer, and Yijin Ren. 2021. "Skeletal Changes in Growing Cleft Patients with Class III Malocclusion Treated with Bone Anchored Maxillary Protraction—A 3.5-Year Follow-Up" Journal of Clinical Medicine 10, no. 4: 750. https://doi.org/10.3390/jcm10040750
APA StyleSteegman, R. M., Klein Meulekamp, A. F., Dieters, A., Jansma, J., van der Meer, W. J., & Ren, Y. (2021). Skeletal Changes in Growing Cleft Patients with Class III Malocclusion Treated with Bone Anchored Maxillary Protraction—A 3.5-Year Follow-Up. Journal of Clinical Medicine, 10(4), 750. https://doi.org/10.3390/jcm10040750






