Inverted U-Curve Association between Serum Indoxyl Sulfate Levels and Cardiovascular Events in Patients on Chronic Hemodialysis
Abstract
1. Introduction
2. Materials and Methods
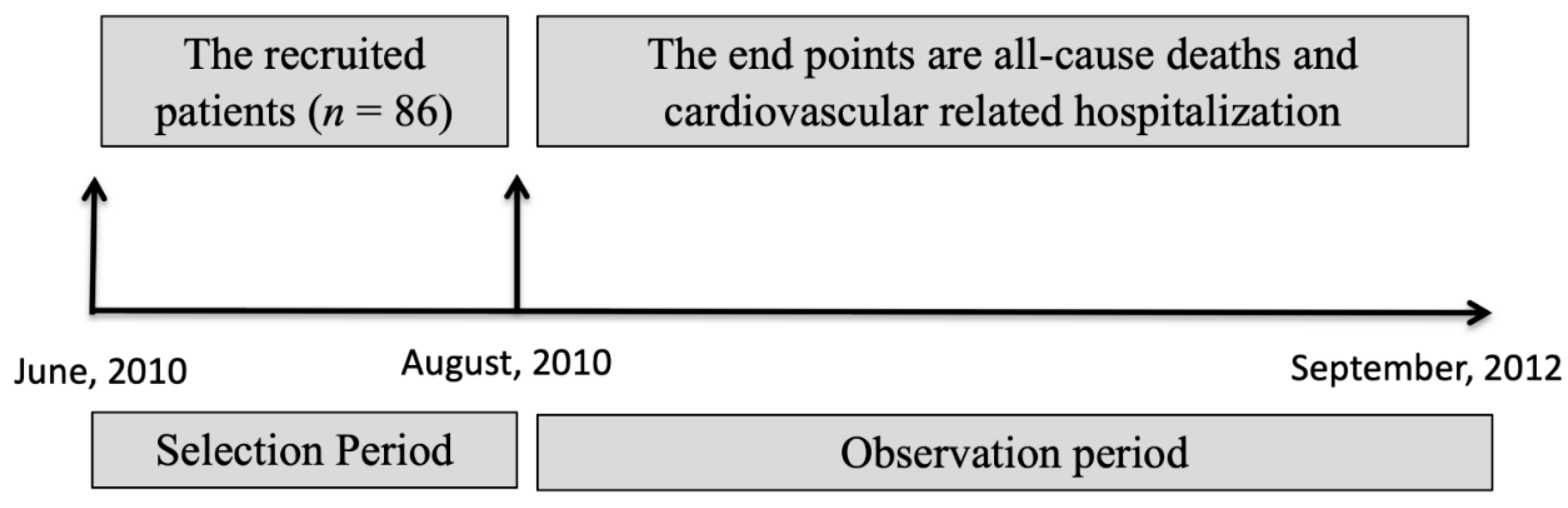
2.1. Study Design and Population
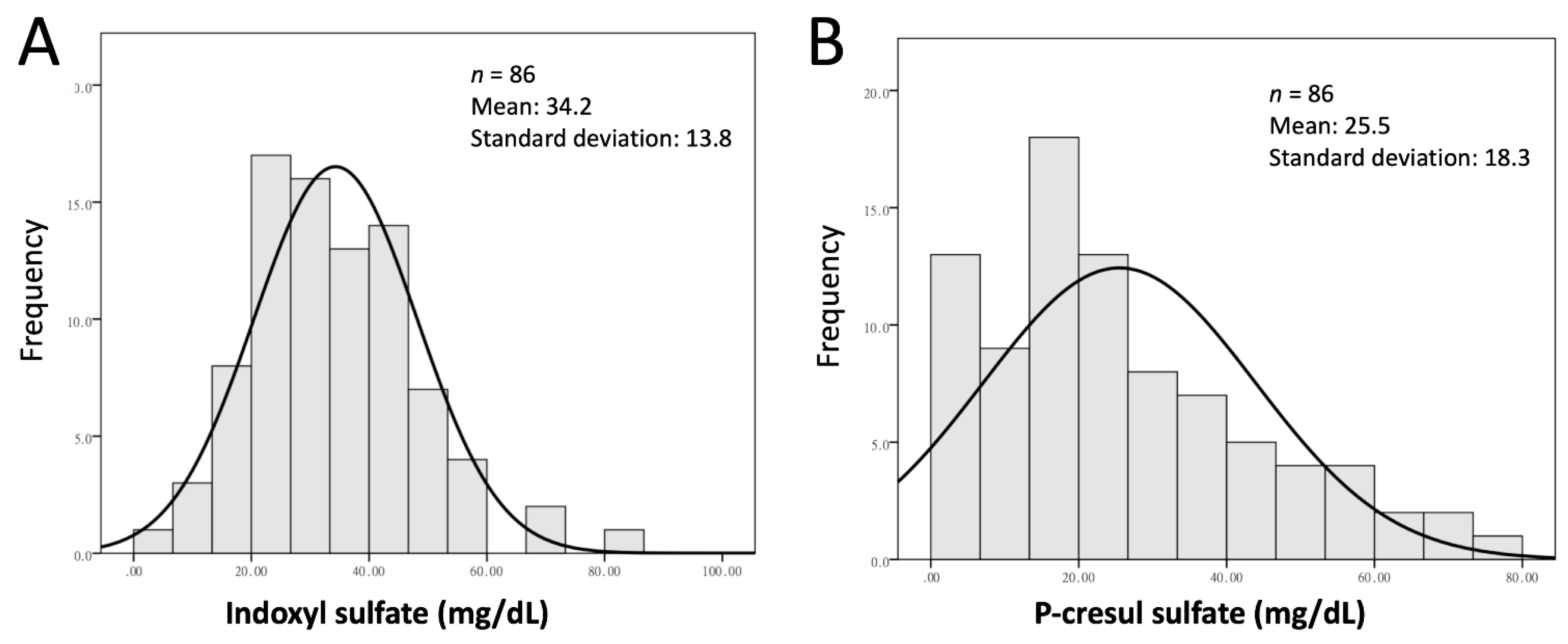
2.2. Laboratory Data
2.3. Event Evaluation
2.4. Statistical Analysis
2.5. Patient and Public Involvement
3. Results
3.1. Study Population Characteristics
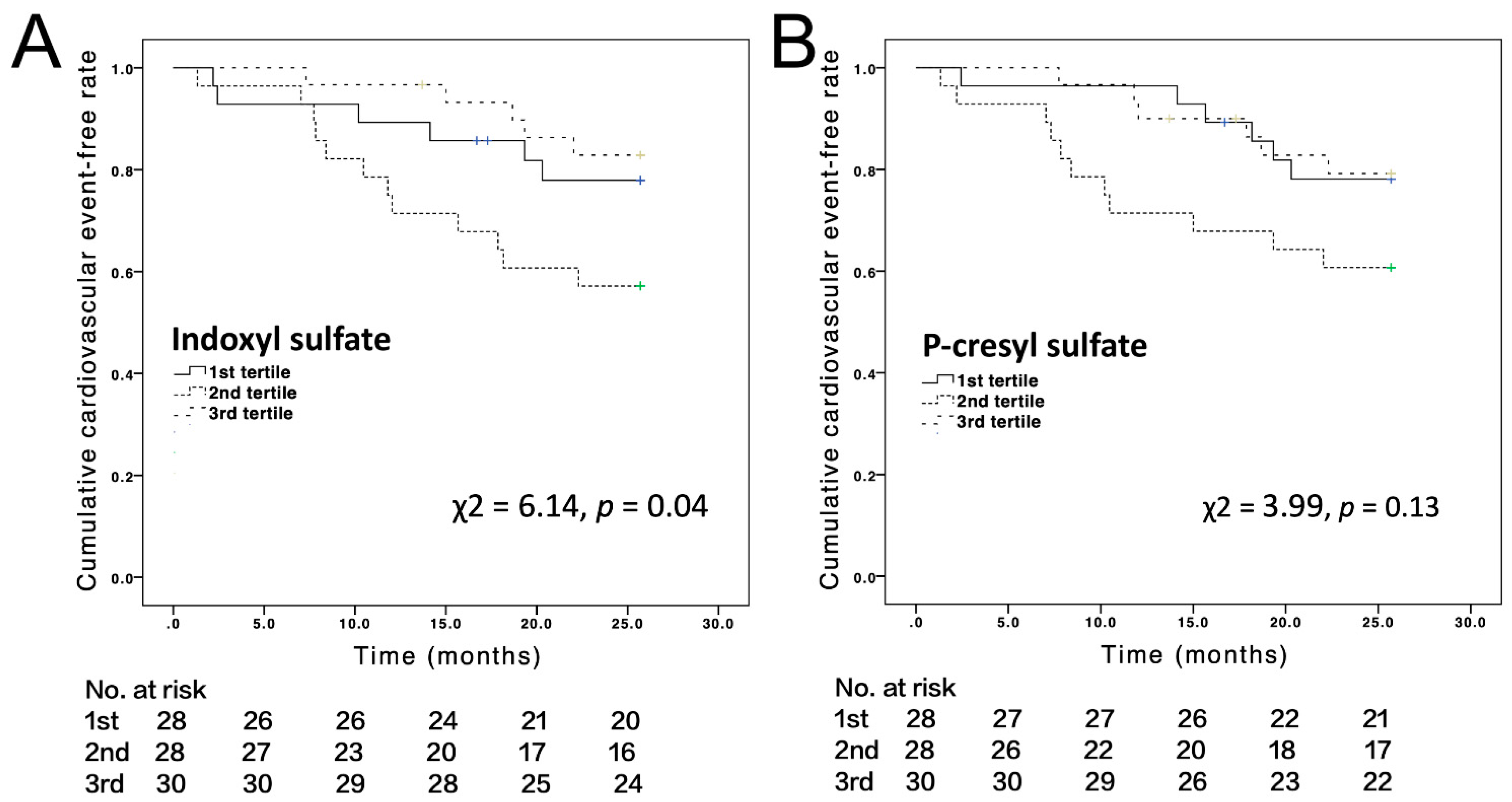
3.2. Cardiovascular Events
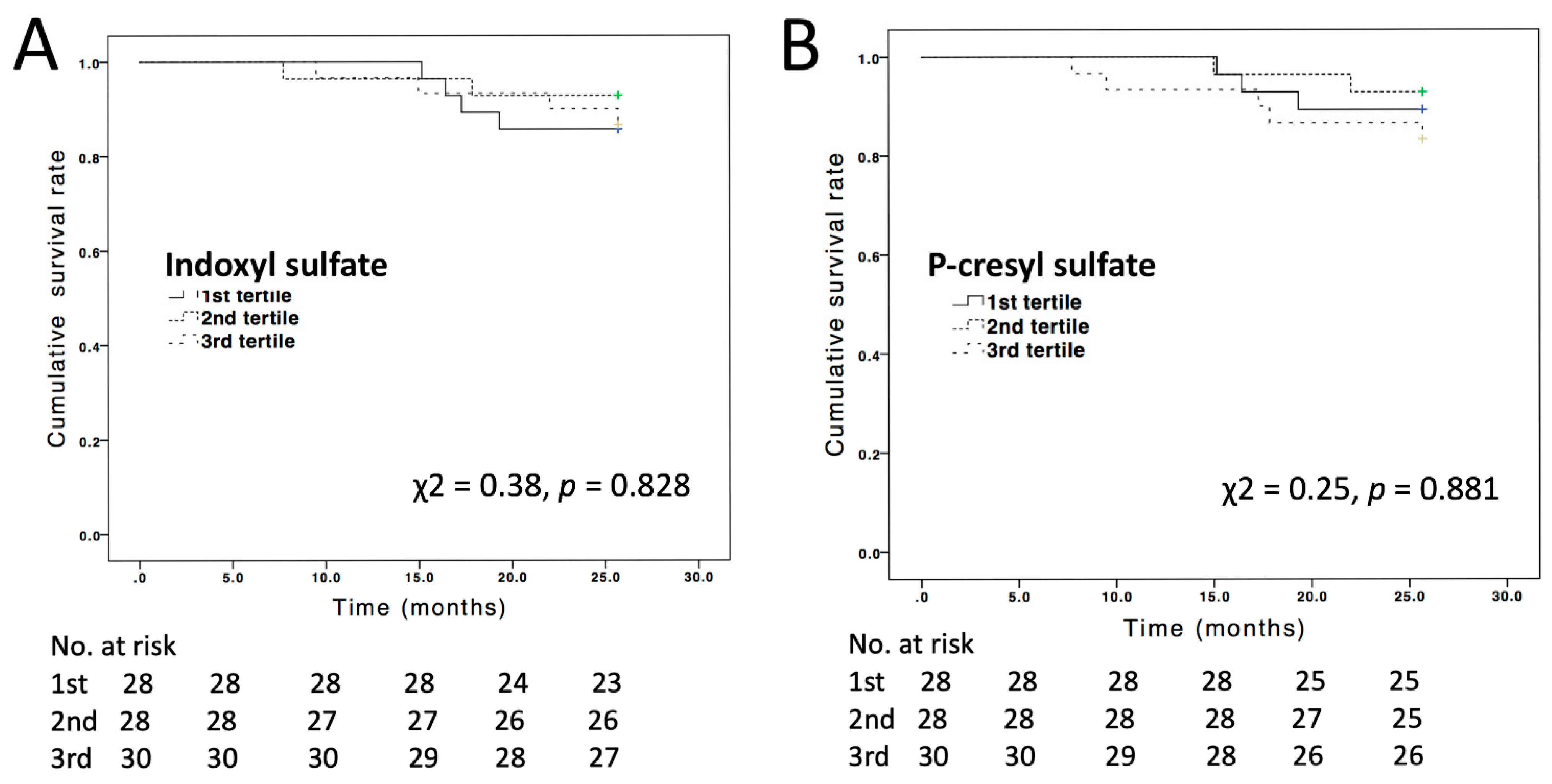
3.3. All-Cause Mortality Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, M.H.; Liou, H.H.; Leu, J.G.; Yen, M.F.; Chen, H.H. Sites of peripheral artery occlusive disease as a predictor for all-cause and cardiovascular mortality in chronic hemodialysis. PLoS ONE 2015, 10, e0128968. [Google Scholar] [CrossRef][Green Version]
- Chen, S.C.; Huang, J.C.; Su, H.M.; Chiu, Y.W.; Chang, J.M.; Hwang, S.J.; Chen, H.C. Prognostic cardiovascular markers in chronic kidney disease. Kidney Blood Press Res. 2018, 43, 1388–1407. [Google Scholar] [CrossRef]
- Ravarotto, V.; Simioni, F.; Pagnin, E.; Davis, P.A.; Calo, L.A. Oxidative stress-chronic kidney disease-cardiovascular disease: A vicious circle. Life Sci. 2018, 210, 125–131. [Google Scholar] [CrossRef]
- Liu, W.C.; Tomino, Y.; Lu, K.C. Impacts of indoxyl sulfate and p-cresol sulfate on chronic kidney disease and mitigating effects of ast-120. Toxins 2018, 10, 367. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.R.; Dehghani, N.L.; Narsimhan, V.; Ronco, C. Uremic toxins and their relation to dialysis efficacy. Blood Purif. 2019, 48, 299–314. [Google Scholar] [CrossRef]
- Deltombe, O.; Van Biesen, W.; Glorieux, G.; Massy, Z.; Dhondt, A.; Eloot, S. Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins 2015, 7, 3933–3946. [Google Scholar] [CrossRef]
- Leong, S.C.; Sirich, T.L. Indoxyl sulfate-review of toxicity and therapeutic strategies. Toxins 2016, 8, 358. [Google Scholar] [CrossRef]
- Dias, G.F.; Bonan, N.B.; Steiner, T.M.; Tozoni, S.S.; Rodrigues, S.; Nakao, L.S.; Kuntsevich, V.; Pecoits Filho, R.; Kotanko, P.; Moreno-Amaral, A.N. Indoxyl sulfate, a uremic toxin, stimulates reactive oxygen species production and erythrocyte cell death supposedly by an organic anion transporter 2 (oat2) and nadph oxidase activity-dependent pathways. Toxins 2018, 10, 280. [Google Scholar] [CrossRef]
- Lano, G.; Burtey, S.; Sallee, M. Indoxyl sulfate, a uremic endotheliotoxin. Toxins 2020, 12, 229. [Google Scholar] [CrossRef]
- Guo, C.C.; Xia, W.W.; Zhang, A.H. [research progress of the uremic toxin indoxyl sulfate in cardiovascular complication of end-stage renal diseases]. Sheng Li Xue Bao 2018, 70, 657–662. [Google Scholar] [PubMed]
- He, X.; Jiang, H.; Gao, F.; Liang, S.; Wei, M.; Chen, L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the pi3k/akt/nf-kappab signaling pathway. Microsc. Res. Tech. 2019, 82, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Sitkin, S.I.; Tkachenko, E.I.; Vakhitov, T.Y. Metabolic dysbiosis of the gut microbiota and its biomarkers. Eksp. Klin. Gastroenterol. 2016, 12, 6–29. [Google Scholar] [PubMed]
- Chao, C.T.; Chiang, C.K. Uremic toxins, oxidative stress, and renal fibrosis: An interwined complex. J. Ren. Nutr. 2015, 25, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Wang, C.Y.; Hsu, C.Y.; Wu, C.H.; Kuo, C.C.; Wang, K.C.; Yang, C.C.; Wu, M.T.; Chuang, F.R.; Lee, C.T. Free p-cresol sulfate is associated with survival and function of vascular access in chronic hemodialysis patients. Kidney Blood Press Res. 2012, 35, 583–588. [Google Scholar] [CrossRef]
- van Gelder, M.K.; Middel, I.R.; Vernooij, R.W.M.; Bots, M.L.; Verhaar, M.C.; Masereeuw, R.; Grooteman, M.P.; Nube, M.J.; van den Dorpel, M.A.; Blankestijn, P.J.; et al. Protein-bound uremic toxins in hemodialysis patients relate to residual kidney function, are not influenced by convective transport, and do not relate to outcome. Toxins 2020, 12, 234. [Google Scholar] [CrossRef]
- Shafi, T.; Sirich, T.L.; Meyer, T.W.; Hostetter, T.H.; Plummer, N.S.; Hwang, S.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Powe, N.R. Results of the hemo study suggest that p-cresol sulfate and indoxyl sulfate are not associated with cardiovascular outcomes. Kidney Int. 2017, 92, 1484–1492. [Google Scholar] [CrossRef]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef]
- Zha, Y.; Qian, Q. Protein nutrition and malnutrition in ckd and esrd. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef]
- Hung, S.C.; Kuo, K.L.; Wu, C.C.; Tarng, D.C. Indoxyl sulfate: A novel cardiovascular risk factor in chronic kidney disease. J. Am. Heart Assoc. 2017, 6, e005022. [Google Scholar] [CrossRef]
- Meijers, B.K.; Bammens, B.; De Moor, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008, 73, 1174–1180. [Google Scholar] [CrossRef]
- Lin, C.J.; Wu, C.J.; Pan, C.F.; Chen, Y.C.; Sun, F.J.; Chen, H.H. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol. Dial. Transpl. 2010, 25, 3693–3700. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.W.; Hsu, K.H.; Hsu, H.J.; Lee, C.C.; Sun, C.Y.; Tsai, C.J.; Wu, M.S. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—A prospective cohort study. Nephrol. Dial. Transpl. 2012, 27, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Marcelli, D.; Usvyat, L.A.; Kotanko, P.; Bayh, I.; Canaud, B.; Etter, M.; Gatti, E.; Grassmann, A.; Wang, Y.; Marelli, C.; et al. Body composition and survival in dialysis patients: Results from an international cohort study. Clin. J. Am. Soc. Nephrol. 2015, 10, 1192–1200. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, M.; Zhang, Y.; Nie, L.; He, T.; Wang, Y.; Huang, Y.; Feng, B.; Zhang, J.; Zhao, J. Association of geriatric nutritional risk index with mortality in hemodialysis patients: A meta-analysis of cohort studies. Kidney Blood Press Res. 2018, 43, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Karger, A.B.; Steffen, B.T.; Nomura, S.O.; Guan, W.; Garg, P.K.; Szklo, M.; Budoff, M.J.; Tsai, M.Y. Association between homocysteine and vascular calcification incidence, prevalence, and progression in the mesa cohort. J. Am. Heart Assoc. 2020, 9, e013934. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.C.; Chen, C.Y.; Lin, C.F.; Yeh, H.I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark Res. 2017, 5, 31. [Google Scholar] [CrossRef]
- Asai, M.; Kumakura, S.; Kikuchi, M. Review of the efficacy of ast-120 (kremezin((r))) on renal function in chronic kidney disease patients. Ren. Fail 2019, 41, 47–56. [Google Scholar] [CrossRef]
- Lee, C.T.; Hsu, C.Y.; Tain, Y.L.; Ng, H.Y.; Cheng, B.C.; Yang, C.C.; Wu, C.H.; Chiou, T.T.; Lee, Y.T.; Liao, S.C. Effects of ast-120 on blood concentrations of protein-bound uremic toxins and biomarkers of cardiovascular risk in chronic dialysis patients. Blood Purif. 2014, 37, 76–83. [Google Scholar] [CrossRef]
- Ueda, H.; Shibahara, N.; Takagi, S.; Inoue, T.; Katsuoka, Y. Ast-120, an oral adsorbent, delays the initiation of dialysis in patients with chronic kidney diseases. Ther. Apher. Dial. 2007, 11, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Shibahara, N.; Takagi, S.; Inoue, T.; Katsuoka, Y. Ast-120 treatment in pre-dialysis period affects the prognosis in patients on hemodialysis. Ren. Fail. 2008, 30, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.D.; Lin, D.Y. Time-dependent covariates in the cox proportional-hazards regression model. Annu. Rev. Public Health 1999, 20, 145–157. [Google Scholar] [CrossRef] [PubMed]
| Parameters | All (n = 86) | Serum IS | Serum PCS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Tertile (n = 28) | 2nd Tertile (n = 28) | 3rd Tertile (n = 30) | p Value | 1st Tertile (n = 28) | 2nd Tertile (n = 28) | 3rd Tertile (n = 30) | p Value | ||
| IS (mg/dL) | 34.2 ± 13.8 | 5.5–26.2 | 26.6–39.1 | 39.5–81 | - | - | - | - | - |
| PCS (mg/dL) | 25.5 ± 13.8 | - | - | - | - | 0.5–15.5 | 15.7–28. | 29.1–78.1 | - |
| Age (year) | 61.8 ± 12.4 | 63.0 ± 10.4 | 58.9 ± 14.6 | 63.3 ± 11.8 | 0.330 | 62 ± 12.3 | 59.3 ± 13.0 | 63.8 ± 11.8 | 0.388 |
| Male gender (n %) | 53 (61%) | 17 (60%) | 16 (57%) | 20 (67%) | 0.752 | 13 (46%) | 20 (71%) | 20 (67%) | 0.123 |
| Vintage (year) | 9.1 ± 5.4 | 10.1 ± 5.8 | 8.9 ± 5.4 | 8.4 ± 5.0 | 0.591 | 10.5 ± 5.6 | 8.6 ± 5.0 | 8.3 ± 5.5 | 0.262 |
| Body mass index (kg/m2) | 22.4 ± 3.9 | 22.2 ± 2.7 | 22.3 ± 4.1 | 23.0 ± 4.8 | 0.867 | 22.6 ± 4.0 | 23.0 ± 4.2 | 22.0 ± 3.8 | 0.606 |
| Diabetes mellitus (n %) | 36 (42%) | 12 (43%) | 10 (35%) | 14 (46%) | 0.694 | 9 (32%) | 12 (42%) | 15 (50%) | 0.384 |
| Presence of CVD (n %) | 26 (30%) | 7 (25%) | 10 (36%) | 9 (30%) | 0.683 | 7 (25%) | 10 (36%) | 9 (30%) | 0.683 |
| Systolic BP (mmHg) | 148 ± 27 | 150 ± 26 | 145 ± 28 | 151 ± 27 | 0.600 | 146 ± 25 | 157 ± 28 | 145 ± 28 | 0.178 |
| Diastolic BP (mmHg) | 71 ± 17 | 71 ± 17 | 75 ± 16 | 69 ± 18 | 0.424 | 71 ± 11 | 77 ± 21 | 69 ± 18 | 0.204 |
| Ca P product (mg2/dL2) | 50.1 ± 15.5 | 40.6 ± 12.4 | 51.6 ± 18.3 | 52.1 ± 15.2 | 0.375 | 51.4 ± 16.1 | 52.2 ± 14.0 | 47.0 ± 16.2 | 0.271 |
| iPTH (pg/mL) | 186 ± 210 | 154 ± 250 a | 172 ± 179 | 229 ± 194 | 0.027 | 230 ± 274 | 182 ± 190 | 149 ± 149 | 0.349 |
| Albumin (g/dL) | 4.2 ± 0.4 | 4.1 ± 0.4 a | 4.1 ± 0.4 a | 4.4 ± 0.3 | 0.012 | 4.2 ± 0.4 | 4.3 ± 0.4 | 4.3 ± 0.5 | 0.737 |
| hsCRP (mg/dL) | 1.0 ± 1.9 | 0.8 ± 1.1 | 1.5 ± 3.0 | 0.9 ± 1.2 | 0.813 | 1.1 ± 1.2 | 0.9 ± 1.3 | 1.2 ± 2.8 | 0.846 |
| Homocysteine (μmol/L) | 26.6 ± 12.0 | 31.3 ± 17.0 a | 26.8 ± 7.6 | 22.2 ± 8.0 | 0.014 | 29.6 ± 17.3 | 25.4 ± 8.4 | 25.0 ± 8.4 | 0.284 |
| Hemoglobin (g/dL) | 10.4 ± 1.2 | 10.3 ± 1.2 | 10.4 ± 1.4 | 10.6 ± 1.3 | 0.639 | 10.2 ± 1.0 | 10.7 ± 1.6 | 10.3 ± 1.2 | 0.280 |
| Ferritin (ng/mL) | 566 ± 311 | 573 ± 329 | 481 ± 263 | 639 ± 328 | 0.084 | 651 ± 357 | 523 ± 286 | 525 ± 280 | 0.595 |
| Uric acid (mg/dL) | 7.0 ± 1.3 | 7.0 ± 1.3 | 7.2 ± 1.5 | 7.0 ± 1.2 | 0.675 | 7.2 ± 1.5 | 7.1 ± 1.1 | 6.8 ± 1.3 | 0.672 |
| Total cholesterol (mg/dL) | 184 ± 43 | 183 ± 47 | 174 ± 46 | 193 ± 35 | 0.119 | 181 ± 47 | 187 ± 45 | 183 ± 37 | 0.833 |
| LDL (mg/dL) | 109 ± 34 | 103 ± 32 | 105 ± 36 | 117 ± 33 | 0.275 | 97 ± 30 | 117 ± 37 | 111 ± 31 | 0.078 |
| HDL (mg/dL) | 49 ± 18 | 45 ± 14 | 45 ± 15 | 55 ± 22 | 0.105 | 50 ± 16 | 45 ± 21 | 51 ± 16 | 0.461 |
| Triglycerides (mg/dL) | 175 ± 215 | 229 ± 329 | 156 ± 157 | 141 ± 83 | 0.534 | 227 ± 356 | 166 ± 87 | 134 ± 75 | 0.249 |
| Kt/V | 1.38 ± 0.17 | 1.37 ± 0.13 | 1.40 ± 0.23 | 1.38 ± 0.17 | 0.970 | 1.40 ± 1.52 | 1.33 ± 1.64 | 1.40 ± 0.21 | 0.274 |
| Parameters | Cardiovascular Event | All-Cause Mortality | ||
|---|---|---|---|---|
| Crude | Crude | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| IS (per mg/dL) | 1.00 (0.97–1.03) | 0.899 | 1.00 (0.95–1.06) | 0.96 |
| 1st tertile (vs. 3rd tertile) | 1.39 (0.42–4.56) | 0.585 | 1.63 (0.27–9.78) | 0.591 |
| 2nd tertile (vs. 3rd tertile) | 3.14 (1.10–8.94) | 0.031 | 0.53 (0.14–7.51) | 0.955 |
| PCS (per mg/dL) | 0.99 (0.97–1.02) | 0.474 | 1.00 (0.97–1.04) | 0.844 |
| 1st tertile (vs. 3rd tertile) | 1.05 (0.34–3.27) | 0.972 | 0.68 (0.11–4.11) | 0.682 |
| 2nd tertile (vs. 3rd tertile) | 2.31 (0.85–6.25) | 0.099 | 0.67 (0.11–4.05) | 0.669 |
| Age (per year) | 1.01 (0.97–1.04) | 0.562 | 1.03 (0.96–1.10) | 0.319 |
| Male versus female | 0.58 (0.26–1.33) | 0.205 | 0.77 (0.17–3.47) | 0.741 |
| Vintage (per year) | 1.02 (0.94–1.10) | 0.554 | 1.11 (0.97–1.27) | 0.115 |
| BMI (per kg/m2) | 0.97 (0.87–1.09) | 0.689 | 0.94 (0.76–1.16) | 0.587 |
| Diabetes | 0.68 (0.29–1.62) | 0.395 | 0.21 (0.02–1.78) | 0.154 |
| Presence of CVD | 2.64 (1.16–5.99) | 0.020 | 0.36 (0.04–2.99) | 0.345 |
| Systolic BP (per mmHg) | 0.99 (0.98–1.01) | 0.488 | 0.99 (0.97–1.02) | 0.892 |
| Diastolic BP (per mmHg) | 1.00 (0.97–1.02) | 0.886 | 0.98 (0.94–1.03) | 0.611 |
| Ca P product (per mg2/dL2) | 0.99 (0.96–1.02) | 0.754 | 0.97 (0.91–1.03) | 0.342 |
| iPTH (per pg/mL) | 0.99 (0.99–1.00) | 0.473 | 0.99 (0.99–1.00) | 0.287 |
| Albumin (per g/dL) | 0.29 (0.11–0.75) | 0.011 | 0.05 (0.01–0.28) | 0.001 |
| hsCRP (per mg/dL) | 1.26 (1.11–1.44) | <0.001 | 2.15 (1.41–3.30) | <0.001 |
| Homocysteine (per μmol/L) | 0.97 (0.93–1.01) | 0.253 | 0.98 (0.91–1.06) | 0.698 |
| Hemoglobin (per g/dL) | 0.92 (0.65–1.31) | 0.675 | 0.52 (0.22–1.22) | 0.135 |
| Ln(Ferritin) (per 1 unit) | 1.08 (0.69–1.70) | 0.732 | 1.18 (0.48–2.89) | 0.716 |
| Uric acid (per mg/dL) | 0.93 (0.68–1.25) | 0.616 | 0.51 (0.28–0.90) | 0.021 |
| TC (per mg/dL) | 0.99 (0.99–1.00) | 0.351 | 0.97 (0.94–0.99) | 0.027 |
| LDL (per mg/dL) | 0.98 (0.97–1.00) | 0.022 | 0.97 (0.95–1.00) | 0.060 |
| HDL (per mg/dL) | 1.01 (0.99–1.04) | 0.116 | 1.01 (0.98–1.04) | 0.509 |
| Ln(Triglyceride) (per unit) | 0.67 (0.36–1.24) | 0.198 | 0.40 (0.12–1.35) | 0.139 |
| Kt/V (per unit) | 4.69 (0.44–49.7) | 0.199 | 4.64 (0.07–284) | 0.464 |
| Models | HR | 95% CI | p Value |
|---|---|---|---|
| Unadjusted | |||
| 1st tertile | 1.39 | 0.42–4.56 | 0.58 |
| 2nd tertile | 3.14 | 1.10–8.94 | 0.03 |
| 3rd tertile | 1 | ||
| Model 1 | |||
| 1st tertile | 1.35 | 0.41–4.46 | 0.61 |
| 2nd tertile | 3.70 | 1.25–10.92 | 0.01 |
| 3rd tertile | 1 | ||
| Model 2 | |||
| 1st tertile | 1.76 | 0.52–5.97 | 0.36 |
| 2nd tertile | 4.27 | 1.37–13.22 | 0.01 |
| 3rd tertile | 1 | ||
| Model 3 | |||
| 1st tertile | 2.25 | 0.67–8.04 | 0.214 |
| 2nd tertile | 4.63 | 1.40–15.37 | 0.012 |
| 3rd tertile | 1 | ||
| Model 4 | |||
| 1st tertile | 1.76 | 0.43–7.19 | 0.430 |
| 2nd tertile | 3.31 | 0.87–12.60 | 0.079 |
| 3rd tertile | 1 | ||
| Model 5 * | |||
| 1st tertile | 2.19 | 0.63–7.64 | 0.218 |
| 2nd tertile | 5.42 | 1.67–17.60 | 0.005 |
| 3rd tertile | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-H.; Chang, C.-H.; Liou, H.-H.; Fang, Y.-W. Inverted U-Curve Association between Serum Indoxyl Sulfate Levels and Cardiovascular Events in Patients on Chronic Hemodialysis. J. Clin. Med. 2021, 10, 744. https://doi.org/10.3390/jcm10040744
Tsai M-H, Chang C-H, Liou H-H, Fang Y-W. Inverted U-Curve Association between Serum Indoxyl Sulfate Levels and Cardiovascular Events in Patients on Chronic Hemodialysis. Journal of Clinical Medicine. 2021; 10(4):744. https://doi.org/10.3390/jcm10040744
Chicago/Turabian StyleTsai, Ming-Hsien, Chung-Hsin Chang, Hung-Hsiang Liou, and Yu-Wei Fang. 2021. "Inverted U-Curve Association between Serum Indoxyl Sulfate Levels and Cardiovascular Events in Patients on Chronic Hemodialysis" Journal of Clinical Medicine 10, no. 4: 744. https://doi.org/10.3390/jcm10040744
APA StyleTsai, M.-H., Chang, C.-H., Liou, H.-H., & Fang, Y.-W. (2021). Inverted U-Curve Association between Serum Indoxyl Sulfate Levels and Cardiovascular Events in Patients on Chronic Hemodialysis. Journal of Clinical Medicine, 10(4), 744. https://doi.org/10.3390/jcm10040744






