New Surgical Technique for Robotic Myomectomy: Continuous Locking Suture on Myoma (LSOM) Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
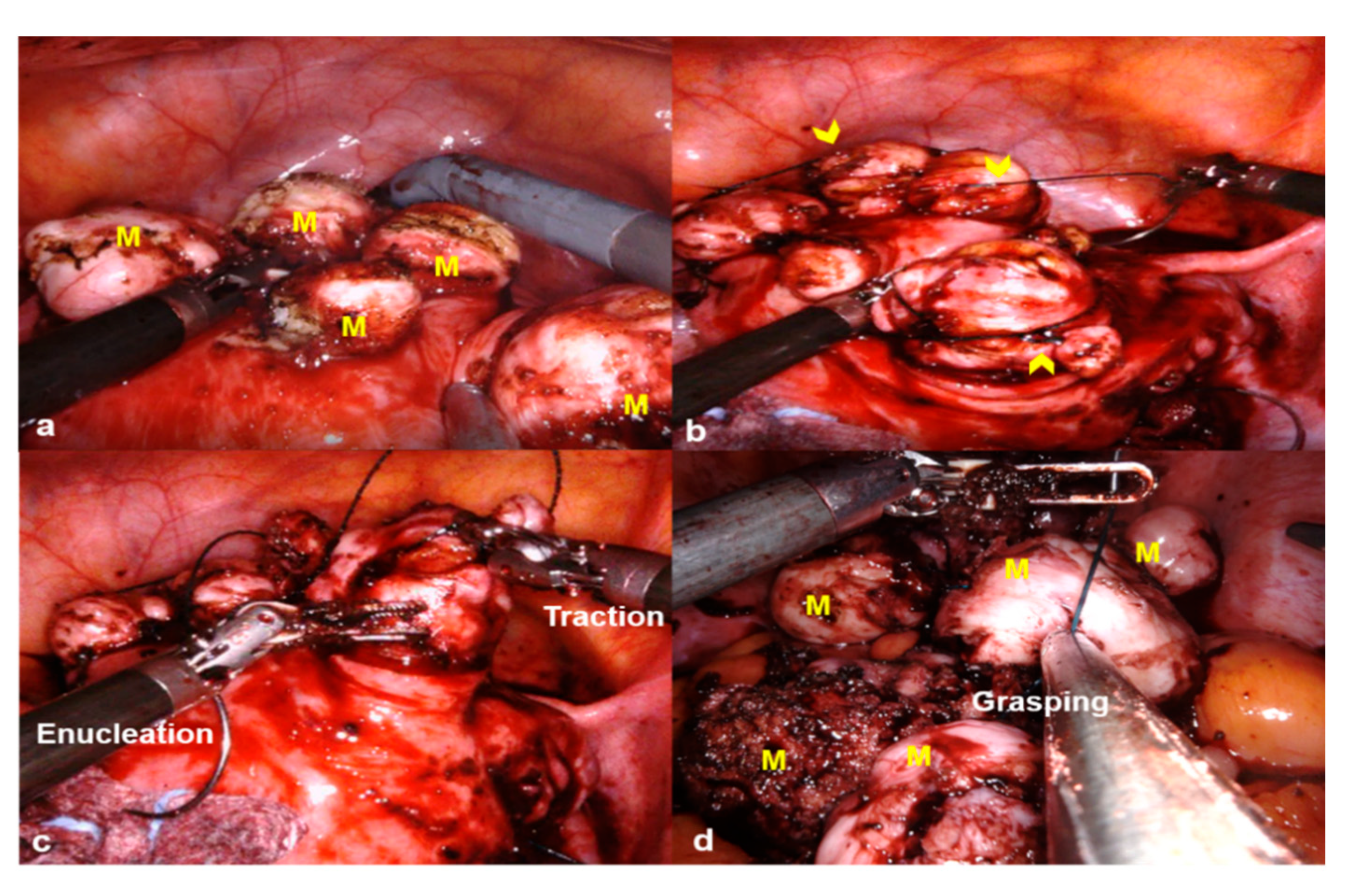
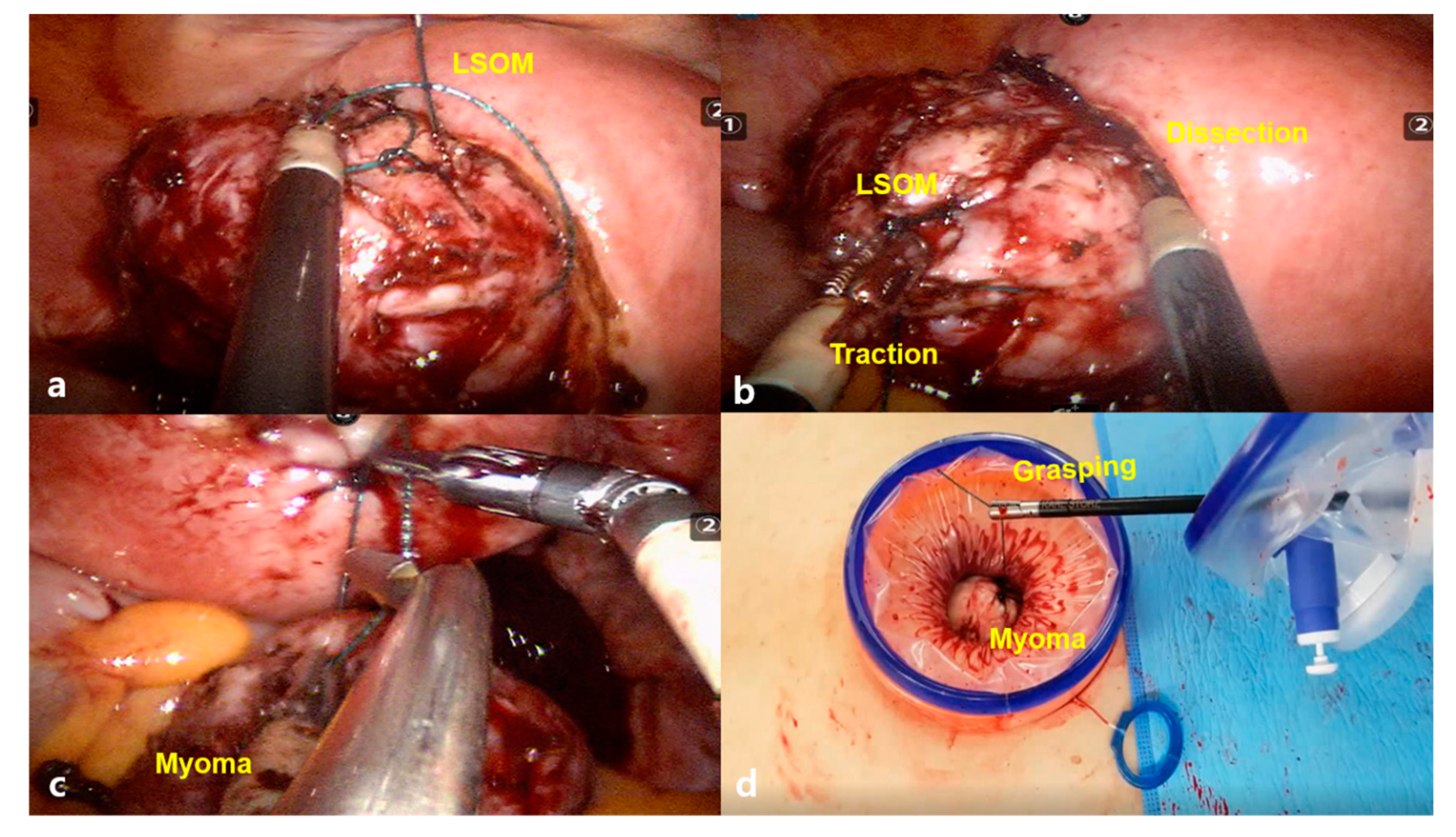
2.2. Surgical Procedures
2.3. Statistical Analysis
3. Results
3.1. Patient Baseline Characteristics
3.2. Comparison of Single Variables between the LSOM and Non-LSOM Groups
3.3. Results of Multiple Linear Regression Analysis
4. Discussion
4.1. Feasibility of the LSOM Technique in RALM
4.2. Application of LSOM Technique in Single Incision RALM
4.3. LSOM Technique for Safe Minimally Invasive Myomectomy
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, E.A. Clinical practice. Uterine fibroids. N. Engl. J. Med. 2015, 372, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Cookson, C.L.; Gandolfo, R.A.; Schulze-Rath, R. Epidemiology of uterine fibroids: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1501–1512. [Google Scholar] [CrossRef]
- Cezar, C.; Becker, S.; di Spiezio Sardo, A.; Herrmann, A.; Larbig, A.; Tanos, V.; de la Roche, L.A.; Verhoeven, H.C.; Wallwiener, M.; De Wilde, R.L. Laparoscopy or laparotomy as the way of entrance in myoma enucleation. Arch. Gynecol. Obstet. 2017, 296, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Stanhiser, J.; Mouille, B.; Flyckt, R.; Goldberg, J.; Falcone, T.; Goodman, L.R. Trends Over Time and Surgical Outcomes of Abdominal, Mini-Laparotomy, and Traditional and Robotic-Assisted Laparoscopy With and Without Tandem Mini-Laparotomy: A Comparison of Myomectomy Techniques. J. Minim. Invasive Gynecol. 2015, 22, S1. [Google Scholar] [CrossRef] [PubMed]
- Advincula, A.P.; Xu, X.; Goudeau, S., IV; Ransom, S.B. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: A comparison of short-term surgical outcomes and immediate costs. J. Minim. Invasive Gynecol. 2007, 14, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.R.; Seong, S.J.; Paek, J.; Lee, Y.S.; Nam, E.J.; Kim, Y.M.; Park, Y.H.; Kim, T.J.; Kim, Y.B.; et al. Trends in robotic surgery in Korean gynecology. Gyne Robot Surg. 2020, 1, 50–56. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, E.S.; Lee, Y.J.; Lee, S.W.; Park, J.Y.; Kim, D.Y.; Kim, S.H.; Kim, Y.M.; Suh, D.S.; Kim, Y.T. Robot-Assisted Laparoscopic Myomectomy versus Abdominal Myomectomy for Large Myomas Sized over 10 cm or Weighing 250 g. Yonsei Med. J. 2020, 61, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.F.; Nam, S.H.; Kim, J.M. Robot-assisted laparoscopic surgery versus conventional laparoscopic surgery in randomized controlled trials: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0191628. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.; Doglioli, M.; Fader, A.N. Updates and Controversies of Robotic-Assisted Surgery in Gynecologic Surgery. Clin. Obstet. Gynecol. 2019, 62, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Tapper, A.M.; Hannola, M.; Zeitlin, R.; Isojärvi, J.; Sintonen, H.; Ikonen, T.S. A systematic review and cost analysis of robot-assisted hysterectomy in malignant and benign conditions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 177, 1–10. [Google Scholar] [CrossRef]
- Behera, M.A.; Likes, C.E., III; Judd, J.P.; Barnett, J.C.; Havrilesky, L.J.; Wu, J.M. Cost analysis of abdominal, laparoscopic, and robotic-assisted myomectomies. J. Minim. Invasive Gynecol. 2012, 19, 52–57. [Google Scholar] [CrossRef]
- Truong, M.; Kim, J.H.; Scheib, S.; Patzkowsky, K. Advantages of robotics in benign gynecologic surgery. Curr. Opin. Obstet. Gynecol. 2016, 28, 304–310. [Google Scholar] [CrossRef]
- Winter, M.L.; Leu, S.Y.; Lagrew, D.C., Jr.; Bustillo, G. Cost comparison of robotic-assisted laparoscopic hysterectomy versus standard laparoscopic hysterectomy. J. Robot Surg. 2015, 9, 269–275. [Google Scholar] [CrossRef]
- Gingold, J.A.; Gueye, N.A.; Falcone, T. Minimally Invasive Approaches to Myoma Management. J. Minim. Invasive Gynecol. 2018, 25, 237–250. [Google Scholar] [CrossRef]
- Tulandi, T.; Einarsson, J.I. The use of barbed suture for laparoscopic hysterectomy and myomectomy: A systematic review and meta-analysis. J. Minim. Invasive Gynecol. 2014, 21, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Naval, S.; Naval, R.; Naval, S.; Rane, J. Tips for Safe Laparoscopic Multiple Myomectomy. J. Minim. Invasive Gynecol. 2017, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Zullo, F.; Venturella, R.; Raffone, A.; Saccone, G. In-bag manual versus uncontained power morcellation for laparoscopic myomectomy. Cochrane Database Syst. Rev. 2020, 5, CD013352. [Google Scholar] [CrossRef] [PubMed]
- Frascà, C.; Degli Esposti, E.; Arena, A.; Tuzzato, G.; Moro, E.; Martelli, V.; Seracchioli, R. Can In-Bag Manual Morcellation Represent an Alternative to Uncontained Power Morcellation in Laparoscopic Myomectomy? A Randomized Controlled Trial. Gynecol. Obstet. Investig. 2018, 83, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Jeong, K.; Moon, H.S. A Simple Continuous Locking Suturing Technique of Myoma Traction for Easy Robotic Single-Site Myomectomy on Large-Sized Uterine Myomas. J. Minim. Invasive Gynecol. 2017, 24, S127. [Google Scholar] [CrossRef]
- Sizzi, O.; Rossetti, A.; Malzoni, M.; Minelli, L.; La Grotta, F.; Soranna, L.; Panunzi, S.; Spagnolo, R.; Imperato, F.; Landi, S.; et al. Italian multicenter study on complications of laparoscopic myomectomy. J. Minim. Invasive Gynecol. 2007, 14, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Frascà, C.; Tuzzato, G.; Arena, A.; Degli Esposti, E.; Zanello, M.; Raimondo, D.; Seracchioli, R. The Role of Pelvic Ultrasound in Preoperative Evaluation for Laparoscopic Myomectomy. J. Minim. Invasive Gynecol. 2018, 25, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S. FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynaecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 19 September 2020).
- Kongnyuy, E.J.; Wiysonge, C.S. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst. Rev. 2014, 8, CD005355. [Google Scholar] [CrossRef]
- Fusca, L.; Perelman, I.; Fergusson, D.; Boutet, M.; Chen, I. The Effectiveness of Tranexamic Acid at Reducing Blood Loss and Transfusion Requirement for Women Undergoing Myomectomy: A Systematic Review and Meta-analysis. J. Obstet. Gynaecol. Can. 2019, 41, 1185–1192.e1. [Google Scholar] [CrossRef] [PubMed]
- Conforti, A.; Mollo, A.; Alviggi, C.; Tsimpanakos, I.; Strina, I.; Magos, A.; De Placido, G. Techniques to reduce blood loss during open myomectomy: A qualitative review of literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 192, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Cho, Y.M.; Im, K.S.; Yoo, S.B.; Hyung, S.W. Transient Occlusion of Uterine Arteries in Procedures with High Risk of Uterine Bleeding. JSLS 2019, 23, e2018.00072. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mahey, R.; Kachhawa, G.; Bhatla, N.; Upadhyay, A.D.; Kriplani, A. Comparison of intramyometrial vasopressin plus rectal misoprostol with intramyometrial vasopressin alone to decrease blood loss during laparoscopic myomectomy: Randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 279–283. [Google Scholar] [CrossRef]
- Venturella, R.; Rocca, M.L.; Lico, D.; La Ferrera, N.; Cirillo, R.; Gizzo, S.; Morelli, M.; Zupi, E.; Zullo, F. In-bag manual versus uncontained power morcellation for laparoscopic myomectomy: Randomized controlled trial. Fertil. Steril. 2016, 105, 1369–1376. [Google Scholar] [CrossRef]
- Falcone, T.; Flyckt, R. Tissue extraction technique at the time of laparoscopic myomectomy. Fertil. Steril. 2016, 105, 1158–1159. [Google Scholar] [CrossRef][Green Version]
- Sinha, R.; Hegde, A.; Warty, N.; Mahajan, C. Laparoscopic myomectomy: Enucleation of the myoma by morcellation while it is attached to the uterus. J. Minim. Invasive Gynecol. 2005, 12, 284–289. [Google Scholar] [CrossRef]
- Won, S.; Lee, N.; Kim, M.; Kim, M.K.; Kim, M.L.; Jung, Y.W.; Yun, B.S.; Seong, S.J. Comparison of surgical outcomes between robotic & laparoscopic single-site myomectomies. Gyne Robot Surg. 2020, 1, 14–20. [Google Scholar]
- Di Saverio, S. Emergency laparoscopy: A new emerging discipline for treating abdominal emergencies attempting to minimize costs and invasiveness and maximize outcomes and patients’ comfort. J. Trauma Acute Care Surg. 2014, 77, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.intuitive.com/en-us/-/media/Project/Intuitive-surgical/files/pdf (accessed on 19 September 2020).
- Pepin, K.; Dmello, M.; Sandberg, E.; Hill-Verrochi, C.; Maghsoudlou, P.; Ajao, M.; Cohen, S.L.; Einarsson, J.I. Reproductive outcomes following use of barbed suture during laparoscopic myomectomy. J. Minim. Invasive Gynecol. 2020, 27, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Degli Esposti, E.; Cristani, G.; Orsini, B.; Moro, E.; Raimondo, D.; Del Forno, S.; Lenzi, J.; Casadio, P.; Seracchioli, R. Comparison of fertility outcomes after laparoscopic myomectomy for barbed versus nonbarbed sutures. Fertil Steril 2021, 115, 248–255. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | LSOM (n = 160) | Non-LSOM (n = 177) | p-Value |
|---|---|---|---|
| Age (years, mean ± SD) | 37.23 ± 6.17 | 38.07 ± 5.89 | 0.21 |
| BMI (kg/m2, mean ± SD) | 23.03 ± 4.04 | 23.46 ± 4.20 | 0.86 |
| Parity (median (range)) | 0 (0–3) | 0 (0–3) | <0.001 |
| Gravidity (median (range)) | 0 (0–7) | 0 (0–6) | <0.001 |
| Previous pelvic surgery | |||
| Cesarean section, n (%) | 6 (3.8%) | 20 (11.4%) | 0.02 |
| Other pelvic surgery, n (%) | 20 (12.5%) | 24 (13.6%) | 0.90 |
| Outcomes | LSOM (n = 160) | Non-LSOM (n = 177) | p-Value |
|---|---|---|---|
| Type of main myoma * | 0.26 | ||
| Intramural (FIGO type 3, 4) | 114 (71.25) | 124 (70.05) | |
| Subserosal (FIGO type 5, 6) | 30 (18.75) | 34 (19.20) | |
| Pedunculated subserosal (FIGO type 7) | 1 (0.62) | 12 (14.12) | |
| Submucosal (FIGO type 0–2) | 6 (3.75) | 4 (2.25) | |
| Intraligamentary (FIGO type 8) | 7 (4.37) | 3 (1.69) | |
| Cervical (FIGO type 8) | 1 (0.62) | 0 | |
| Parasitic (FIGO type 8) | 1 (0.62) | 0 | |
| Maximal myoma diameter (cm, mean ± SD) | 9.20 ± 3.17 | 7.94 ± 2.49 | <0.001 |
| Number of removed myomas (median (range)) | 2 (1–34) | 2 (1–10) | <0.001 |
| Multiple myomas | 135 (84.4%) | 176 (99.4%) | <0.001 |
| Number of myomas >3 cm (median (range)) | 1 (1–10) | 1 (1–6) | <0.001 |
| Weight of the removed myomas (g, mean ± SD) † | 359.52 ± 313.44 | 241.88 ± 202.10 | <0.001 |
| Total operative time, (min, mean ± SD) ‡ | 141.43 ± 72.47 | 147.71 ± 58.06 | 0.38 |
| Concomitant surgery | 6 (3.8%) | 20 (11.4%) | 0.02 |
| Estimated blood loss (mL, mean ± SD) | 411.78 ± 806.64 | 241.16 ± 222.42 | 0.01 |
| Preoperative Hb (g/dL) | 12.64 ± 1.36 | 12.34 ± 1.40 | 0.04 |
| Postoperative Hb (g/dL) | 9.48 ± 1.66 | 9.56 ± 1.63 | 0.66 |
| Difference in Hb (g/dL) | 3.16 ± 1.69 | 2.78 ± 1.58 | 0.03 |
| Transfusion | 27 (25.8%) | 35 (35.1%) | 0.59 |
| Number of transfused packs (median (range)) | 0 (0–17) | 0 (0–5) | 0.42 |
| Length of hospital stay (days, mean ± SD) | 2 (2–22) | 3 (0–8) | <0.001 |
| Postoperative fever (within 48 h) | 21 (13.1%) | 12 (6.8%) | 0.08 |
| Factors | Coefficient (95% CI) | p Value |
|---|---|---|
| Age | −7.41 (−21.57~6.76) | 0.31 |
| BMI | 1.34 (−21.96~24.65) | 0.91 |
| History of pelvic surgery | −35.43 (−295.96~225.10) | 0.79 |
| Concomitant surgery | −102.01 (−428.03~224.01) | 0.54 |
| Multiple myomas | −152.35 (−485.80~181.09) | 0.37 |
| Maximal myoma diameter | −12.36 (−51.14~26.41) | 0.53 |
| Number of myomas | 6.24 (−21.32~33.81) | 0.66 |
| Number of myomas >3 cm | −13.58 (−90.24~63.07) | 0.73 |
| Weight of the removed myomas | 0.37 (−0.07~0.82) | 0.10 |
| Total operative time | 0.52 (−0.82~1.87) | 0.45 |
| Type of surgery * | 165.13 (−17.64~347.91) | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.R.; Lee, E.S.; Eum, H.R.; Lee, Y.-J.; Lee, S.-W.; Park, J.Y.; Suh, D.-S.; Kim, D.-Y.; Kim, S.H.; Kim, Y.-M.; et al. New Surgical Technique for Robotic Myomectomy: Continuous Locking Suture on Myoma (LSOM) Technique. J. Clin. Med. 2021, 10, 654. https://doi.org/10.3390/jcm10040654
Lee SR, Lee ES, Eum HR, Lee Y-J, Lee S-W, Park JY, Suh D-S, Kim D-Y, Kim SH, Kim Y-M, et al. New Surgical Technique for Robotic Myomectomy: Continuous Locking Suture on Myoma (LSOM) Technique. Journal of Clinical Medicine. 2021; 10(4):654. https://doi.org/10.3390/jcm10040654
Chicago/Turabian StyleLee, Sa Ra, Eun Sil Lee, Hye Rim Eum, Young-Jae Lee, Shin-Wha Lee, Jeong Yeol Park, Dae-Shik Suh, Dae-Yeon Kim, Sung Hoon Kim, Yong-Man Kim, and et al. 2021. "New Surgical Technique for Robotic Myomectomy: Continuous Locking Suture on Myoma (LSOM) Technique" Journal of Clinical Medicine 10, no. 4: 654. https://doi.org/10.3390/jcm10040654
APA StyleLee, S. R., Lee, E. S., Eum, H. R., Lee, Y.-J., Lee, S.-W., Park, J. Y., Suh, D.-S., Kim, D.-Y., Kim, S. H., Kim, Y.-M., & Kim, Y.-T. (2021). New Surgical Technique for Robotic Myomectomy: Continuous Locking Suture on Myoma (LSOM) Technique. Journal of Clinical Medicine, 10(4), 654. https://doi.org/10.3390/jcm10040654






