Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Details
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Quantitative Synthesis
3. Results
3.1. Search Results
3.2. Characteristics of Included Studies
3.3. Quality Assessment
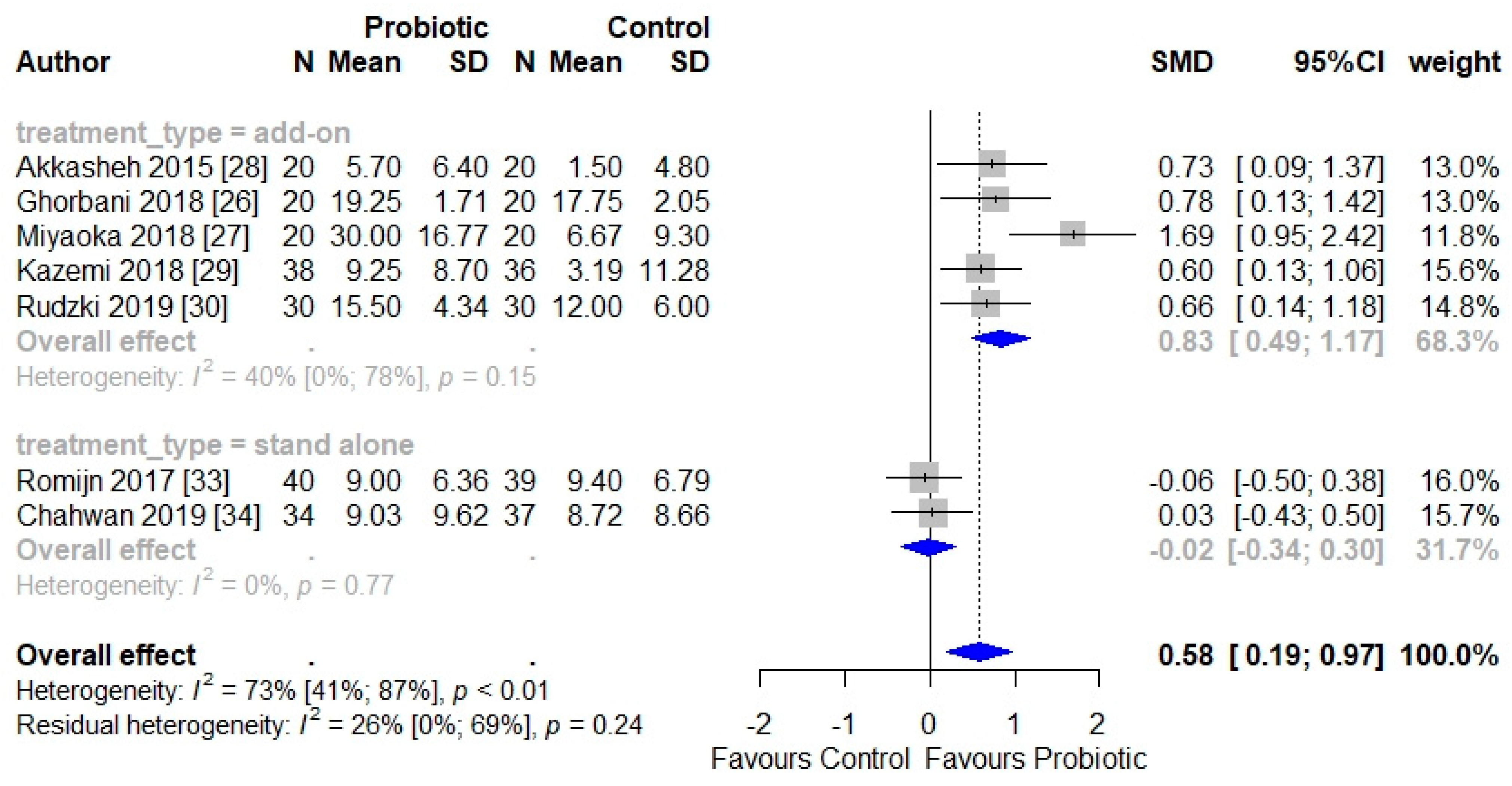
3.4. Efficacy of Probiotics for the Treatment of Depressive Symptoms
3.5. Probiotics and Biomarkers of Depression
3.6. Tolerability and Compliance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolova, V.L.; Zaidi, S.Y.; Young, A.H.; Cleare, A.J.; Stone, J.M. Gut feeling: Randomized controlled trials of probiotics for the treatment of clinical depression: Systematic review and meta-analysis. Ther. Adv. Psychopharmacol. 2019, 9, 204512531985996. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; David, C.M.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Primers 2016, 2, 1–20. [Google Scholar] [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Fava, M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 2003, 53, 649–659. [Google Scholar] [CrossRef]
- Strawbridge, R.; Young, A.H.; Cleare, A.J. Biomarkers for depression: Recent insights, current challenges and future prospects. Neuropsychiatr. Dis. Treat. 2017, 13, 1245–1262. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol. Motil. 2013, 25, 713–719. [Google Scholar] [CrossRef]
- Cheung, S.G.; Goldenthal, A.R.; Uhlemann, A.-C.; Mann, J.J.; Miller, J.M.; Sublette, M.E. Systematic Review of Gut Microbiota and Major Depression. Front. Psychiatry 2019, 10, 34. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizaw, Y.; Salas-Valer, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and me-ta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Evans, S.J.; Bassis, C.M.; Hein, R.; Assari, S.; Flowers, S.A.; Kelly, M.B.; Young, V.B.; Ellingrod, V.E.; McInnis, M.G. The gut microbiome composition associates with bipolar disorder and illness severity. J. Psychiatr. Res. 2017, 87, 23–29. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef]
- Rios, A.C.; Maurya, P.K.; Pedrini, M.; Zeni-Graiff, M.; Asevedo, E.; Mansur, R.B.; Wieck, A.; Grassi-Oliveira, R.; McIntyre, R.S.; Hayashi, M.A.F.; et al. Microbiota abnormalities and the therapeutic potential of probiotics in the treatment of mood disorders. Rev. Neurosci. 2017, 28, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef]
- Smith, K.; Greene, M.W.; Babu, J.R.; Frugé, A.D. Psychobiotics as treatment for anxiety, depression, and related symptoms: A systematic review. Nutr. Neurosci. 2019, 1–15. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Maleki, V.; Behrooz, M.; Ranjbar, F.; Ebrahimi-Mameghani, M. Can psychobiotics ‘mood’ ify gut? An update systematic review of ran-domized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin. Nutr. Edinb. Scotl. 2020, 39, 1395–1410. [Google Scholar] [CrossRef]
- Amirani, E.; Milajerdi, A.; Mirzaei, H.; Jamilian, H.; Mansournia, M.A.; Hallajzadeh, J.; Ghaderi, A. The effects of probiotic supplementation on mental health, biomarkers of inflam-mation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 49, 102361. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cumpston, M.; Changler, J. Chapter IV: Updating a review. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.1; updated September 2020; Cochrane: London, UK; Available online: https://training.cochrane.org/handbook/current/chapter-iv (accessed on 17 December 2020).
- Garner, P.; Hopewell, S.; Chandler, J.; MacLehose, H.; Schünemann, H.J.; Akl, E.; Beyene, J.; Chang, S.; Churchill, R.; Dearness, K.; et al. When and how to update systematic reviews: Consensus and checklist. BMJ 2016, 354, i3507. [Google Scholar] [CrossRef]
- Strawbridge, R.; Carter, B.; Marwood, L.; Bandelow, B.; Tsapekos, D.; Nikolova, V.L.; Taylor, R.; Mantingh, T.; De Angel, V.; Patrick, F.; et al. Augmentation therapies for treatment-resistant depression: Systematic review and meta-analysis. Br. J. Psychiatry 2018, 214, 42–51. [Google Scholar] [CrossRef]
- Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: http://kcl.eblib.com/patron/FullRecord.aspx?p=366838 (accessed on 17 December 2020).
- Hirschberg, R.; Cohen, A.H.; Kopple, J.D. Effects of Keto Acid Supplements on Renal Function and Histology in Azotemic Rats Fed High-Protein Diets. Am. J. Nephrol. 1988, 8, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Stovold, E.M.; Beecher, D.; Foxlee, R.; Noel-Storr, A. Study flow diagrams in Cochrane systematic review updates: An adapted PRISMA flow diagram. Syst. Rev. 2014, 3, 54. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Nazari, S.; Etesam, F.; Nourimajd, S.; Ahmadpanah, M.; Jahromi, S.R.M. The Effect of Synbiotic as an Adjuvant Therapy to Fluoxetine in Moderate Depression: A Randomized Multicenter Trial. Arch. Neurosci. 2018, 5, e60507. [Google Scholar] [CrossRef]
- Miyaoka, T.; Kanayama, M.; Wake, R.; Hashioka, S.; Hayashida, M.; Nagahama, M.; Horiguchi, J. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin. Neuropharmacol. 2018, 41, 151–155. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Furukawa, T.A.; Reijnders, M.; Kishimoto, S.; Sakata, M.; DeRubeis, R.J.; Dimidjian, S.; Dozois, D.J.; Hegerl, U.; Hollon, S.D.; Jarrett, R.B.; et al. Translating the BDI and BDI-II into the HAMD and vice versa with equipercentile linking. Epidemiol. Psychiatr. Sci. 2019, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Svanborg, P.; Åsberg, M. A comparison between the Beck Depression Inventory (BDI) and the self-rating version of the Montgomery Åsberg Depression Rating Scale (MADRS). J. Affect. Disord. 2001, 64, 203–216. [Google Scholar] [CrossRef]
- Romijn, A.R.; Rucklidge, J.J.; Kuijer, R.G.; Frampton, C. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust. N. Z. J. Psychiatry 2017, 51, 810–821. [Google Scholar] [CrossRef]
- Chahwan, B.; Kwan, S.; Isik, A.; Van Hemert, S.; Burke, C.; Roberts, L. Gut feelings: A randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J. Affect. Disord. 2019, 253, 317–326. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Djafarian, K. Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: A double-blind, placebo-controlled randomized clinical trial. J. Funct. Foods 2019, 52, 596–602. [Google Scholar] [CrossRef]
- Heidarzadeh-Rad, N.; Gökmen-Özel, H.; Kazemi, A.; Almasi, N.; Djafarian, K. Effects of a Psychobiotic Supplement on Serum Brain-derived Neu-rotrophic Factor Levels in Depressive Patients: A Post Hoc Analysis of a Randomized Clinical Trial. J. Neurogastroenterol. Motil. 2020, 26, 486–495. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Djafarian, K. Effect of probiotic and prebiotic versus placebo on appetite in patients with major depressive disorder: Post hoc analysis of a randomised clinical trial. J. Hum. Nutr. Diet. 2019, 33, 56–65. [Google Scholar] [CrossRef]
- Chait, Y.A.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef]
- Husain, M.I.; Chaudhry, I.B.; Husain, N.; Khoso, A.B.; Rahman, R.R.; Hamirani, M.M.; Hodsoll, J.; Qurashi, I.; Deakin, J.F.; Young, A.H. Minocycline as an adjunct for treatment-resistant depressive symptoms: A pilot randomised placebo-controlled trial. J. Psychopharmacol. 2017, 31, 1166–1175. [Google Scholar] [CrossRef]
- Husain, M.I.; Cullen, C.; Umer, M.; Carvalho, A.F.; Kloiber, S.; Meyer, J.H.; Ortiz, A.; Knyahnytska, Y.; Husain, M.O.; Giddens, J.; et al. Minocycline as adjunctive treatment for treatment-resistant depression: Study protocol for a double blind, placebo-controlled, randomized trial (MINDEP2). BMC Psychiatry 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Macedo, D.S.; Filho, A.J.M.C.; De Sousa, C.N.S.; Quevedo, J.; Barichello, T.; Júnior, H.V.N.; De Lucena, D.F. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J. Affect. Disord. 2017, 208, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.M.; Greene, M.T.; Young, V.B.; Saint, S.; Langa, K.M.; Kao, J.Y.; Aronoff, D.M. Depression, antidepressant medications, and risk of Clostridium difficileinfection. BMC Med. 2013, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Evans, S.J.; Ward, K.M.; McInnis, M.G.; Ellingrod, V.L. Interaction Between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2017, 37, 261–267. [Google Scholar] [CrossRef]
- Getachew, B.; Aubee, J.I.; Schottenfeld, R.S.; Csoka, A.B.; Thompson, K.M.; Tizabi, Y. Ketamine interactions with gut-microbiota in rats: Relevance to its antidepressant and anti-inflammatory properties. BMC Microbiol. 2018, 18, 222. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Fava, M.; Graves, L.M.; Benazzi, F.; Scalia, M.J.; Iosifescu, D.V.; Alpert, J.E.; Papakostas, G.I. A Cross-Sectional Study of the Prevalence of Cognitive and Physical Symptoms During Long-Term Antidepressant Treatment. J. Clin. Psychiatry 2006, 67, 1754–1759. [Google Scholar] [CrossRef]
- Yee, A.; Chin, S.C.; Hashim, A.H.B.; Singh, M.K.A.H.; Loh, H.S.; Sulaiman, A.H.; Ng, C.G. Anhedonia in depressed patients on treatment with selective serotonin reuptake inhibitor anti-depressant—A two-centered study in Malaysia. Int. J. Psychiatry Clin. Pr. 2015, 19, 182–187. [Google Scholar] [CrossRef]
- Bair, M.J.; Robinson, R.L.; Eckert, G.J.; Stang, P.E.; Croghan, T.W.; Kroenke, K. Impact of Pain on Depression Treatment Response in Primary Care. Psychosom. Med. 2004, 66, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Shilyansky, C.; Williams, L.M.; Gyurak, A.; Harris, A.; Usherwood, T.; Etkin, A. Effect of antidepressant treatment on cognitive impairments associated with depression: A randomised longitudinal study. Lancet Psychiatry 2016, 3, 425–435. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Niciu, M.J.; Richards, E.M.; Zarate, C.A. Pharmacologic Treatment of Dimensional Anxious Depression. Prim. Care Companion CNS Disord. 2014, 16, PCC.13r01621. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive Mechanisms of Probiotics and Their Therapeutic Potential. Front. Neurosci. 2020, 13, 1361. [Google Scholar] [CrossRef] [PubMed]
- Demyttenaere, K.; Fruyt, J.D. Getting What You Ask For: On the Selectivity of Depression Rating Scales. Psychother. Psychosom. 2003, 72, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; MacQueen, G.M.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef]
- O’Sullivan, E.; Barrett, E.; Grenham, S.; Fitzgerald, P.; Stanton, C.; Ross, R.P.; Quigley, E.M.M.; Cryan, J.F.; Dinan, T.G. BDNF expression in the hippocampus of maternally separated rats: Does Bifidobacterium breve 6330 alter BDNF levels? Benef. Microbes 2011, 2, 199–207. [Google Scholar] [CrossRef]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A Randomized Controlled Trial of the Tumor Necrosis Factor Antagonist Infliximab for Treatment-Resistant Depression: The Role of Baseline Inflammatory Biomarkers. JAMA Psychiatry 2013, 70, 31. [Google Scholar] [CrossRef] [PubMed]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; De Jonge, P.; Beekman, A.T.F.; Penninx, B.W.J.H. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 2012, 18, 692–699. [Google Scholar] [CrossRef] [PubMed]

| Study (Year) | Country | Sample | Intervention | Primary | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population, Definition | Severity | Sample Size | Avg. Age, % Female | Type | Duration | Probiotic (CFU/g)/ Dose | Control/ Blinding | Outcome Measure | ||
| Akkasheh (2015) [28] | Iran | MDD, DSM-IV | At least moderate | 40 | 37 years (y) 85% | Add-on to SSRI (citalopram) | 8 weeks | L. acidophilus (2 × 109) L. casei (2 × 109), B. bifidum (2 × 109) 1 capsule daily | Placebo Double-blind | BDI |
| Romijn (2017) [33] | New Zealand | Depressive symptoms, QIDS-SR | At least moderate | 79 | 35 y 79% | Standalone | 8 weeks | L. helveticus + B. longum (≥2 × 109) 1.5 g daily | Placebo Double-blind | MADRS |
| Kazemi (2019) [29] | Iran | MDD, ICD-10 | Mild-moderate | 74 | 36 y 71% | Add-on to SSRI or amitriptiline | 8 weeks | L. helveticus + B. longum (≥2 × 109) 5 g daily | Placebo Double-blind | BDI |
| Ghorbani (2018) [26] | Iran | MDD, DSM-5 | Moderate | 40 | 35 y 70% | Add-on to SSRI (fluoxetine) | 6 weeks | MS probiotic 1, 500 mg + 100 mg prebiotic 1 capsule daily | Placebo Double-blind | HAMD-17 |
| Miyaoka (2018) [27] | Japan | TRD, DSM-IV-TR | At least moderate | 40 | 43 y 52% | Add-on to SSRI or SNRI | 8 weeks | C. butyricum 60 mg daily | TAU Open label | BDI |
| Rudzki (2019) [30] | Poland | MDD, DSM-IV-TR | Moderate | 60 | 39 y 71% | Add-on to SSRI | 8 weeks | L. plantarum (10 × 109) 2 capsules daily | Placebo Double-blind | HAMD-17 |
| Chahwan (2019) [34] | Australia | Depressive symptoms, BDI | Mild–severe | 71 | 36 y 69% | Standalone | 8 weeks | MS probiotic 2 (2.5 × 109), 4 g daily | Placebo Triple-blind | BDI |
| Parameter Assessed | Study | Outcome |
|---|---|---|
| Inflammatory markers (IL-1β, IL-6, TNF-α) | Romijn (2017) [33] | No significant differences. |
| Kazemi (2019) [35] | No significant differences after adjustment for multiple covariates. | |
| Rudzki (2019) [30] | No significant differences. | |
| CRP | Akkasheh (2015) [28] | Significantly reduced in the probiotic group compared to placebo post-treatment. |
| Romijn (2017) [33] | No significant difference. | |
| BDNF | Romijn (2017) [33] | No significant difference. |
| Kazemi (2019) [36] | Significantly increased in the probiotic group compared to placebo post-treatment. This was significantly correlated with reduction in depressive scores. | |
| Tryptophane/kynurenine ratio | Kazemi (2019) [29] | Tryptophan/kynurenine ratio significantly reduced in the probiotic group compared to placebo post-treatment. |
| Rudzki (2019) [30] | No significant difference. | |
| Cortisol (urinary) (plasma) | Kazemi (2019) [35] | Not statistically significant, but potentially clinically relevant reduction in the probiotic group compared to placebo post-treatment |
| Rudzki (2019) [30] | No significant difference. | |
| Metabolic markers (insulin, FPG, lipids, cholesterol) | Akkasheh (2015) [28] | Serum insulin and insulin resistance were significantly reduced in the probiotic group compared to the placebo group post-treatment. |
| Oxidative stress (tac, gsh) | Akkasheh (2015) [28] | GSH levels significantly increased in the probiotic group compared to the placebo group post-treatment. |
| Kynurenine | Rudzki (2019) [30] | Kynurenine significantly decreased in the probiotic group compared to the placebo group post-treatment. |
| Tryptophan | Rudzki (2019) [30] | No significant difference. |
| Other kynurenine ratios | Rudzki (2019) [30] | 3HKYN/KYN ratio significantly decreased in the probiotic group compared to the placebo group post-treatment. |
| Other tryptophan ratios | Kazemi (2019) [29] | Tryptophan/isoleucine ratio significantly reduced in the probiotic group compared to the placebo group post-treatment. |
| Leptin | Kazemi (2019) [37] | No significant difference (p = 0.07). |
| Vitamin D | Romijn (2017) [33] | No significant difference. |
| Gut microbiota (diversity and abundance) | Chahwan (2019) [34] | No significant differences. |
| Study | Drop-Out Rate (n/Total n) | Adherence % (Doses Taken) | Adverse Events n * | ||
|---|---|---|---|---|---|
| Probiotic | Control | Probiotic | Control | ||
| Akkasheh (2015) [28] | 3/20 | 2/20 | 90% | nr | nr |
| Romijn (2017) [33] | 7/40 | 3/39 | 97% | 77 | 91 |
| Kazemi (2019) [29] | 10/38 | 10/36 | 92% | 10 | 1 |
| Ghorbani (2018) [26] | 0/20 | 0/20 | Not mentioned | 13 | 3 |
| Miyaoka (2018) [27] | 0/20 | 0/20 | Not mentioned | 3 | 3 |
| Rudzki (2019) [30] | 10/40 | 9/39 | Monitored, nr | 5 | 7 |
| Chahwan (2019) [34] | 11/34 | 13/37 | Monitored, nr | 65 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment. J. Clin. Med. 2021, 10, 647. https://doi.org/10.3390/jcm10040647
Nikolova VL, Cleare AJ, Young AH, Stone JM. Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment. Journal of Clinical Medicine. 2021; 10(4):647. https://doi.org/10.3390/jcm10040647
Chicago/Turabian StyleNikolova, Viktoriya L., Anthony J. Cleare, Allan H. Young, and James M. Stone. 2021. "Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment" Journal of Clinical Medicine 10, no. 4: 647. https://doi.org/10.3390/jcm10040647
APA StyleNikolova, V. L., Cleare, A. J., Young, A. H., & Stone, J. M. (2021). Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment. Journal of Clinical Medicine, 10(4), 647. https://doi.org/10.3390/jcm10040647








