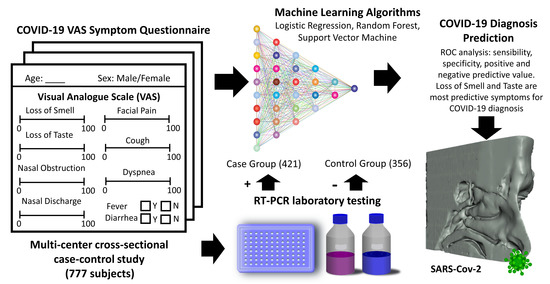
Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Study Variables
2.3. Data Analysis and Statistical Methods
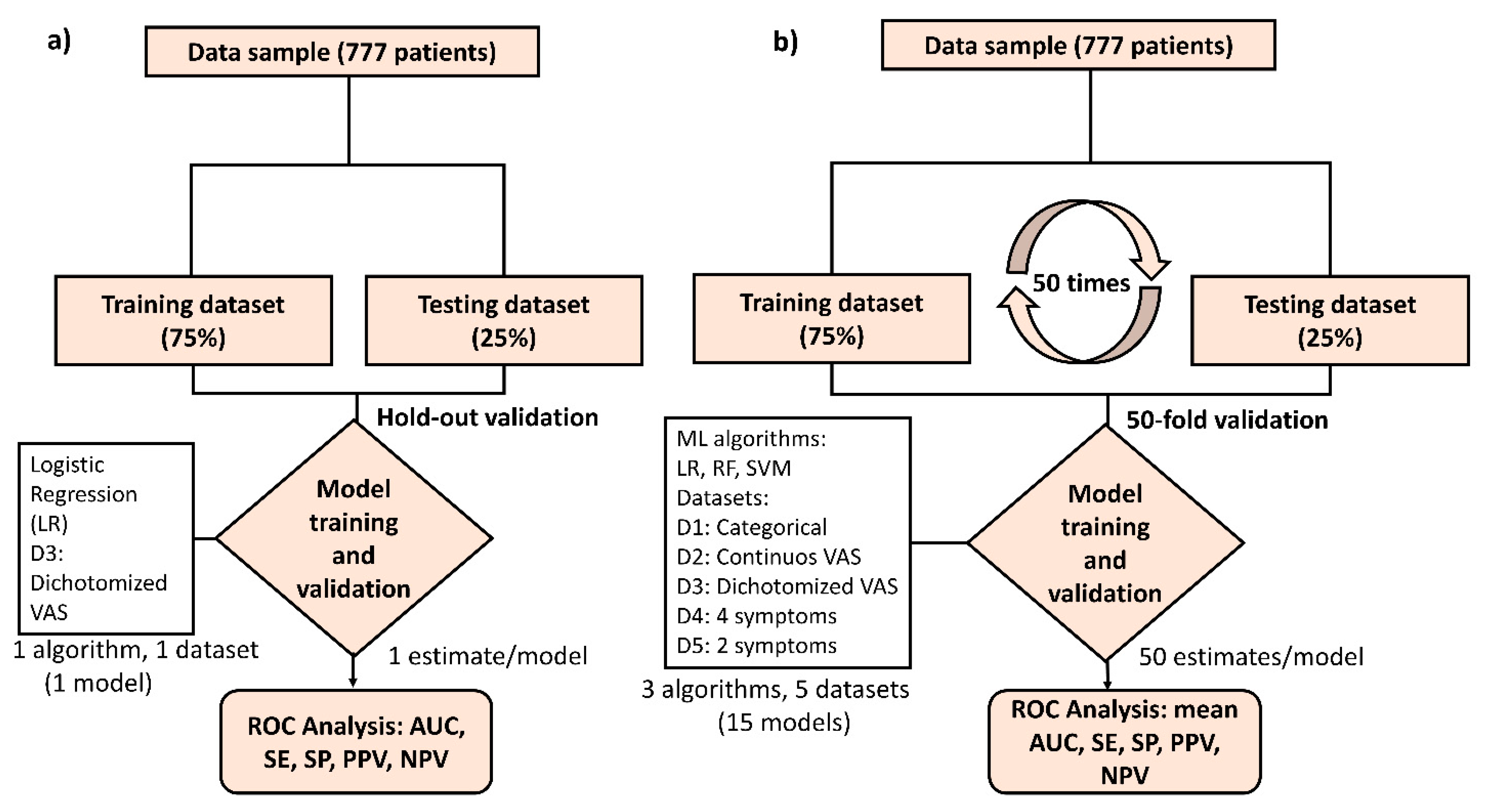
2.4. Machine Leaning (ML) Approach to Predict a COVID-19 Diagnosis
3. Results
3.1. Demographic Characteristics of the Sample
3.2. Prevalence and Intensity of Symptoms between Cases and Controls
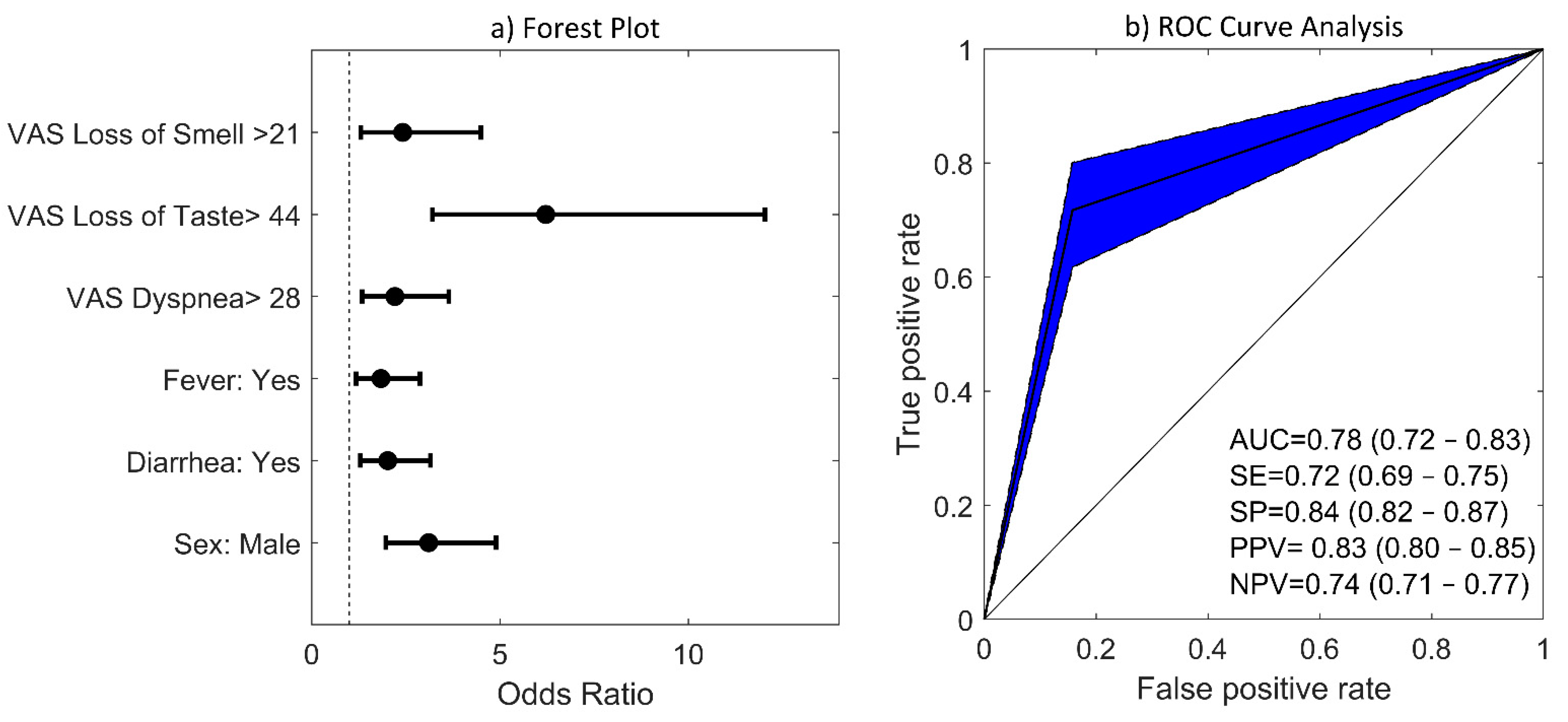
3.3. VAS Cut-off Points That Optimally Predicted COVID-19 Diagnosis
3.4. Multivariate Logistic Regression (LR) Model
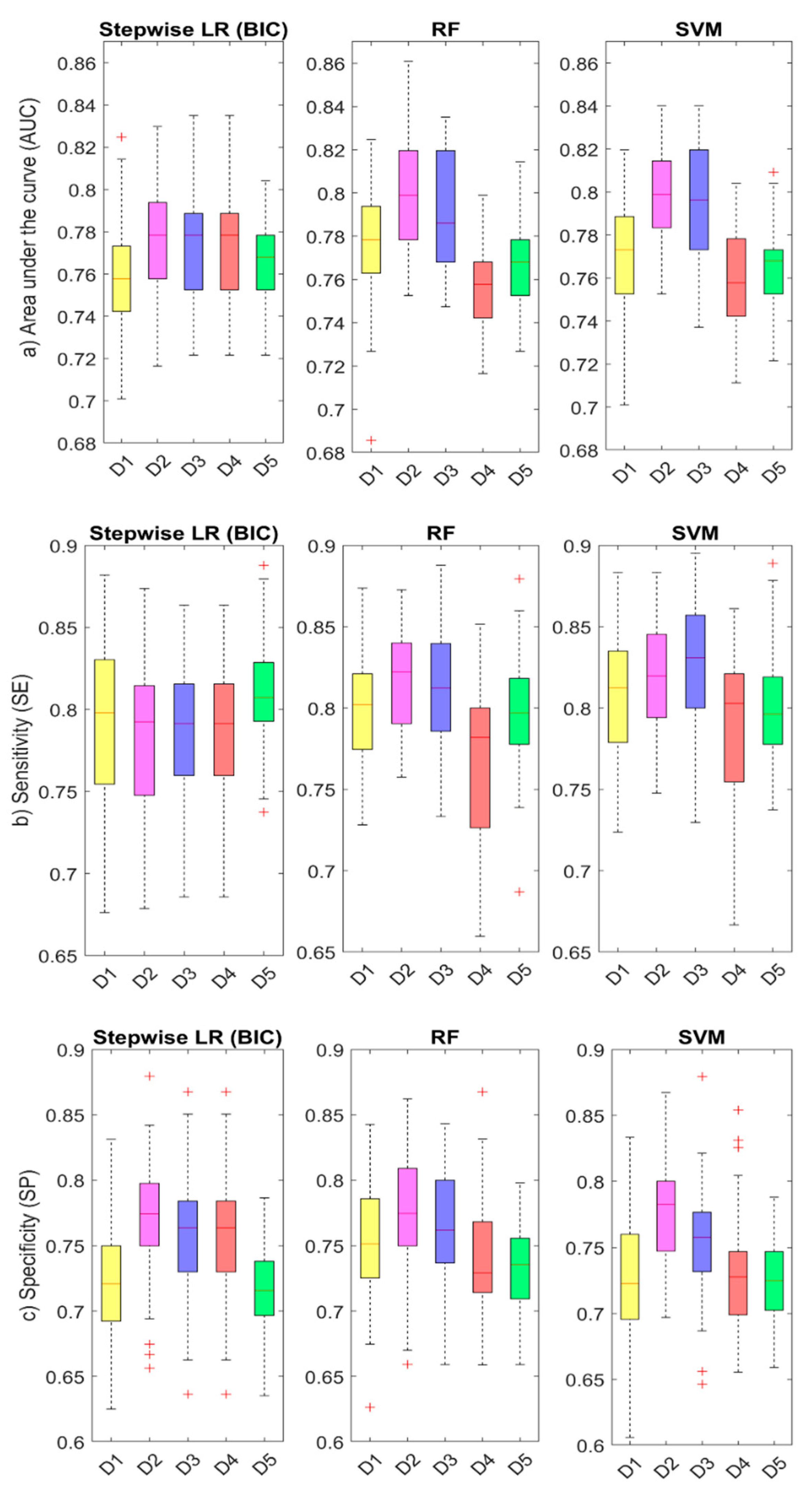
3.5. ML Results: Comparison of Accuracy between Algorithms and Model Datasets
4. Discussion
4.1. Prevalence of Smell and Taste Disorders in COVID-19 Subjects
4.2. Predictive Value of Smell and Taste Disorders in the COVID-19 Disease
4.3. Prediction Models for COVID-19 Diagnosis Based on Smell and Taste Disorders
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.W.; Chee, J.; Subramaniam, S.; Ng, C.L. Frequency and Clinical Utility of Olfactory Dysfunction in COVID-19: A System-atic Review and Meta-analysis. Curr. Allergy Asthma Rep. 2020, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Alobid, I.; Mariño-Sánchez, F.; Izquierdo-Domínguez, A.; Marin, C.; Klimek, L.; Wang, D.-Y.; Liu, Z. The Loss of Smell and Taste in the COVID-19 Outbreak: A Tale of Many Countries. Curr. Allergy Asthma Rep. 2020, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rocke, J.; Hopkins, C.; Philpott, C.M.; Kumar, N. Is loss of sense of smell a diagnostic marker in COVID-19: A systematic review and meta-analysis. Clin. Otolaryngol. 2020, 45, 914–922. [Google Scholar] [CrossRef]
- Izquierdo-Dominguez, A.; Rojas-Lechuga, M.J.; Mullol, J.; Alobid, I. Olfactory Dysfunction in the COVID-19 Outbreak. J. Investig. Allergol. Clin. Immunol. 2020, 30, 317–326. [Google Scholar] [CrossRef]
- Bagheri, S.H.; Asghari, A.; Farhadi, M.; Shamshiri, A.R.; Kabir, A.; Kamrava, S.K.; Jalessi, M.; Mohebbi, A.; Alizadeh, R.; Honarmand, A.A.; et al. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med. J. Islam. Repub. Iran 2020, 34, 62. [Google Scholar]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto Rhino Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Freidin, M.B.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Ganesh, S.; Varsavsky, T.; Cardoso, M.J.; Moustafa, J.S.E.-S.; et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020, 26, 1037–1040. [Google Scholar] [CrossRef]
- Roland, L.T.; Ii, J.G.G.; Loftus, P.A.; Cheung, S.W.; Chang, J.L. Smell and taste symptom-based predictive model for COVID-19 diagnosis. Int. Forum Allergy Rhinol. 2020, 10, 832–838. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Hagen, M.M.; Butowt, R. Prevalence of Chemosensory Dysfunction in COVID-19 Patients: A Systematic Re-view and Meta-analysis Reveals Significant Ethnic Differences. ACS Chem. Neurosci. 2020 11, 2944–2961.
- Beltrán-Corbellini, Á.; Chico-García, J.L.; Martínez-Poles, J.; Rodríguez-Jorge, F.; Natera-Villalba, E.; Gómez-Corral, J.; Gómez-López, A.; Monreal, E.; Parra-Díaz, P.; Cortés-Cuevas, J.L.; et al. Acute-onset smell and taste disorders in the context of COVID-19: A pilot multicentre polymerase chain reaction based case–control study. Eur. J. Neurol. 2020, 27, 1738–1741. [Google Scholar] [CrossRef]
- Villarreal, I.M.; Morato, M.; Martínez-RuizCoello, M.; Navarro, A.; Garcia-Chillerón, R.; Ruiz, Á.; De Almeida, I.V.; Mazón, L.; Plaza, G. Olfactory and taste disorders in healthcare workers with COVID-19 infection. Eur. Arch. Oto-Rhino-Laryngol. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Clemency, B.M.; Varughese, R.; Do, D.K.S.; Ludwig, B.; Welch, J.V.; McCormack, R.F.; Ma, C.; Nan, N.; Rn, T.G.; Raab, T. Symptom Criteria for COVID-19 Testing of Heath Care Workers. Acad. Emerg. Med. 2020, 27, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Gerkin, R.C.; Ohla, K.; Veldhuizen, M.G.; Joseph, P.V.; Kelly, C.; Bakke, A.J.; Steele, K.; Farruggia, M.C.; Pellegrino, R.; Pepino, M.Y.; et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem. Senses 2020. [Google Scholar] [CrossRef]
- Pierron, D.; Pereda-Loth, V.; Mantel, M.; Moranges, M.; Bignon, E.; Alva, O.; Kabous, J.; Heiske, M.; Pacalon, J.; David, R.; et al. Smell and taste changes are early indicators of the COVID-19 pandemic and political decision effectiveness. Nat. Commun. 2020, 11, 5152. [Google Scholar] [CrossRef]
- Ramakrishnan, V.R.; Arbet, J.; Mace, J.C.; Smith, S.S.; Soler, Z.M.; Smith, T.L. Predicting Olfactory Loss In Chronic Rhinosinusitis Using Machine Learning. medRxiv 2020. [Google Scholar]
- Yadaw, A.S.; Li, Y.-C.; Bose, S.; Iyengar, R.; Bunyavanich, S.; Pandey, G. Clinical features of COVID-19 mortality: Development and validation of a clinical prediction model. Lancet Digit. Health 2020, 2, e516–e525. [Google Scholar] [CrossRef]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Yan, C.H.; Faraji, F.; Prajapati, D.; Boone, C.E.; DeConde, A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef]
- Izquierdo-Domínguez, A.; Rojas-Lechuga, M.J.; Chiesa-Estomba, C.; Calvo-Henríquez, C.; Ninchritz-Becerra, E.; Soriano-Reixach, M.; Poletti-Serafini, D.; Villarreal, I.M.; Maza-Solano, J.M.; Moreno-Luna, R.; et al. Smell and Taste Dysfunction in COVID-19 Is Associated With Younger Age in Ambulatory Settings: A Multicenter Cross-Sectional Study. J. Investig. Allergol. Clin. Immunol. 2020, 30, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Hopkins, C.; Philips, V.; Obholzer, R.; Tirelli, G.; Polesel, J.; Calvanese, L.; Boscolo-Rizzo, P. Self-reported alteration of sense of smell or taste in patients with COVID-19: A systematic review and meta-analysis on 3563 patients. Rhinol. J. 2020, 58, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Larco, R.M.; Altez-Fernandez, C. Anosmia and dysgeusia in COVID-19: A systematic review. Wellcome Open Res. 2020, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.V.T.d.; Carnaúba, A.T.L.; Rocha, K.W.; Andrade, K.C.L.d.; Ferreira, S.M.S.; Menezes, P.d.L. Olfactory and taste disorders in COVID-19: A systematic review. Braz. J. Otorhinolaryngol. 2020, 86, 781–792. [Google Scholar] [CrossRef]
- Mercante, G.; Ferreli, F.; De Virgilio, A.; Gaino, F.; Di Bari, M.; Colombo, G.; Russo, E.; Costantino, A.; Pirola, F.; Cugini, G.; et al. Prevalence of Taste and Smell Dysfunction in Coronavirus Disease 2019. JAMA Otolaryngol. Neck Surg. 2020, 146, 723. [Google Scholar] [CrossRef]
- Lovato, A.; De Filippis, C. Clinical Presentation of COVID-19: A Systematic Review Focusing on Upper Airway Symptoms. Ear, Nose Throat J. 2020, 99, 569–576. [Google Scholar] [CrossRef]
- Zhou, Z.; Kang, H.; Li, S.; Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: From neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020, 267, 2179–2184. [Google Scholar] [CrossRef]
- Dubé, M.; Coupanec, A.L.; Wong, A.H.M.; Rini, J.M.; Desforges, M.; Talbot, P.J. Axonal Transport Enables Neuron-to-Neuron Propa-gation of Human Coronavirus OC43. J. Virol. 2018, 92(17), 1–21. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Athmer, J.; Meyerholz, D.K.; Perlman, S. Murine Olfactory Bulb Interneurons Survive Infection with a Neuro-tropic Coronavirus. J. Virol. 2017, 91, 1–10. [Google Scholar] [CrossRef]
- Chen, M.; Shen, W.; Rowan, N.R.; Kulaga, H.; Hillel, A.; Ramanathan, M.; Lane, A.P. Elevated ACE-2 expression in the olfactory neuroepithelium: Implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020, 56, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, T.; Yang, N.; Han, D.; Mi, X.; Li, Y.; Liu, K.; Vuylsteke, A.; Xiang, H.; Guo, X. Neurological manifestations of patients with COVID-19: Potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front. Med. 2020, 14, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of Ocular Findings of Patients With Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Nur, F.; Chainani-Wu, N.; Fortuna, G.; Sroussi, H. Dysgeusia in COVID-19: Possible Mechanisms and Implications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Kowall, B.; Nonnemacher, M.; Brune, B.; Brinkmann, M.; Dudda, M.; Böttcher, J.; Schmidt, B.; Standl, F.; Stolpe, S.; Dittmer, U.; et al. A model to identify individuals with a high probability of a SARS-CoV-2 infection. J. Infect. 2020. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7686708/ (accessed on 6 December 2020). [CrossRef] [PubMed]
- Wynants, L.; Van Calster, B.; Collins, G.S.; Riley, R.D.; Heinze, G.; Schuit, E.; Bonten, M.M.J.; Dahly, D.L.; Damen, J.; Debray, T.P.; et al. Prediction models for diagnosis and prognosis of covid-19: Systematic review and critical appraisal. BMJ 2020, 369, m1328. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.; Jr, F.E.H.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.S.; Van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- Haller, D.M.; Sebo, P.; Tudrej, B.; Maisonneuve, H. Is a COVID-19 prediction model based on symptom tracking through an app applicable in primary care? Fam. Pract. 2020, 37. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7454492/ (accessed on 23 December 2020). [CrossRef] [PubMed]
- Barra, G.B.; Santa Rita, T.H.; Mesquita, P.G.; Jácomo, R.H.; Nery, L.F.A. Analytical Sensitivity and Specificity of Two RT-qPCR Protocols for SARS-CoV-2 Detection Performed in an Automated Workflow. Genes 2020, 11, 1183. [Google Scholar] [CrossRef]

| Symptom | Total (n = 777) | Cases (n = 421) | Controls (n = 356) | Odds Ratio | CI 95% | p-Value |
|---|---|---|---|---|---|---|
| Loss of smell | 312 (40%) | 257 (61%) | 55 (15%) | 8.58 | [6.06, 12.14] | <0.0001 |
| Loss of taste | 332 (43%) | 266 (63%) | 66 (19%) | 7.54 | [5.41, 10.52] | <0.0001 |
| Nasal obstruction | 262 (34%) | 153 (36%) | 109 (31%) | 1.29 | [0.95, 1.74] | 0.0946 |
| Nasal Discharge | 378 (49%) | 210 (50%) | 168 (47%) | 1.11 | [0.84, 1.47] | 0.4718 |
| Facial Pain | 161 (21%) | 114 (27%) | 47 (13%) | 2.44 | [1.67, 3.55] | 0.6173 |
| Cough | 471 (61%) | 307 (73%) | 164 (46%) | 3.15 | [2.34, 4.25] | <0.001 |
| Dyspnea | 260 (33%) | 182 (43%) | 78 (22%) | 2.71 | [1.98, 3.73] | <0.001 |
| Fever | 339 (44%) | 258 (61%) | 81 (23%) | 5.37 | [3.92, 7.37] | <0.001 |
| Diarrhea | 299 (39%) | 220 (53%) | 79 (22%) | 3.84 | [2.80, 5.26] | 0.0011 |
| OR | CI 95% | p-Value | VAS Cutoff Points | AUC | Sensitivity | Specificity | ||
|---|---|---|---|---|---|---|---|---|
| Loss of smell | 10.85 | 7.47 | 15.77 | <0.0001 | 21 | 0.76 | 0.60 | 0.88 |
| Loss of taste | 12.62 | 8.50 | 18.73 | <0.0001 | 44 | 0.76 | 0.59 | 0.90 |
| Nasal obstruction | 1.75 | 1.19 | 2.56 | 0.0046 | 52 | 0.53 | 0.21 | 0.87 |
| Nasal discharge | 1.46 | 1.06 | 2.02 | 0.0232 | 41 | 0.52 | 0.30 | 0.77 |
| Facial pain | 2.54 | 1.73 | 3.74 | <0.0001 | 15 | 0.57 | 0.26 | 0.88 |
| Cough | 3.19 | 2.36 | 4.31 | <0.0001 | 3 | 0.65 | 0.73 | 0.54 |
| Dyspnea | 3.06 | 2.16 | 4.33 | <0.0001 | 28 | 0.61 | 0.36 | 0.84 |
| OR | CI 95% | p-Value | ||
|---|---|---|---|---|
| Loss of smell | 2.42 | 1.30 | 4.50 | 0.0053 |
| Loss of taste | 6.21 | 3.21 | 12.04 | <0.001 |
| Dyspnea | 2.21 | 1.34 | 3.64 | 0.002 |
| Fever | 1.84 | 1.18 | 2.87 | 0.007 |
| Diarrhea | 2.02 | 1.29 | 3.16 | 0.002 |
| Sex | 3.11 | 1.97 | 4.90 | <0.001 |
| AUC | SE | SP | PPV | NPV | ||
|---|---|---|---|---|---|---|
Dataset 1 (11 predictors)
| LR | 0.759 | 0.792 | 0.722 | 0.772 | 0.747 |
| RF | 0.777 | 0.798 | 0.752 | 0.792 | 0.758 | |
| SVM | 0.771 | 0.811 | 0.724 | 0.777 | 0.764 | |
Dataset 2 (11 predictors)
| LR | 0.777 | 0.783 | 0.771 | 0.803 | 0.751 |
| RF | 0.798 | 0.818 | 0.775 | 0.812 | 0.782 | |
| SVM | 0.799 | 0.818 | 0.778 | 0.814 | 0.783 | |
Dataset 3 (11 predictors)
| LR | 0.773 | 0.787 | 0.757 | 0.794 | 0.751 |
| RF | 0.791 | 0.812 | 0.765 | 0.804 | 0.775 | |
| SVM | 0.794 | 0.826 | 0.755 | 0.800 | 0.786 | |
Dataset 4 (4 predictors)
| LR | 0.764 | 0.737 | 0.798 | 0.812 | 0.718 |
| RF | 0.755 | 0.768 | 0.741 | 0.779 | 0.731 | |
| SVM | 0.759 | 0.784 | 0.730 | 0.776 | 0.742 | |
Dataset 5 (2 predictors)
| LR | 0.768 | 0.812 | 0.715 | 0.772 | 0.762 |
| RF | 0.768 | 0.797 | 0.733 | 0.780 | 0.753 | |
| SVM | 0.765 | 0.799 | 0.724 | 0.775 | 0.753 |
| Menni et al. [9] | Roland et al. [10] | Clemency et al. [14] | Kowall et al. [35] | Gerkin et al. [15] | Our Study | |
|---|---|---|---|---|---|---|
| Sample | (UK)6452+/9186− (US)726+/2037− | 145+/157− | 225+/736− | 296+/1641− | 4148+/546− | 421+/356− |
| Demographic Data | (UK) Positive group: Mean Age: 41.25 71.88% female Negative group: Mean Age: 43.2 76.40 % female | Mean age: 39 Sex: 72% female | N/A | Mean age: 53.5 years Sex: 61.3% females in the negative group and 57.8% in the positive group | Positive group: Mean Age: 40.6 74% female Negative group: Mean Age: 43.2 78% female | Positive group: Mean Age: 47.3 61% female Negative group: Mean Age: 45.2 78% female |
| Data collection | App-based symptom tracker | Public survey posted on social media | Nurse call center for healthcare workers (HCW) | Self-administered questionnaire | Online survey | Self-administered questionnaire |
| Variable Types | Categorical | Categorical | Categorical | Categorical | Categorical, continuous VAS | Categorical, Continuous VAS |
| Classification Methods | ● Stepwise (forward and backward) ● Logistic Regression ● Akaike Information Criterion (AIC) Classifier threshold at 0.5 | ● Stepwise Logistic Regression ● (p = 0.05 for entry and 0.10 for removal with maximum iterations set at 20) Classifier threshold at 0.5 | Logistic regression with maximum positive likelihood ratio (PLR) criterion | Stepwise backward logistic regression (p = 0.10 for entry and for removal) | L1 regularized logistic regression (penalty α = 1) | ● Stepwise (forward and backward) ● Logistic Regression ● Bayesian Information Criterion (BIC) ● Random Forest (RF) ● Support Vector Machine (SVM) Classifier threshold at 0.5 |
| Predictors | Age, sex, loss of smell and taste, severe or significant persistent cough, severe fatigue, skipped meals | (1) Smell or taste change, fever, body ache, shortness of breath, sore throat (2) Smell or taste change, fever and/or myalgia | (1) Fever, shortness of breath, dry cough (2) Fever, loss of taste or smell (3) Fever, shortness of breath, dry cough, loss of taste or smell | Age, sex, age, return from abroad, close contact with a confirmed case, the presence of fever, cough, exhaustion, taste or smell disorder, current smoking, general health condition and number of comorbidities | (1) Loss of smell, time duration (2) Model with 70 features | Five model datasets (see Table 4) including different variables among: age, sex, loss of smell, loss of taste, nasal obstruction, nasal discharge, facial pain, cough, dyspnea, fever and diarrhea |
| Validation method | ● Holdout 80:20% ● training/test ● 10-fold cross-validation in the UK sample ● US validation sample | Holdout 75:25% training/test | N/A | Holdout 60:40% training-test | 100-fold cross-validation with 80:20% training-test | ● Holdout 75:25% training-test ● 50-fold cross-validation with ● 75:25% training-test |
| Accuracy Parameters | AUC = 0.76 SE = 0.66 SP = 0.83 PPV = 0.58 NPV = 0.87 | (1) AUC = 0.82 SE = 0.56 (2) AUC = 0.75 SE = 0.70 SP = 0.73 | (1) AUC = 0.63 SE = 0.93 SP = 0.09 (2) AUC = 0.75 SE = 0.89 SP = 0.48 (3) AUC = 0.77 SE = 0.98 SP = 0.08 | AUC = 0.821 | AUC = 0.72 SE = 0.85 SP = 0.75 | AUC = 0.80 SE = 0.82 SP = 0.78 PPV = 0.81 NPV = 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callejon-Leblic, M.A.; Moreno-Luna, R.; Del Cuvillo, A.; Reyes-Tejero, I.M.; Garcia-Villaran, M.A.; Santos-Peña, M.; Maza-Solano, J.M.; Martín-Jimenez, D.I.; Palacios-Garcia, J.M.; Fernandez-Velez, C.; et al. Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach. J. Clin. Med. 2021, 10, 570. https://doi.org/10.3390/jcm10040570
Callejon-Leblic MA, Moreno-Luna R, Del Cuvillo A, Reyes-Tejero IM, Garcia-Villaran MA, Santos-Peña M, Maza-Solano JM, Martín-Jimenez DI, Palacios-Garcia JM, Fernandez-Velez C, et al. Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach. Journal of Clinical Medicine. 2021; 10(4):570. https://doi.org/10.3390/jcm10040570
Chicago/Turabian StyleCallejon-Leblic, María A, Ramon Moreno-Luna, Alfonso Del Cuvillo, Isabel M Reyes-Tejero, Miguel A Garcia-Villaran, Marta Santos-Peña, Juan M Maza-Solano, Daniel I Martín-Jimenez, Jose M Palacios-Garcia, Carlos Fernandez-Velez, and et al. 2021. "Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach" Journal of Clinical Medicine 10, no. 4: 570. https://doi.org/10.3390/jcm10040570
APA StyleCallejon-Leblic, M. A., Moreno-Luna, R., Del Cuvillo, A., Reyes-Tejero, I. M., Garcia-Villaran, M. A., Santos-Peña, M., Maza-Solano, J. M., Martín-Jimenez, D. I., Palacios-Garcia, J. M., Fernandez-Velez, C., Gonzalez-Garcia, J., Sanchez-Calvo, J. M., Solanellas-Soler, J., & Sanchez-Gomez, S. (2021). Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach. Journal of Clinical Medicine, 10(4), 570. https://doi.org/10.3390/jcm10040570