Serum N-Glycomics Stratifies Bacteremic Patients Infected with Different Pathogens
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Chemicals and Reagents
2.1.2. Sample Cohort
2.2. Methods
2.2.1. Blood Collection
2.2.2. N-glycan Release and Preparation
2.2.3. N-glycome Profiling
2.2.4. Statistics
3. Results
3.1. Quantitative N-glycome Profiling
3.2. Serum N-glycomics Separates Bacteremic Patients from Healthy Donors without Prior Knowledge
3.3. Pathogen-Specific Alterations of the Serum N-glycome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACN | acetonitrile; |
| ANOVA | analysis of variance; |
| AUC | area-under-the-curve; |
| CID | collision-induced dissociation; |
| CRP | C-reactive protein; |
| DTT | dithiothreitol; |
| EIC | extracted ion chromatogram; |
| FDR | false discovery rate; |
| GlcNAc | N-acetylglucosamine; |
| HREC | human research ethics committee; |
| LC | liquid chromatography; |
| LSD | least significance difference; |
| MS | mass spectrometry; |
| MS/MS | tandem mass spectrometry; |
| PCA | principal component analysis; |
| PGC | porous graphitized carbon; |
| PNGase F | peptide-N-glycosidase F; |
| RAH | Royal Adelaide Hospital; |
| ROC | receiver operating characteristic; |
| SD | standard deviation; |
| SPE | solid-phase extraction |
References
- Christaki, E.; Giamarellos-Bourboulis, E.J. The complex pathogenesis of bacteremia: From antimicrobial clearance mechanisms to the genetic background of the host. Virulence 2014, 5, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mayr, F.B.; Yende, S.; Angus, D.C. Epidemiology of severe sepsis. Virulence 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.; Klee, Y.; Popov, A.F.; Erlenwein, J.; Ghadimi, M.; Beissbarth, T.; Bauer, M.; Hinz, J. Primary bacteraemia is associated with a higher mortality risk compared with pulmonary and intra-abdominal infections in patients with sepsis: A prospective observational cohort study. BMJ Open 2015, 5, e006616. [Google Scholar] [CrossRef] [PubMed]
- Sante, L.; Aguirre-Jaime, A.; Miguel, M.A.; Ramos, M.J.; Pedroso, Y.; Lecuona, M. Epidemiological study of secondary bloodstream infections: The forgotten issue. J. Infect. Public Health 2019, 12, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Franceschini, E.; Koppel, F.; Turjeman, A.; Babich, T.; Bitterman, R.; Neuberger, A.; Ghanem-Zoubi, N.; Santoro, A.; Eliakim-Raz, N.; et al. Seven Versus 14 Days of Antibiotic Therapy for Uncomplicated Gram-negative Bacteremia: A Noninferiority Randomized Controlled Trial. Clin. Infect. Dis. 2019, 69, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Oda, S.; Sadahiro, T.; Nakamura, M.; Hirayama, Y.; Tateishi, Y.; Shinozaki, K.; Hirasawa, H. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit. Care 2010, 14, R27. [Google Scholar] [CrossRef] [PubMed]
- Wujtewicz, M.A.; Śledzińska, A.; Owczuk, R.; Wujtewicz, M. Escherichia coli bacteraemias in intensive care unit patients. Anaesthesiol. Intensive Ther. 2016, 48, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Kot, B. Antibiotic Resistance Among Uropathogenic Escherichia coli. Pol. J. Microbiol. 2019, 68, 403–415. [Google Scholar] [CrossRef]
- Lund-Palau, H.; Turnbull, A.R.; Bush, A.; Bardin, E.; Cameron, L.; Soren, O.; Wierre-Gore, N.; Alton, E.W.; Bundy, J.G.; Connett, G.; et al. Pseudomonas aeruginosa infection in cystic fibrosis: Pathophysiological mechanisms and therapeutic approaches. Expert. Rev. Respir. Med. 2016, 10, 685–697. [Google Scholar] [CrossRef]
- Bachman, M.A.; Breen, P.; Deornellas, V.; Mu, Q.; Zhao, L.; Wu, W.; Cavalcoli, J.D.; Mobley, H.L.T. Genome-Wide Identification of Klebsiella pneumoniae Fitness Genes during Lung Infection. mBio 2015, 6, e00775-15. [Google Scholar] [CrossRef]
- Cervera, C.; Almela, M.; Martínez-Martínez, J.A.; Moreno, A.; Miró, J.M. Risk factors and management of Gram-positive bacteraemia. Int. J. Antimicrob. Agents 2009, 34, S26–S30. [Google Scholar] [CrossRef]
- Vos, F.J.; Kullberg, B.J.; Sturm, P.D.; Krabbe, P.F.M.; Van Dijk, A.P.J.; Wanten, G.J.A.; Oyen, W.J.G.; Bleeker-Rovers, C.P. Metastatic Infectious Disease and Clinical Outcome in Staphylococcus aureus and Streptococcus species Bacteremia. Medicine 2012, 91, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Wolk, D.M. Bloodstream Infections. Microbiol. Spectr. 2016, 4, 653–689. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.; Syndergaard, C.; Damas, C.; Trubey, R.; Mukindaraj, A.; Qian, S.; Jin, X.; Breslow, S.; Niemz, A. Sepsis Pathogen Identification. J. Lab. Autom. 2015, 20, 539–561. [Google Scholar] [CrossRef] [PubMed]
- Uhle, F.; Lichtenstern, C.; Brenner, T.; Weigand, M.A. Pathophysiology of sepsis. Anasthesiol. Intensivmed Notf Schmerzther 2015, 50, 114–122. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Lee, A.; Mirrett, S.; Reller, L.B.; Weinstein, M.P. Detection of Bloodstream Infections in Adults: How Many Blood Cultures Are Needed? J. Clin. Microbiol. 2007, 45, 3546–3548. [Google Scholar] [CrossRef]
- Kethireddy, S.; Bilgili, B.; Sees, A.; Kirchner, H.L.; Ofoma, U.R.; Light, R.B.; Mirzanejad, Y.; Maki, D.; Kumar, A.; Layon, A.J.; et al. Culture-Negative Septic Shock Compared With Culture-Positive Septic Shock: A Retrospective Cohort Study. Crit. Care. Med. 2018, 46, 506–512. [Google Scholar] [CrossRef]
- Scerbo, M.H.; Kaplan, H.B.; Dua, A.; Litwin, D.B.; Ambrose, C.G.; Moore, L.J.; Murray, C.C.K.; Wade, C.E.; Holcomb, J.B. Beyond Blood Culture and Gram Stain Analysis: A Review of Molecular Techniques for the Early Detection of Bacteremia in Surgical Patients. Surg. Infect. 2016, 17, 294–302. [Google Scholar] [CrossRef]
- Fang, W.-F.; Chen, Y.-M.; Wang, Y.-H.; Huang, C.-H.; Hung, K.-Y.; Fang, Y.-T.; Chang, Y.-C.; Lin, C.-Y.; Chang, Y.-T.; Chen, H.-C.; et al. Incorporation of dynamic segmented neutrophil-to-monocyte ratio with leukocyte count for sepsis risk stratification. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lim, G.; Kang, S.Y.; Lee, W.-I.; Suh, J.-T.; Lee, H.J. Utility of Procalcitonin as an Early Diagnostic Marker of Bacteremia in Patients with Acute Fever. Yonsei Med, J. 2011, 52, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Zarkesh, M.; Sedaghat, F.; Heidarzadeh, A.; Tabrizi, M.; Bolooki-Moghadam, K.; Ghesmati, S. Diagnostic value of IL-6, CRP, WBC, and absolute neutrophil count to predict serious bacterial infection in febrile infants. Acta Medica Iran. 2015, 53, 408–411. [Google Scholar]
- Sweeney, T.E.; Liesenfeld, O.; May, L. Diagnosis of bacterial sepsis: Why are tests for bacteremia not sufficient? Expert Rev. Mol. Diagn. 2019, 19, 959–962. [Google Scholar] [CrossRef]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Cvetko, A.; Kifer, D.; Gornik, O.; Klaric, L.; Visser, E.; Lauc, G.; Wilson, J.F.; Štambuk, T. Glycosylation Alterations in Multiple Sclerosis Show Increased Proinflammatory Potential. Biomedicines 2020, 8, 410. [Google Scholar] [CrossRef]
- Stavenhagen, K.; Gahoual, R.; Domínguez-Vega, E.; Palmese, A.; Ederveen, A.L.H.; Cutillo, F.; Palinsky, W.; Bierau, H.; Wuhrer, M. Site-specific N- and O-glycosylation analysis of atacicept. mAbs 2019, 11, 1053–1063. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, L.Y.; Kawahara, R.; Abrahams, J.L.; Adamczyk, B.; Anugraham, M.; Ashwood, C.; Sumer-Bayraktar, Z.; Briggs, M.T.; Chik, J.H.L.; et al. Protein Paucimannosylation Is an Enriched N -Glycosylation Signature of Human Cancers. Proteomics 2019, 19, e1900010. [Google Scholar] [CrossRef]
- Kawahara, R.; Recuero, S.; Srougi, M.; Leite, K.R.M.; Thaysen-Andersen, M.; Palmisano, G. The complexity and dynamics of the tissue glycoproteome associated with prostate cancer progression. Mol. Cell. Proteomics 2020, 100026. [Google Scholar] [CrossRef]
- Hinneburg, H.; Korać, P.; Schirmeister, F.; Gasparov, S.; Seeberger, P.H.; Zoldoš, V.; Kolarich, D. Unlocking Cancer Glycomes from Histopathological Formalin-fixed and Paraffin-embedded (FFPE) Tissue Microdissections. Mol. Cell. Proteomics 2017, 16, 524–536. [Google Scholar] [CrossRef]
- Möginger, U.; Grunewald, S.; Hennig, R.; Kuo, C.-W.; Schirmeister, F.; Voth, H.; Rapp, E.; Khoo, K.-H.; Seeberger, P.H.; Simon, J.C.; et al. Alterations of the Human Skin N- and O-Glycome in Basal Cell Carcinoma and Squamous Cell Carcinoma. Front. Oncol. 2018, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Ugonotti, J.; Chatterjee, S.; Thaysen-Andersen, M. Structural and functional diversity of neutrophil glycosylation in innate immunity and related disorders. Mol. Asp. Med. 2020, 100882. [Google Scholar] [CrossRef] [PubMed]
- Loke, I.; Østergaard, O.; Heegaard, N.H.H.; Packer, N.H.; Thaysen-Andersen, M. Paucimannose-RichN-glycosylation of Spatiotemporally Regulated Human Neutrophil Elastase Modulates Its Immune Functions. Mol. Cell. Proteomics 2017, 16, 1507–1527. [Google Scholar] [CrossRef] [PubMed]
- Reiding, K.R.; Franc, V.; Huitema, M.G.; Brouwer, E.; Heeringa, P.; Heck, A.J. Neutrophil myeloperoxidase harbors distinct site-specific peculiarities in its glycosylation. J. Biol. Chem. 2019, 294, 20233–20245. [Google Scholar] [CrossRef] [PubMed]
- Hare, N.J.; Lee, L.Y.; Loke, I.; Britton, W.J.; Saunders, B.M.; Thaysen-Andersen, M. Mycobacterium tuberculosis Infection Manipulates the Glycosylation Machinery and the N-Glycoproteome of Human Macrophages and Their Microparticles. J. Proteome Res. 2016, 16, 247–263. [Google Scholar] [CrossRef]
- Venkatakrishnan, V.; Thaysen-Andersen, M.; Chen, S.C.; Nevalainen, H.; Packer, N.H. Cystic fibrosis and bacterial colonization define the sputum N-glycosylation phenotype. Glycobiology 2015, 25, 88–100. [Google Scholar] [CrossRef]
- Pučić, M.; Pinto, S.; Novokmet, M.; Knežević, A.; Gornik, O.; Polašek, O.; Vlahoviček, K.; Wang, W.; Rudd, P.M.; Wright, A.F.; et al. Common aberrations from the normal human plasma N-glycan profile. Glycobiology 2010, 20, 970–975. [Google Scholar] [CrossRef]
- Dotz, V.; Wuhrer, M. N-glycome signatures in human plasma: Associations with physiology and major diseases. FEBS Lett. 2019, 593, 2966–2976. [Google Scholar] [CrossRef]
- Knežević, A.; Polašek, O.; Gornik, O.; Rudan, I.; Campbell, H.; Hayward, C.; Wright, A.; Kolčić, I.; O’Donoghue, N.; Bones, J.; et al. Variability, Heritability and Environmental Determinants of Human Plasma N-Glycome. J. Proteome Res. 2009, 8, 694–701. [Google Scholar] [CrossRef]
- Reiding, K.R.; Bondt, A.; Hennig, R.; Gardner, R.A.; O’Flaherty, R.; Trbojević-Akmačić, I.; Shubhakar, A.; Hazes, J.M.W.; Reichl, U.; Fernandes, D.L.; et al. High-throughput Serum N-Glycomics: Method Comparison and Application to Study Rheumatoid Arthritis and Pregnancy-associated Changes. Mol. Cell. Proteomics. 2019, 18, 3–15. [Google Scholar] [CrossRef]
- Gui, H.-L.; Gao, C.-F.; Wang, H.; Liu, X.; Xie, Q.; Dewaele, S.; Wang, L.; Zhuang, H.; Contreras, R.; Libert, C.; et al. Altered serumN-glycomics in chronic hepatitis B patients. Liver Int. 2010, 30, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Gornik, O.; Royle, L.; Harvey, D.J.; Radcliffe, C.M.; Saldova, R.; Dwek, R.A.; Rudd, P.; Lauc, G. Changes of Serum Glycans During Sepsis and Acute Pancreatitis. Glycobiology 2007, 17, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yoneyama, T.; Noro, D.; Imanishi, K.; Kojima, Y.; Hatakeyama, S.; Tobisawa, Y.; Mori, K.; Yamamoto, H.; Imai, A.; et al. Aberrant N-Glycosylation Profile of Serum Immunoglobulins is a Diagnostic Biomarker of Urothelial Carcinomas. Int. J. Mol. Sci. 2017, 18, 2632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Westhrin, M.; Bondt, A.; Wuhrer, M.; Standal, T.; Holst, S. Serum protein N-glycosylation changes in multiple myeloma. Biochim.Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 960–970. [Google Scholar] [CrossRef]
- Jensen, P.H.; Karlsson, N.G.; Kolarich, D.; Packer, N.H. Structural analysis of N- and O-glycans released from glycoproteins. Nat. Protoc. 2012, 7, 1299–1310. [Google Scholar] [CrossRef]
- Ashwood, C.; Lin, C.-H.; Thaysen-Andersen, M.; Packer, N.H. Discrimination of Isomers of Released N- and O-Glycans Using Diagnostic Product Ions in Negative Ion PGC-LC-ESI-MS/MS. J. Am. Soc. Mass Spectrom. 2018, 29, 1194–1209. [Google Scholar] [CrossRef]
- Ashwood, C.; Pratt, B.; MacLean, B.X.; Gundry, R.L.; Packer, N.H. Standardization of PGC-LC-MS-based glycomics for sample specific glycotyping. Analyst 2019, 144, 3601–3612. [Google Scholar] [CrossRef]
- Stavenhagen, K.; Kolarich, D.; Wuhrer, M. Clinical Glycomics Employing Graphitized Carbon Liquid Chromatography–Mass Spectrometry. Chromatographia 2015, 78, 307–320. [Google Scholar] [CrossRef]
- Kolarich, D.; Windwarder, M.; Alagesan, K.; Altmann, F. Isomer-Specific Analysis of Released N-Glycans by LC-ESI MS/MS with Porous Graphitized Carbon. Methods Mol. Biol. 2015, 1321, 427–435. [Google Scholar] [CrossRef]
- Everest-Dass, A.V.; Kolarich, D.; Campbell, M.P.; Packer, N.H. Tandem mass spectra of glycan substructures enable the multistage mass spectrometric identification of determinants on oligosaccharides. Rapid Commun. Mass Spectrom. 2013, 27, 931–939. [Google Scholar] [CrossRef]
- Palmisano, G.; Larsen, M.R.; Packer, N.H.; Thaysen-Andersen, M. Structural analysis of glycoprotein sialylation–part II: LC-MS based detection. RSC Adv. 2013, 3, 22706–22726. [Google Scholar] [CrossRef]
- Hinneburg, H.; Chatterjee, S.; Schirmeister, F.; Nguyen-Khuong, T.; Packer, N.H.; Rapp, E.; Thaysen-Andersen, M. Post-Column Make-Up Flow (PCMF) Enhances the Performance of Capillary-Flow PGC-LC-MS/MS-Based Glycomics. Anal. Chem. 2019, 91, 4559–4567. [Google Scholar] [CrossRef] [PubMed]
- Thaysen-Andersen, M.; Packer, N.H. Advances in LC–MS/MS-based glycoproteomics: Getting closer to system-wide site-specific mapping of the N- and O-glycoproteome. Biochim. et Biophys. Acta (BBA) Proteins Proteom. 2014, 1844, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Joenvaara, S.; Saraswat, M.; Kuusela, P.; Saraswat, S.; Agarwal, R.; Kaartinen, J.; Järvinen, A.; Renkonen, R. Quantitative N-glycoproteomics reveals altered glycosylation levels of various plasma proteins in bloodstream infected patients. PLoS ONE 2018, 13, e0195006. [Google Scholar] [CrossRef] [PubMed]
- Nenke, M.; Lewis, J.G.; Rankin, W.; Shaw, D.; Torpy, D.J. Corticosteroid-binding globulin cleavage may be pathogen-dependent in bloodstream infection. Clin. Chim. Acta 2017, 464, 176–181. [Google Scholar] [CrossRef]
- Nenke, M.A.; Lewis, J.G.; Rankin, W.; Torpy, D.J. Evidence of Reduced CBG Cleavage in Abdominal Obesity: A Potential Factor in Development of the Metabolic Syndrome. Horm. Metab. Res. 2016, 48, 620. [Google Scholar] [CrossRef]
- Cooper, C.A.; Gasteiger, E.; Packer, N.H. GlycoMod--a software tool for determining glycosylation compositions from mass spectrometric data. Proteomics 2001, 1, 340–349. [Google Scholar] [CrossRef]
- Abrahams, J.L.; Campbell, M.P.; Packer, N.H. Building a PGC-LC-MS N-glycan retention library and elution mapping resource. Glycoconj. J. 2018, 35, 15–29. [Google Scholar] [CrossRef]
- Ramus, C.; Hovasse, A.; Marcellin, M.; Hesse, A.-M.; Mouton-Barbosa, E.; Bouyssié, D.; Vaca, S.; Carapito, C.; Chaoui, K.; Bruley, C.; et al. Spiked proteomic standard dataset for testing label-free quantitative software and statistical methods. Data Brief 2016, 6, 286–294. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Hinneburg, H.; Pedersen, J.L.; Bokil, N.J.; Pralow, A.; Schirmeister, F.; Kawahara, R.; Rapp, E.; Saunders, B.M.; Thaysen-Andersen, M. High-resolution longitudinal N- and O-glycoprofiling of human monocyte-to-macrophage transition. Glycobiology 2020, 30, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Neelamegham, S.; Aoki-Kinoshita, K.; Bolton, E.; Frank, M.; Lisacek, F.; Lütteke, T.; O#x2019;Boyle, N.; Packer, N.H.; Stanley, P.; Toukach, F.V.; et al. Updates to the Symbol Nomenclature for Glycans guidelines. Glycobiology 2019, 29, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Schiøtz, P.O.; Høiby, N.; Permin, H.; Wiik, A. IgA and IgG antibodies against surface antigens of Pseudomonas aeruginosa in sputum and serum from patients with cystic fibrosis. Acta Pathol. Microbiol. Scand.C 1979, 87c, 229–233. [Google Scholar]
- Pedersen, S.S.; Espersen, F.; Høiby, N.; Jensen, T. Immunoglobulin A and immunoglobulin G antibody responses to alginates from Pseudomonas aeruginosa in patients with cystic fibrosis. J. Clin. Microbiol. 1990, 28, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Y.; Wan, Q.-Q. Pseudomonas aeruginosa bacteremia among liver transplant recipients. Infect. Drug Resist. 2018, 11, 2345–2356. [Google Scholar] [CrossRef]
- Bang, J.H.; Jung, Y.; Cheon, S.; Kim, C.-J.; Song, K.-H.; Choe, P.G.; Park, W.B.; Kim, E.S.; Park, S.W.; Kim, W.J.; et al. Pseudomonas aeruginosa bacteremia in patients with liver cirrhosis: A comparison with bacteremia caused by Enterobacteriaceae. BMC Infect. Dis. 2013, 13, 332. [Google Scholar] [CrossRef]
- Thaysen-Andersen, M.; Venkatakrishnan, V.; Loke, I.; Laurini, C.; Diestel, S.; Parker, B.L.; Packer, N.H. Human Neutrophils Secrete Bioactive Paucimannosidic Proteins from Azurophilic Granules into Pathogen-Infected Sputum. J. Biol. Chem. 2015, 290, 8789–8802. [Google Scholar] [CrossRef]
- Swathi Raju, M.; Jahnavi, V.; Kamaraju, R.S.; Sritharan, V.; Rajkumar, K.; Natarajan, S.; Kumar, A.D.; Burgula, S. Continuous evaluation of changes in the serum proteome from early to late stages of sepsis caused by Klebsiella pneumoniae. Mol. Med. Rep. 2016, 13, 4835–4844. [Google Scholar] [CrossRef]
- Dietz, S.; Lautenschläger, C.; Müller-Werdan, U.; Pilz, G.; Fraunberger, P.; Päsler, M.; Ebelt, H.; Walli, A.K.; Werdan, K.; Nuding, S. Serum IgG levels and mortality in patients with severe sepsis and septic shock. Med. Klin. Intensivmed. Notf. 2017, 112, 462–470. [Google Scholar] [CrossRef]
- Arnold, J.N.; Wormald, M.R.; Sim, R.B.; Rudd, P.M.; Dwek, R.A. The Impact of Glycosylation on the Biological Function and Structure of Human Immunoglobulins. Annu. Rev. Immunol. 2007, 25, 21–50. [Google Scholar] [CrossRef]
- Stöckmann, H.; Adamczyk, B.; Hayes, J.; Rudd, P.M. Automated, high-throughput IgG-antibody glycoprofiling platform. Anal. Chem. 2013, 85, 8841–8849. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.P.; Hulen, C. Influence of sialic acid and bacterial sialidase on differential adhesion of Pseudomonas aeruginosa to epithelial cells. Colloids Surf. B: Biointerfaces 2006, 52, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Khatua, B.; Bhattacharya, K.; Mandal, C. Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J. Leukoc. Biol. 2012, 91, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Grünwald-Gruber, C.; Thader, A.; Maresch, D.; Dalik, T.; Altmann, F. Determination of true ratios of different N-glycan structures in electrospray ionization mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Kenny, D.T.; Skoog, E.C.; Padra, M.; Adamczyk, B.; Vitizeva, V.; Thorell, A.; Venkatakrishnan, V.; Lindén, S.K.; Karlsson, N.G. Structural Diversity of Human Gastric Mucin Glycans. Mol. Cell. Proteomics 2017, 16, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Nie, H.; Sun, X.; Sun, W.; Qu, Y.; Liu, X.; Yao, Y.; Liang, X.; Chen, C.C.; Li, Y. Human serum N-glycan profiles are age and sex dependent. Age Ageing 2011, 40, 568–575. [Google Scholar] [CrossRef]
- De Vroome, S.W.; Holst, S.; Girondo, M.R.; Van Der Burgt, Y.E.M.; Mesker, W.E.; Tollenaar, R.A.E.M.; Wuhrer, M. Serum N-glycome alterations in colorectal cancer associate with survival. Oncotarget 2018, 9, 30610–30623. [Google Scholar] [CrossRef]
- Vučković, F.; Theodoratou, E.; Thaçi, K.; Timofeeva, M.; Vojta, A.; Štambuk, J.; Pučić-Baković, M.; Rudd, P.M.; Đerek, L.; Servis, D.; et al. IgG Glycome in Colorectal Cancer. Clin. Cancer Res. 2016, 22, 3078–3086. [Google Scholar] [CrossRef]
- Clerc, F.; Reiding, K.R.; Jansen, B.C.; Kammeijer, G.S.M.; Bondt, A.; Wuhrer, M. Human plasma protein N-glycosylation. Glycoconj. J. 2016, 33, 309–343. [Google Scholar] [CrossRef]
- Tjondro, H.C.; Loke, I.; Chatterjee, S.; Thaysen-Andersen, M. Human protein paucimannosylation: Cues from the eukaryotic kingdoms. Biol. Rev. 2019, 94, 2068–2100. [Google Scholar] [CrossRef]
- Venkatakrishnan, V.; Dieckmann, R.; Loke, I.; Tjondro, H.C.; Chatterjee, S.; Bylund, J.; Thaysen-Andersen, M.; Karlsson, N.G.; Karlsson-Bengtsson, A. Glycan analysis of human neutrophil granules implicates a maturation-dependent glycosylation machinery. J. Biol. Chem. 2020, 295, 12648–12660. [Google Scholar] [CrossRef] [PubMed]
- Tjondro, H.C.; Ugonotti, J.; Kawahara, R.; Chatterjee, S.; Loke, I.; Chen, S.; Soltermann, F.; Hinneburg, H.; Parker, B.L.; Venkatakrishnan, V.; et al. Hyper-truncated Asn355- and Asn391-glycans modulate the activity of neutrophil granule myeloperoxidase. J. Biol. Chem. 2020, 296, 100144. [Google Scholar] [CrossRef] [PubMed]
- Pavić, T.; Dilber, D.; Kifer, D.; Selak, N.; Keser, T.; Ljubičić, Đ.; Vukić Dugac, A.; Lauc, G.; Rumora, L.; Gornik, O. N-glycosylation patterns of plasma proteins and immunoglobulin G in chronic obstructive pulmonary disease. J. Transl. Med. 2018, 16, 323. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Quelhas, D.; Critchley, A.J.; Carchon, H.; Hebestreit, H.F.; Hibbert, R.G.; Vilarinho, L.; Teles, E.; Matthijs, G.; Schollen, E.; et al. Detailed glycan analysis of serum glycoproteins of patients with congenital disorders of glycosylation indicates the specific defective glycan processing step and provides an insight into pathogenesis. Glycobiology 2003, 13, 601–622. [Google Scholar] [CrossRef] [PubMed]
- Thaysen-Andersen, M.; Packer, N.H.; Schulz, B.L. Maturing Glycoproteomics Technologies Provide Unique Structural Insights into the N-glycoproteome and Its Regulation in Health and Disease. Mol. Cell. Proteomics 2016, 15, 1773–1790. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Li, L. Recent advances in mass spectrometry (MS)-based glycoproteomics in complex biological samples. Trends Anal. Chem. 2019, 118, 880–892. [Google Scholar] [CrossRef]
- Chernykh, A.; Kawahara, R.; Thaysen-Andersen, M. Towards structure-focused glycoproteomics. Biochem. Soc. Trans. 2021. [Google Scholar] [CrossRef]

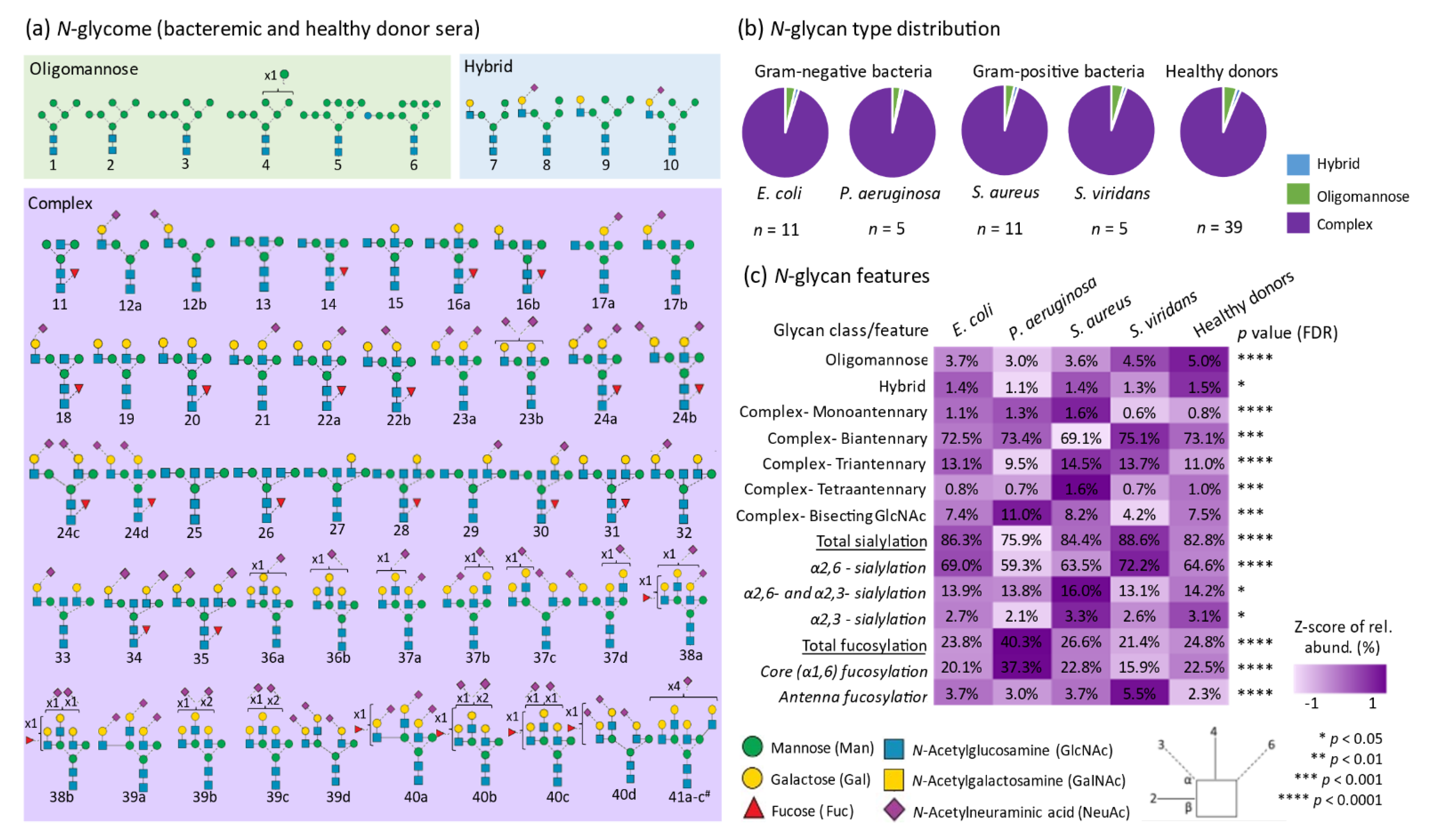
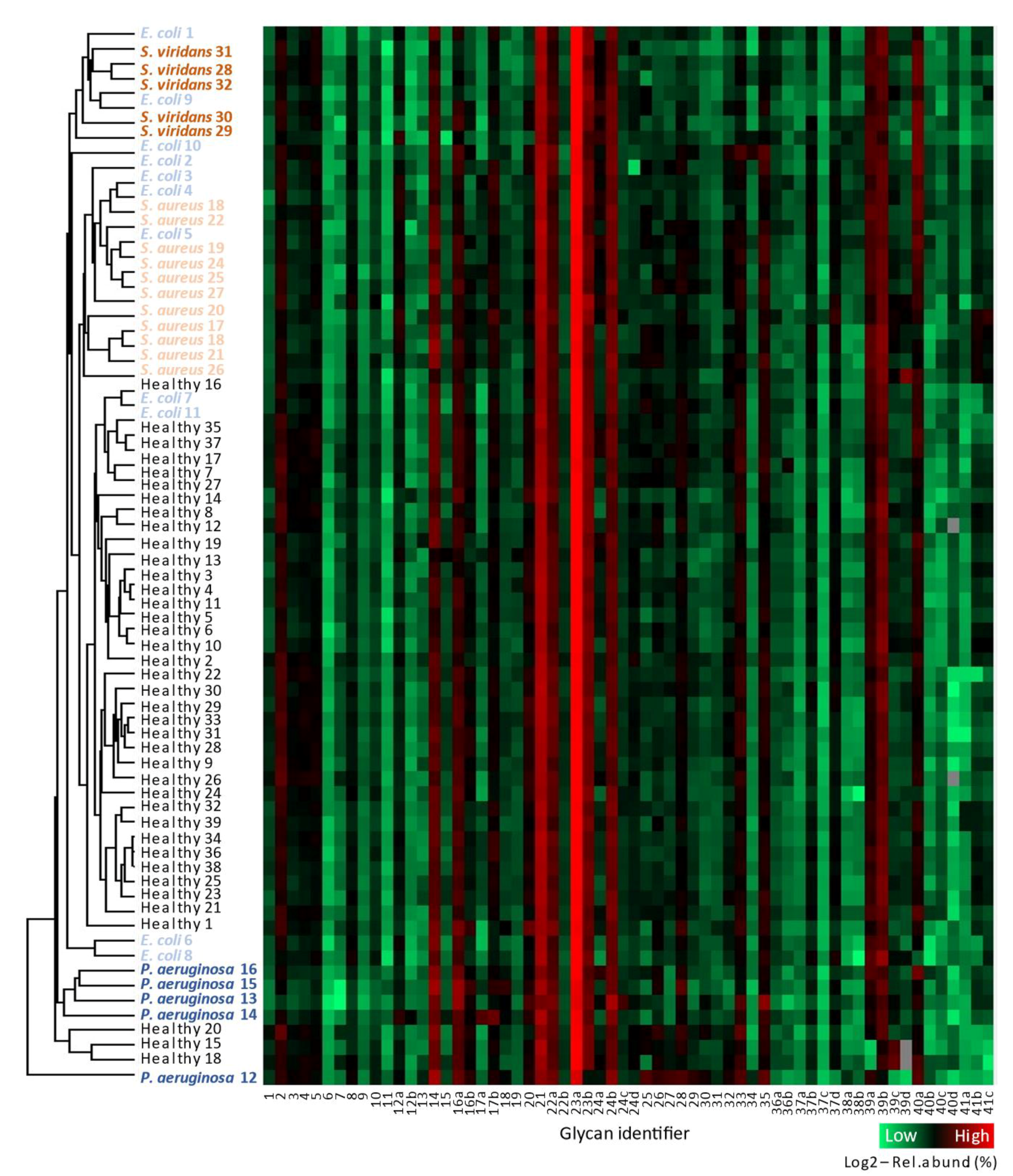
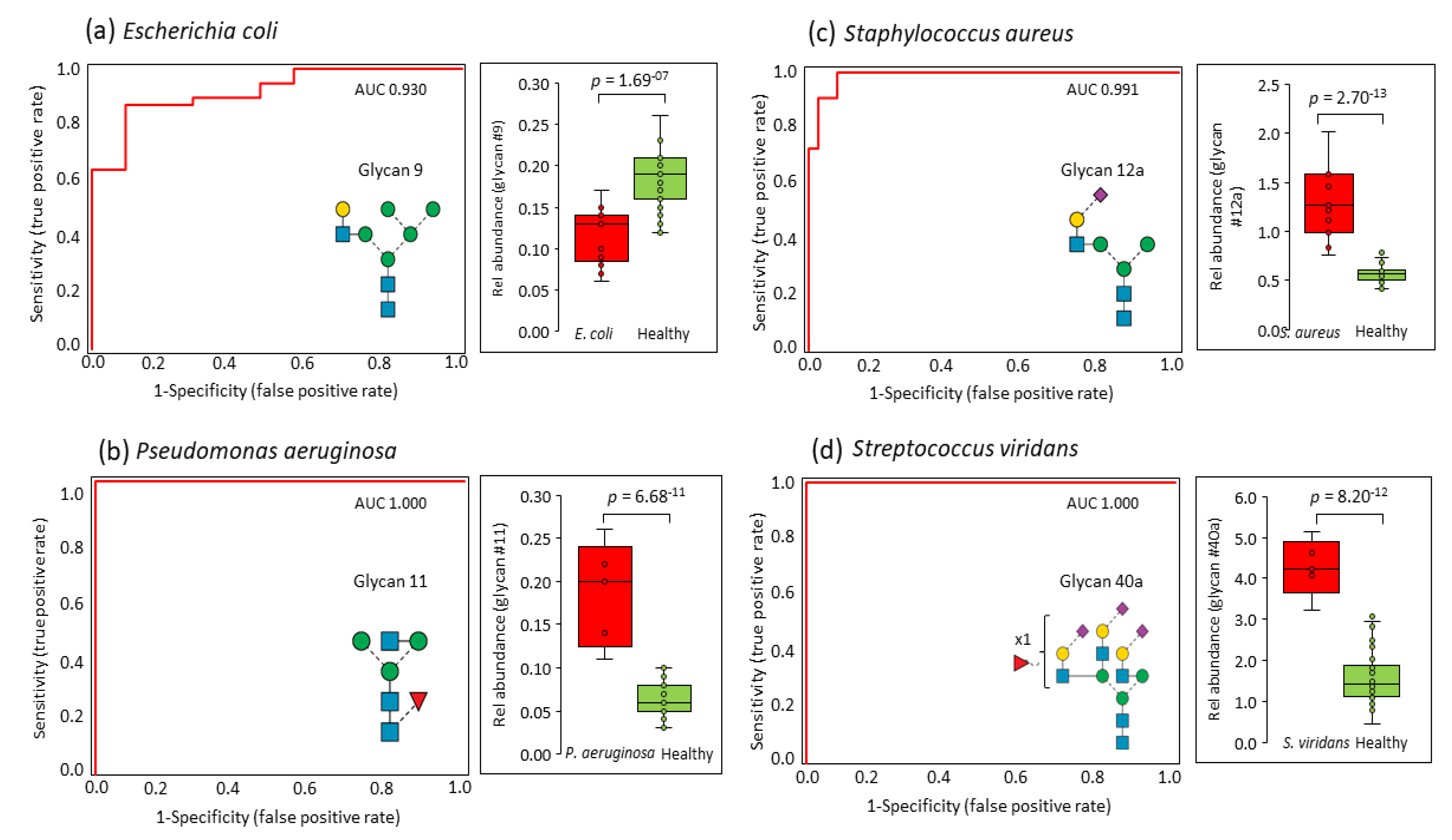
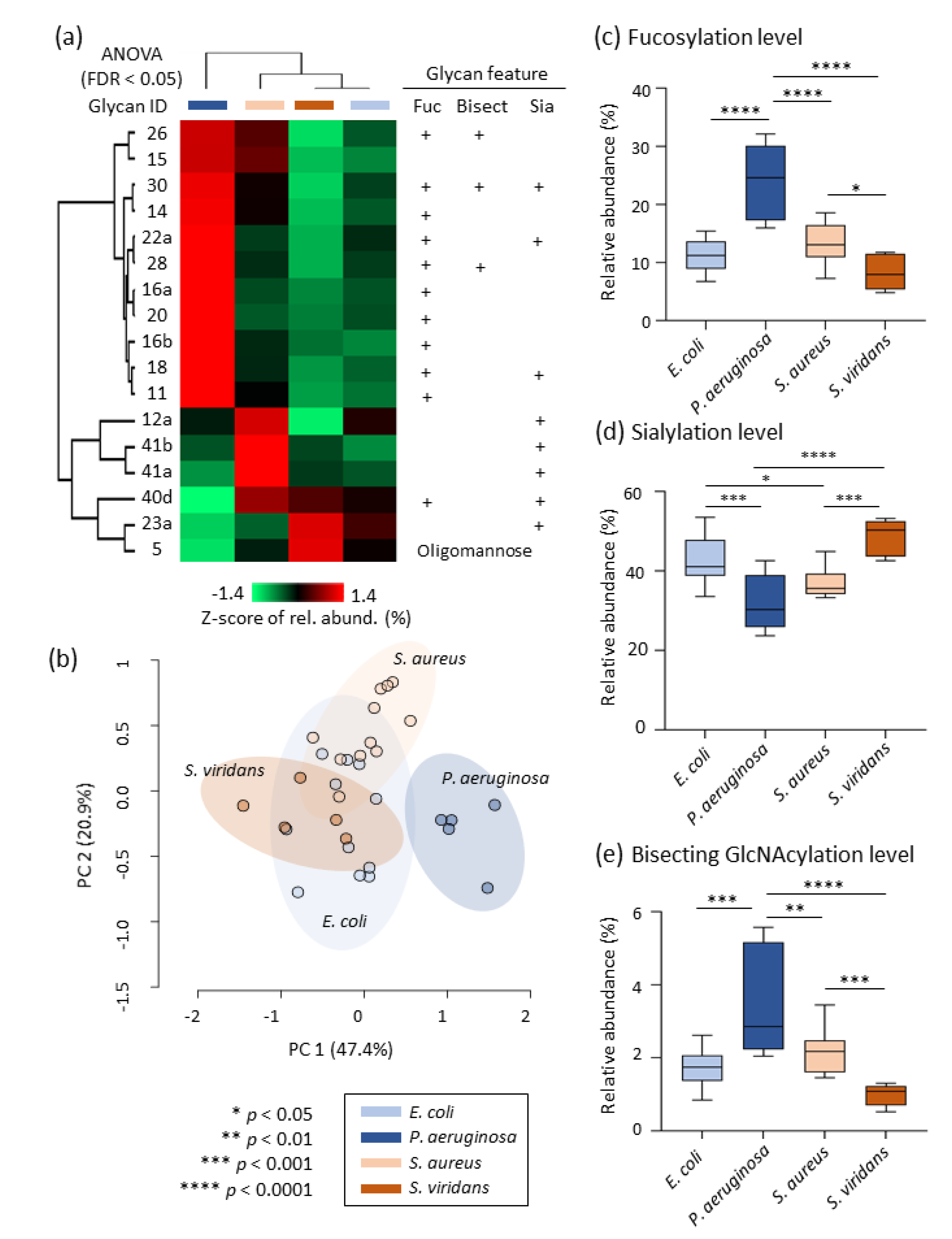
| n | Pathogen (Gram Character) | Age (Years) | Sex (F, Female; M, Male) | Pitt Severity Score | White Cell Count (109/L) | Neutrophil Count (109/L) | C-Reactive Protein (mg/L) |
|---|---|---|---|---|---|---|---|
| 39 | Healthy donors | 54.9 ± 17.5 | F = 19 M = 20 | N/A | 4.0–11.0 * | 2.0–8.0 * | N/A |
| 11 | E. coli (negative) | 61.4 ± 24.8 | F = 9 M = 2 | 1.1 ± 1.0 | 18.5 ± 11.3 | 16.7 ± 10.1 | 186.7 ± 89.8 |
| 5 | P. aeruginosa (negative) | 73.2 ± 4.4 | F = 0 M = 5 | 2.0 ± 0.0 | 7.8 ± 9.9 | 6.7 ± 9.5 | 130.4 ± 49.3 |
| 11 | S. aureus (positive) | 49.1 ± 18.8 | F = 5 M = 6 | 0.9 ± 1.2 | 12.6 ± 3.6 | 10.7 ± 3.5 | 161.0 ± 123.3 |
| 5 | S. viridans (positive) | 43.4 ± 17.5 | F = 2 M = 3 | 0.4 ± 0.5 | 8.1 ± 7.4 | 11.2 ± 2.2 | 127.0 ± 68.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, S.; Kawahara, R.; Tjondro, H.C.; Shaw, D.R.; Nenke, M.A.; Torpy, D.J.; Thaysen-Andersen, M. Serum N-Glycomics Stratifies Bacteremic Patients Infected with Different Pathogens. J. Clin. Med. 2021, 10, 516. https://doi.org/10.3390/jcm10030516
Chatterjee S, Kawahara R, Tjondro HC, Shaw DR, Nenke MA, Torpy DJ, Thaysen-Andersen M. Serum N-Glycomics Stratifies Bacteremic Patients Infected with Different Pathogens. Journal of Clinical Medicine. 2021; 10(3):516. https://doi.org/10.3390/jcm10030516
Chicago/Turabian StyleChatterjee, Sayantani, Rebeca Kawahara, Harry C. Tjondro, David R. Shaw, Marni A. Nenke, David J. Torpy, and Morten Thaysen-Andersen. 2021. "Serum N-Glycomics Stratifies Bacteremic Patients Infected with Different Pathogens" Journal of Clinical Medicine 10, no. 3: 516. https://doi.org/10.3390/jcm10030516
APA StyleChatterjee, S., Kawahara, R., Tjondro, H. C., Shaw, D. R., Nenke, M. A., Torpy, D. J., & Thaysen-Andersen, M. (2021). Serum N-Glycomics Stratifies Bacteremic Patients Infected with Different Pathogens. Journal of Clinical Medicine, 10(3), 516. https://doi.org/10.3390/jcm10030516







