Post-Operative Permanent Hypoparathyroidism and Preoperative Vitamin D Prophylaxis
Abstract
1. Introduction
2. Methods
3. Results
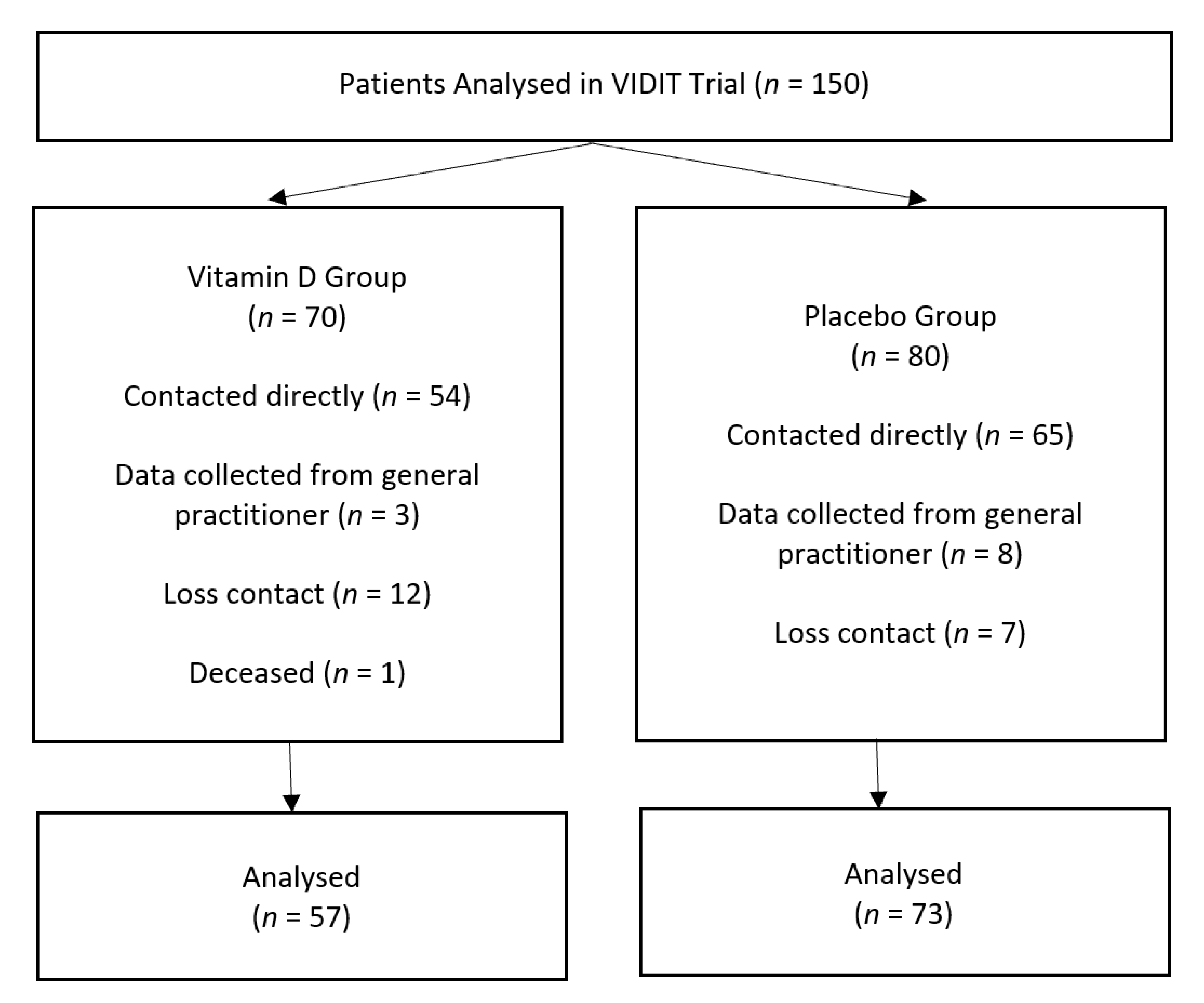
3.1. Participant Flow
3.2. Baseline Characteristics
3.3. Complete Case Analysis
3.4. Loss to Follow-Up
3.5. Primary Outcome
3.6. Secondary Findings—Sub-Group Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Francis, D.O.; Randolph, G.; Davies, L. Nationwide variation in rates of thyroidectomy among US medicare beneficiaries. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Edafe, O.; Prasad, P.; Harrison, B.J.; Balasubramanian, S.P. Incidence and predictors of post-thyroidectomy hypocalcaemia in a tertiary endocrine surgical unit. Ann. R. Coll. Surg. Engl. 2014, 96, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Khairy, G.A.; Al-Saif, A. Incidental parathyroidectomy during thyroid resection: Incidence, risk factors, and outcome. Ann. Saudi Med. 2011, 31, 274–278. [Google Scholar] [CrossRef]
- Chadwick, D.R. Hypocalcaemia and permanent hypoparathyroidism after total/bilateral thyroidectomy in the BAETS registry. Gland Surg. 2017, 6, S69. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.K.; Shonka, D.C.; Robinson, D.J.; Levine, P.A. Association of preoperative calcium and calcitriol therapy with postoperative hypocalcemia after total thyroidectomy. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 679–684. [Google Scholar] [CrossRef]
- Kirkby-Bott, J.; Markogiannakis, H.; Skandarajah, A.; Cowan, M.; Fleming, B.; Palazzo, F. Preoperative vitamin D deficiency predicts postoperative hypocalcemia after total thyroidectomy. World J. Surg. 2011, 35, 324–330. [Google Scholar] [CrossRef]
- Puig-Domingo, M.; Diaz, G.; Nicolau, J.; Fernández, C.; Rueda, S.; Halperin, I. Successful treatment of vitamin D unresponsive hypoparathyroidism with multipulse subcutaneous infusion of teriparatide. Eur. J. Endocrinol. 2008, 159, 653. [Google Scholar] [CrossRef][Green Version]
- Almquist, M.; Ivarsson, K.; Nordenström, E.; Bergenfelz, A. 2018 Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. Br. J. Surg. 2018, 105, 1313–1318. [Google Scholar] [CrossRef]
- Genser, L.; Trésallet, C.; Godiris-Petit, G.; Fui, S.L.S.; Salepcioglu, H.; Royer, C.; Menegaux, F. Randomized controlled trial of alfacalcidol supplementation for the reduction of hypocalcemia after total thyroidectomy. Am. J. Surg. 2014, 207, 39–45. [Google Scholar] [CrossRef]
- Mitchell, D.M.; Regan, S.; Cooley, M.R.; Lauter, K.B.; Vrla, M.C.; Becker, C.B.; Burnett-Bowie, S.A.M.; Mannstadt, M. Long-term follow-up of patients with hypoparathyroidism. J. Clin. Endocrinol. Metab. 2012, 97, 4507–4514. [Google Scholar] [CrossRef]
- Stack Jr, B.C.; Bimston, D.N.; Bodenner, D.L.; Brett, E.M.; Dralle, H.; Orloff, L.A.; Pallota, J.; Snyder, S.K.; Wong, R.J.; Randolph, G.W. American association of clinical endocrinologists and American college of endocrinology disease state clinical review: Postoperative hypoparathyroidism-definitions and management. Endocr. Pract. 2015, 21, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.W.; Arthurs, S.; O’Neill, C.J.; Hawthorne, J.; Carroll, R.; Wynne, K.; Bendinelli, C. High-dose preoperative cholecalciferol to prevent post-thyroidectomy hypocalcaemia: A randomized, double-blinded placebo-controlled trial. Clin. Endocrinol. 2019, 90, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.T.; Chang, J.W.; Jin, J.; Lim, Y.C.; Rha, K.S.; Koo, B.S. Transient and permanent hypocalcemia after total thyroidectomy: Early predictive factors and long-term follow-up results. Surgery 2015, 158, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, P.V.; Ramalingam, K. Postoperative PTH measurement is not a reliable predictor for hypocalcemia after total thyroidectomy in vitamin D deficiency: Prospective study of 203 cases. World J. Surg. 2014, 38, 564–567. [Google Scholar] [CrossRef]
- Agarwal, G.; Aggarwal, V. Is total thyroidectomy the surgical procedure of choice for benign multinodular goiter? An evidence-based review. World J. Surg. 2008, 32, 1313. [Google Scholar] [CrossRef]
- Moalem, J.; Suh, I.; Duh, Q.Y. Treatment and prevention of recurrence of multinodular goiter: An evidence-based review of the literature. World J. Surg. 2008, 32, 1301–1312. [Google Scholar] [CrossRef]
- Palazzo, F.F.; Sywak, M.S.; Sidhu, S.B.; Barraclough, B.H.; Delbridge, L.W. 2005 Parathyroid autotransplantation during total thyroidectomy—Does the number of glands transplanted affect outcome? World J. Surg. 2005, 29, 629–631. [Google Scholar] [CrossRef]
- Zedenius, J.; Wadstrom, C.; Delbridge, L. Routine autotransplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. Aust. N. Z. J. Surg. 1999, 69, 794–797. [Google Scholar] [CrossRef]
- Almquist, M.; Hallgrimsson, P.; Nordenström, E.; Bergenfelz, A. Prediction of permanent hypoparathyroidism after total thyroidectomy. World J. Surg. 2014, 38, 2613–2620. [Google Scholar] [CrossRef]
- Chow, T.L.; Choi, C.Y.; Chiu, A.N.K. Postoperative PTH monitoring of hypocalcemia expedites discharge after thyroidectomy. Am. J. Otolaryngol. 2014, 35, 736–740. [Google Scholar] [CrossRef]
- Karamanakos, S.N.; Markou, K.B.; Panagopoulos, K.; Karavias, D.; Vagianos, C.E.; Scopa, C.D.; Fotopoulou, V.; Liava, A.; Vagenas, K. Complications and risk factors related to the extent of surgery in thyroidectomy. Results from 2,043 procedures. Hormones 2010, 9, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, I.; Pragacz, A.; Pragacz, K.; Grzesiuk, W.; Barczyński, M. Total thyroidectomy is associated with increased prevalence of permanent hypoparathyroidism. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 1675. [Google Scholar] [CrossRef]
- Thomusch, O.; Machens, A.; Sekulla, C.; Ukkat, J.; Brauckhoff, M.; Dralle, H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: A multivariate analysis of 5846 consecutive patients. Surgery 2003, 133, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Ritter, K.; Elfenbein, D.; Schneider, D.F.; Chen, H.; Sippel, R.S. Hypoparathyroidism after total thyroidectomy: Incidence and resolution. J. Surg. Res. 2015, 197, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Bollerslev, J.; Rejnmark, L.; Marcocci, C.; Shoback, D.M.; Sitges-Serra, A.; Van Biesen, W.; Dekkers, O.M. European society of endocrinology clinical guideline: Treatment of chronic hypoparathyroidism in adults. Eur. J. Endocrinol. 2015, 173, G1–G20. [Google Scholar] [CrossRef]
- Testa, A.; Fant, V.; De Rosa, A.; Fiore, G.F.; Grieco, V.; Castaldi, P.; Persiani, R.; Rausei, S.; D’ugo, D.; De Rosa, G. Calcitriol plus hydrochlorothiazide prevents transient post-thyroidectomy hypocalcemia. Horm. Metab. Res. 2006, 38, 821–826. [Google Scholar] [CrossRef]
| n = 130 | Intervention (n = 57) | Placebo (n = 73) |
|---|---|---|
| Age: median (Q1, Q3) | 53 (34.5, 61.5) | 56 (40.5, 66.5) |
| Female: n (%) | 43 (75.4%) | 57 (78%) |
| Body mass index: median (Q1, Q3) | 28.3 (24.9, 32.3) | 30.0 (26.3, 34.9) |
| Length of hospital stay: median (Q1, Q3) | 2 (2, 2) | 2 (2, 3) |
| Graves’ Disease: n (%) | 18 (31.6%) | 21 (28.8%) |
| Goitre: n (%) | 24 (42.1%) | 37 (50.7%) |
| Thyroid cancer: n (%) | 15 (26.3%) | 15 (20.5%) |
| Pre-dose 25-OH vitamin D (nmol/L) median (Q1, Q3) | 73.2 (58, 90) | 72.5 (58, 90) |
| Lost Contact | Analysed | p-Value | |
|---|---|---|---|
| n = 150 | 20 | 130 | |
| Age: median (Q1, Q3) | 53 (45.5, 60.2) | 55 (38, 63.3) | 0.78 |
| Female: (%) | 14 (70%) | 100 (78%) | |
| Body mass index: median (Q1, Q3) | 28.1 (24.7, 34.1) | 29.3 (25.5, 33.7) | 0.75 |
| Length of hospital stay: median (Q1, Q3) | 2 (2, 2.75) | 2 (2, 2) | 0.68 |
| Graves’ Disease: n (%) | 7 (35%) | 39 (30%) | 0.70 |
| Goitre: n (%) | 10 (50%) | 61 (46.9%) | |
| Thyroid cancer: n (%) | 3 (15%) | 30 (23.1%) | |
| Pre-dose 25-OH vitamin D (nmol/L): median (Q1, Q3) | 72 (54.25, 79.75) | 72 (57.75, 90) | 0.4 |
| n = 130 | Day 1 PTH < 10 | Day 1 PTH ≥ 10 | p-Value | |
|---|---|---|---|---|
| n | 36 | 94 | ||
| Permanent Hypoparathyroidism | Yes = 11 (8.4%) No = 119 (91.6%) | 3 (8.3%) 33 (91.7%) | 8 (8.5%) 86 (91.5%) | >0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannan, T.; Foster, Y.; Ho, D.J.; Gelzinnis, S.J.; Merakis, M.; Wynne, K.; Balogh, Z.J.; Bendinelli, C. Post-Operative Permanent Hypoparathyroidism and Preoperative Vitamin D Prophylaxis. J. Clin. Med. 2021, 10, 442. https://doi.org/10.3390/jcm10030442
Kannan T, Foster Y, Ho DJ, Gelzinnis SJ, Merakis M, Wynne K, Balogh ZJ, Bendinelli C. Post-Operative Permanent Hypoparathyroidism and Preoperative Vitamin D Prophylaxis. Journal of Clinical Medicine. 2021; 10(3):442. https://doi.org/10.3390/jcm10030442
Chicago/Turabian StyleKannan, Tara, Yasmin Foster, David J. Ho, Scott J. Gelzinnis, Michael Merakis, Katie Wynne, Zsolt J. Balogh, and Cino Bendinelli. 2021. "Post-Operative Permanent Hypoparathyroidism and Preoperative Vitamin D Prophylaxis" Journal of Clinical Medicine 10, no. 3: 442. https://doi.org/10.3390/jcm10030442
APA StyleKannan, T., Foster, Y., Ho, D. J., Gelzinnis, S. J., Merakis, M., Wynne, K., Balogh, Z. J., & Bendinelli, C. (2021). Post-Operative Permanent Hypoparathyroidism and Preoperative Vitamin D Prophylaxis. Journal of Clinical Medicine, 10(3), 442. https://doi.org/10.3390/jcm10030442







