The Prevention of Periprosthetic Joint Infection in Primary Total Hip Arthroplasty Using Pre-Operative Chlorhexidine Bathing
Abstract
1. Introduction
2. Materials and Methods
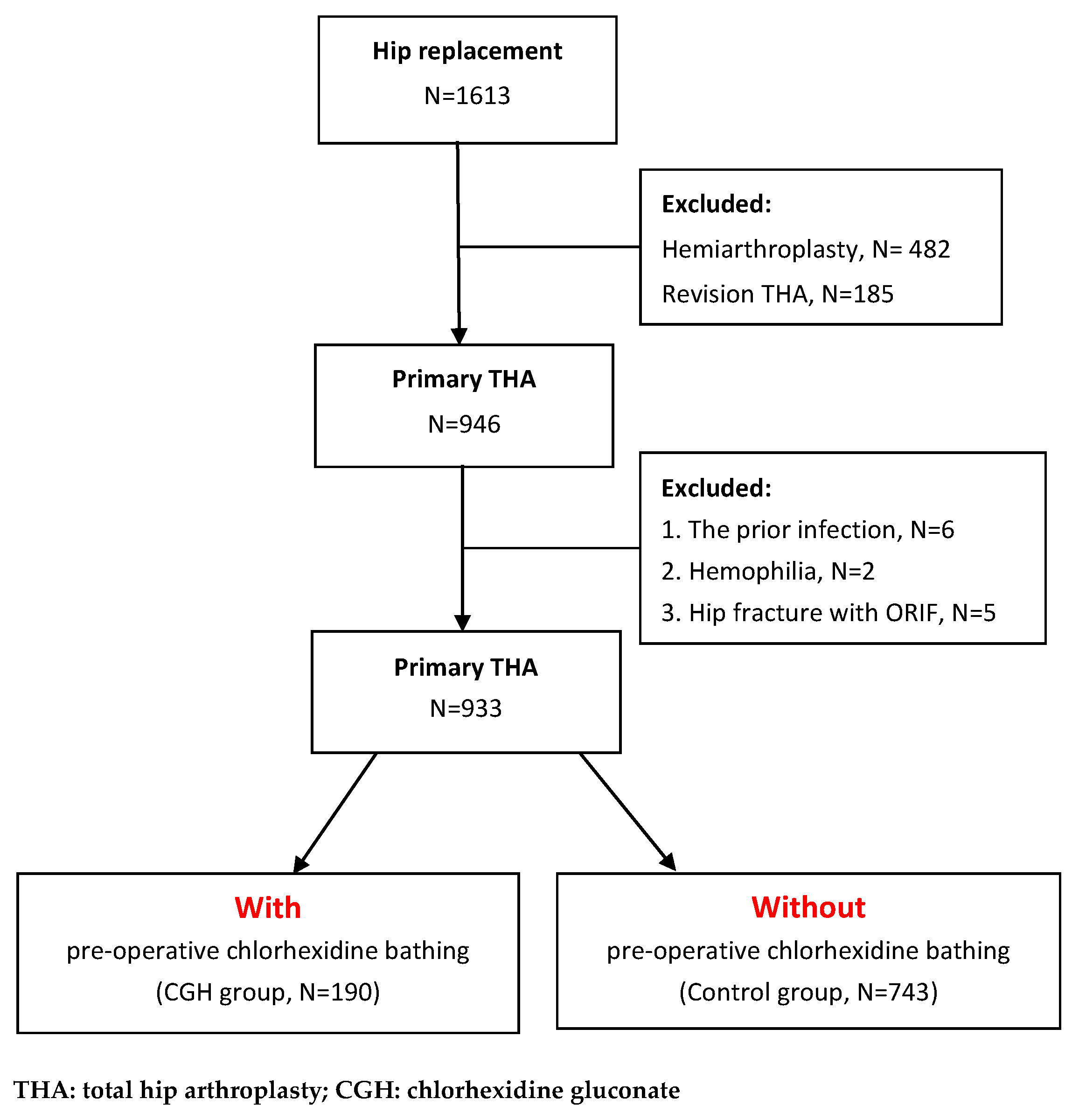
2.1. Study Participants
2.2. Anti-Infective Protocol
2.3. The Definition of PJI
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rienstra, W.; Van Der Veen, H.C.; Akker-Scheek, I.V.D.; Van Raay, J.J. Clinical Outcome, Survival and Polyethylene Wear of an Uncemented Total Hip Arthroplasty. J. Arthroplast. 2013, 28, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Ottink, K.D.; Barnaart, L.; Westerbeek, R.; Van Kampen, K.; Bulstra, S.; Van Jonbergen, H.-P. Survival, Clinical and Radiological Outcome of the Zweymüller SL/Bicon-Plus Total Hip Arthroplasty: A 15-Year Follow-up Study. HIP Int. 2015, 25, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Mohaddes, M.; Nauclér, E.; Kärrholm, J.; Malchau, H.; Odin, D.; Rolfson, O. Implant survival and patient-reported outcome following total hip arthroplasty in patients 30 years or younger: A matched cohort study of 1,008 patients in the Swedish Hip Arthroplasty Register. Acta Orthop. 2019, 90, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-S.; Moon, Y.-W.; Lim, S.-J.; Oh, I.; Lim, J.-S. Prognostic Factors Influencing the Functional Outcome of Total Hip Arthroplasty for Hip Infection Sequelae. J. Arthroplast. 2005, 20, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.E.; Crane, T.P.; Noy, M.; Elliott, T.S.J.; Grimer, R.J. The incidence of deep prosthetic infections in a specialist orthopaedic hospital a 15 year prospective survey. J. Bone Jt. Surg. 2006, 88, 943–948. [Google Scholar] [CrossRef]
- Biau, D.J.; Leclerc, P.; Marmor, S.; Zeller, V.; Graff, W.; Lhotellier, L.; Leonard, P.; Mamoudy, P. Monitoring the one year postoperative infection rate after primary total hip replacement. Int. Orthop. 2011, 36, 1155–1161. [Google Scholar] [CrossRef]
- Fender, D.H.; Harper, W.M.; Gregg, P.J. Outcome of Charnley total hip replacement across a single health region in England. J. Bone Jt. Surg. 1999, 81, 577–581. [Google Scholar] [CrossRef]
- Pulido, L.; Ghanem, E.; Joshi, A.; Purtill, J.J.; Parvizi, J. Periprosthetic Joint Infection: The Incidence, Timing, and Predisposing Factors. Clin. Orthop. Relat. Res. 2008, 466, 1710–1715. [Google Scholar] [CrossRef]
- Culliford, D.; Maskell, J.; Judge, A.; Cooper, C.; Prieto-Alhambra, D.; Arden, N.K. Future projections of total hip and knee arthroplasty in the UK: Results from the UK Clinical Practice Research Datalink. Osteoarthr. Cartil. 2015, 23, 594–600. [Google Scholar] [CrossRef]
- Klouche, S.; Sariali, E.; Mamoudy, P. Analyse du coût des reprises des prothèses totales de hanche infectées. Rev. Chir. Orthopédique Traumatol. 2010, 96, 167–175. [Google Scholar] [CrossRef]
- Vanhegan, I.S.; Malik, A.K.; Jayakumar, P.; Ul Islam, S.; Haddad, F.S. A financial analysis of revision hip arthroplasty the eco-nomic burden in relation to the national. J. Bone Jt. Surg. 2012, 94, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, N.; Anis, H.K.; Garbarino, L.J.; Gold, P.A.; Kurtz, S.M.; Higuera, C.A.; Hepinstall, M.S.; Mont, M.A. Have We Actually Reduced Our 30-Day Short-Term Surgical Site Infection Rates in Primary Total Hip Arthroplasty in the United States? J. Arthroplast. 2019, 34, 2102–2106. [Google Scholar] [CrossRef] [PubMed]
- Sukeik, M.; Haddad, F.S. Periprosthetic joint infections after total hip replacement: An algorithmic approach. SICOT-J. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.W.; Cook, M.J.; O’Neill, T.W.; Board, T.N. Is the use of antibiotic-loaded bone cement associated with a lower risk of revision after primary total hip arthroplasty? Bone Jt. J. 2020, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Cheng, A.C. Impact of non-rinse skin cleansing with chlorhexidine gluconate on prevention of healthcare-associated infections and colonization with multi-resistant organisms: A systematic review. J. Hosp. Infect. 2012, 82, 71–84. [Google Scholar] [CrossRef]
- Dumville, J.C.; McFarlane, E.; Edwards, P.; Lipp, A.; Holmes, A.; Liu, Z. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst. Rev. 2015, 2015, CD003949. [Google Scholar] [CrossRef]
- Tanner, J.; Dumville, J.C.; Norman, G.; Fortnam, M. Surgical hand antisepsis to reduce surgical site infection. Cochrane Database Syst. Rev. 2016, CD004288. [Google Scholar] [CrossRef]
- George, J.; Klika, A.K.; Higuera, C.A. Use of Chlorhexidine Preparations in Total Joint Arthroplasty. J. Bone Jt. Infect. 2017, 2, 15–22. [Google Scholar] [CrossRef]
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control. 2004, 32, 470–485. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Elmallah, R.K.; Mont, M.A. A Randomized, Clinical Trial of Preadmission Chlorhexidine Skin Preparation for Lower Extremity Total Joint Arthroplasty. J. Arthroplast. 2016, 31, 2856–2861. [Google Scholar] [CrossRef]
- Frisch, M.M.N.B.; Kadri, O.M.; Tenbrunsel, B.T.; Abdul-Hak, B.A.; Qatu, B.M.; Davis, J.J. Intraoperative chlorhexidine irrigation to prevent infection in total hip and knee arthroplasty. Arthroplast. Today 2017, 3, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, K.; Hou, W.; Yang, Z.; Xu, P. Preoperative chlorhexidine reduces the incidence of surgical site infections in total knee and hip arthroplasty: A systematic review and meta-analysis. Int. J. Surg. 2017, 39, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, B.H.; Johnson, A.J.; Daley, J.A.; Issa, K.; Mont, M.A. Pre-admission Cutaneous Chlorhexidine Preparation Reduces Surgical Site Infections in Total Hip Arthroplasty. J. Arthroplast. 2013, 28, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, B.H.; Jauregui, J.J.; Murray, D.P.; Mont, M.A. Does Preadmission Cutaneous Chlorhexidine Preparation Reduce Surgical Site Infections After Total Hip Arthroplasty? Clin. Orthop. Relat. Res. 2016, 474, 1583–1588. [Google Scholar] [CrossRef]
- Franco, L.M.D.C.; Cota, G.F.; Pinto, T.S.; Ercole, F.F. Preoperative bathing of the surgical site with chlorhexidine for infection prevention: Systematic review with meta-analysis. Am. J. Infect. Control. 2017, 45, 343–349. [Google Scholar] [CrossRef]
- Chlebicki, M.P.; Safdar, N.; O’Horo, J.C.; Maki, D.G. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: A meta-analysis. Am. J. Infect. Control. 2013, 41, 167–173. [Google Scholar] [CrossRef]
- Markatos, K.; Kaseta, M.; Nikolaou, V.S. Perioperative Skin Preparation and Draping in Modern Total Joint Arthroplasty: Current Evidence. Surg. Infect. 2015, 16, 221–225. [Google Scholar] [CrossRef]
- Webster, J.; Osborne, S. Preoperative bathing or showering with skin antiseptics to prevent surgical site infection. Cochrane Database Syst. Rev. 2015, CD004985. [Google Scholar] [CrossRef]
- Driesman, A.; Shen, M.; Feng, J.E.; Waren, D.; Slover, J.; Bosco, J.; Schwarzkopf, R. Perioperative Chlorhexidine Gluconate Wash During Joint Arthroplasty Has Equivalent Periprosthetic Joint Infection Rates in Comparison to Betadine Wash. J. Arthroplast. 2020, 35, 845–848. [Google Scholar] [CrossRef]
- Parvizi, J.; Gehrke, T. Definition of Periprosthetic Joint Infection. J. Arthroplast. 2014, 29, 1331. [Google Scholar] [CrossRef] [PubMed]
- Zywiel, M.G.; Daley, J.A.; Delanois, R.E.; Naziri, Q.; Johnson, A.J.; Mont, M.A. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int. Orthop. 2010, 35, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, B.H.; Zhou, P.L.; Jauregui, J.J.; Mont, M.A. Does Preadmission Cutaneous Chlorhexidine Preparation Reduce Surgical Site Infections After Total Knee Arthroplasty? Clin. Orthop. Relat. Res. 2016, 474, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Lipke, V.L.; Hyott, A.S. Reducing surgical site infections by bundling multiple risk reduction strategies and active surveillance. Aorn J. 2010, 92, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.M.; Carver, R.L. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am. J. Infect. Control. 2010, 38, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, J.; Rn, A.P.; Wann-Hansson, C. Searching for Evidence Regarding Using Preoperative Disinfection Showers to Prevent Surgical Site Infections: A Systematic Review. Worldviews Evid. Based Nurs. 2011, 8, 143–152. [Google Scholar] [CrossRef] [PubMed]
| Major Criteria | Two positive periprosthetic cultures with phenotypically identical organisms, OR |
| A sinus tract communicating with the joint, OR | |
| Minor Criteria | (1) Elevated serum C-reactive protein (CRP) AND erythrocyte sedimentation rate (ESR) |
| (2) Elevated synovial fluid white blood cell (WBC) count OR ++change on leukocyte esterase test strip | |
| (3) Elevated synovial fluid polymorphonuclear neutrophil percentage (PMN%) | |
| (4) Positive histological analysis of periprosthetic tissue | |
| (5) A single positive culture |
| CHG (N = 190) | Control (N = 743) | p-Value | |
|---|---|---|---|
| Age (mean ± SD) | 57.71 ± 15.23 | 59.63 ± 15.42 | 0.170 |
| Sex (N (%)) | 0.189 | ||
| Female | 112 (58.9%) | 396 (53.3%) | |
| Male | 78 (41.0%) | 347 (46.7%) | |
| Laterality (N (%)) | 0.212 | ||
| Rt side | 112 (58.9%) | 398 (53.5%) | |
| Lt side | 78 (41.0%) | 345 (46.4%) |
| Case No. | Pathogens | Onset time (Days) | Cemented |
|---|---|---|---|
| 1 | Staphylococcus aureus | 54 | No |
| 2 | Acinetobacter baumannii | 43 | Yes |
| 3 | Pseudomonas aeruginosa | 21 | No |
| 4 | Staphylococcus epidermidis | 30 | No |
| CHG (N = 190) | Control (N = 743) | Total (N = 933) | p-Value | |
|---|---|---|---|---|
| PJI cases | 0 (0%) | 4 (0.54%) | 4 (0.43%) | 0.588 |
| Within 90 days | 0 (0%) | 4 (0.54%) | 4 (0.43%) | |
| Beyond 90 days | 0 (0%) | 0 (0%) | 0 (0%) | |
| No PJI cases | 190 (100%) | 739 (99.46%) | 929 (99.57%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-C.; Lai, Y.-C.; Lee, C.-H.; Shih, C.-M.; Chen, C.-P.; Hung, L.-L.; Wang, S.-P. The Prevention of Periprosthetic Joint Infection in Primary Total Hip Arthroplasty Using Pre-Operative Chlorhexidine Bathing. J. Clin. Med. 2021, 10, 434. https://doi.org/10.3390/jcm10030434
Su W-C, Lai Y-C, Lee C-H, Shih C-M, Chen C-P, Hung L-L, Wang S-P. The Prevention of Periprosthetic Joint Infection in Primary Total Hip Arthroplasty Using Pre-Operative Chlorhexidine Bathing. Journal of Clinical Medicine. 2021; 10(3):434. https://doi.org/10.3390/jcm10030434
Chicago/Turabian StyleSu, Wen-Chi, Yu-Chin Lai, Cheng-Hung Lee, Cheng-Min Shih, Chao-Ping Chen, Li-Ling Hung, and Shun-Ping Wang. 2021. "The Prevention of Periprosthetic Joint Infection in Primary Total Hip Arthroplasty Using Pre-Operative Chlorhexidine Bathing" Journal of Clinical Medicine 10, no. 3: 434. https://doi.org/10.3390/jcm10030434
APA StyleSu, W.-C., Lai, Y.-C., Lee, C.-H., Shih, C.-M., Chen, C.-P., Hung, L.-L., & Wang, S.-P. (2021). The Prevention of Periprosthetic Joint Infection in Primary Total Hip Arthroplasty Using Pre-Operative Chlorhexidine Bathing. Journal of Clinical Medicine, 10(3), 434. https://doi.org/10.3390/jcm10030434






