Risk Factors for Surgical Treatment of Endometrial Cancer Using Traditional and Laparoscopic Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Endpoints
2.2. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Selected Anthropometric and Anatomical Measurements
3.3. Tumor Staging and Grading
3.4. Procedure-Related Complications
3.5. Duration of Hospitalization
3.6. Duration of Surgery
3.7. Procedure-Related Hemoglobin Loss
4. Discussion
4.1. Risk Factors for Surgical Treatment
4.2. Clinical Staging
4.3. Obesity
4.4. Pelvic Dimensions
4.5. Previous Abdominal Surgeries and Deliveries
4.6. Method of Surgery
4.7. Lymphadenectomy
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García Pineda, V.; Hernández Gutiérrez, A.; Gracia Segovia, M.; Siegrist Ridruejo, J.; Diestro Tejeda, M.D.; Zapardiel, I. Low-Volume nodal metastasis in endometrial cancer: Risk factors and prognostic significance. J. Clin. Med. 2020, 9, 1999. [Google Scholar] [CrossRef]
- Uterine Cancer Incidence Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/incidence (accessed on 1 December 2020).
- Von Gruenigen, V.E.; Tian, C.; Frasure, H.; Waggoner, S.; Keys, H.; Barakat, R.R. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma: A gynecologic oncology group study. Cancer 2006, 107, 2786–2791. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Spiegel, G.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic oncology group study LAP2. J. Clin. Oncol. 2009, 27, 5331–5336. [Google Scholar] [CrossRef]
- Scaletta, G.; Dinoi, G.; Capozzi, V.; Cianci, S.; Pelligra, S.; Ergasti, R.; Fagotti, A.; Scambia, G.; Fanfani, F. Comparison of minimally invasive surgery with laparotomic approach in the treatment of high risk endometrial cancer: A systematic review. Eur. J. Surg. Oncol. 2020, 46, 782–788. [Google Scholar] [CrossRef]
- Fondrinier, E.; Rodier, J.F.; Morice, P.; Le Bouëdec, G.; Descamps, P.; Lefranc, J.P. Surgical treatment for endometrial adenocarcinoma: First approaches. Review of the literature. Gynecol. Obstet. Fertil. 2003, 31, 456–464. [Google Scholar] [CrossRef]
- Polan, R.M.; Rossi, E.C.; Barber, E.L. Extent of lymphadenectomy and postoperative major complications among women with endometrial cancer treated with minimally invasive surgery. Am. J. Obstet. Gynecol. 2019, 220, 263.e1–263.e8. [Google Scholar] [CrossRef] [PubMed]
- Obermair, A.; Manolitsas, T.P.; Leung, Y.; Hammond, I.G.; McCartney, A.J. Total laparoscopic hysterectomy versus total abdominal hysterectomy for obese women with endometrial cancer. Int. J. Gynecol. Cancer 2005, 15, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chu, T.; Ghodoussipour, S.; Bowman, C.; Patel, H.; King, K.; Hung, A.J. Effect of Surgeon Experience and Bony Pelvic Dimensions on Surgical Performance and Patient Outcomes in Robot-Assisted Radical Prostatectomy. BJU Int. 2019, 124. [Google Scholar] [CrossRef]
- Yu, Y.-l.; Liu, X.-h.; Liu, H.-s.; Ke, J.; Zou, Y.-f.; Cao, W.-t.; Xiao, J.; Zhou, Z.-y.; Lan, P.; Wu, X.-j.; et al. Impact of Pelvic Dimensions on Anastomotic Leak after Anterior Resection for Patients with Rectal Cancer. Surg. Endosc. 2020. [Google Scholar] [CrossRef]
- Sznurkowski, J.; Knapp, P.; Bodnar, L.; Bidziński, M.; Jach, R.; Misiek, M.; Bieńkiewicz, A.; Blecharz, P.; Kojs, Z.; Kotarski, J.; et al. Recommendations of the Polish Gynecological Oncology Society for the diagnosis and treatment of endometrial cancer. Curr. Gynecol. Oncol. 2017, 15, 34–44. [Google Scholar] [CrossRef]
- Laparoscopic Sentinel Node Detection with Indocyanine Green in Endometrial Cancer. 2016. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6135175/ (accessed on 27 December 2020).
- Anton, C.; Truffa Kleine, R.; Mayerhoff, E.; del Pilar Esteves Diz, M.; de Freitas, D.; de Andrade Carvalho, H.; de Carvalho, J.P.M.; Silva e Silva, A.; Nogueira Dias Genta, M.L.; de Faria e Silva, A.L.; et al. Ten years of experience with endometrial cancer treatment in a single Brazilian institution: Patient characteristics and outcomes. PLoS ONE 2020. [Google Scholar] [CrossRef]
- Bennich, G.; Rudnicki, M.; Lassen, P.D. Laparoscopic surgery for early endometrial cancer. Acta Obstet. Gynecol. Scand. 2016, 95, 894–900. [Google Scholar] [CrossRef]
- Santoso, J.T.; Barton, G.; Riedley-Malone, S.; Wan, J.Y. Obesity and perioperative outcomes in endometrial cancer surgery. Arch. Gynecol. Obstet. 2012, 285, 1139–1144. [Google Scholar] [CrossRef]
- Uccella, S.; Bonzini, M.; Palomba, S.; Fanfani, F.; Ceccaroni, M.; Seracchioli, R.; Vizza, E.; Ferrero, A.; Roviglione, G.; Casadio, P.; et al. Impact of obesity on surgical treatment for endometrial cancer: A multicenter study comparing laparoscopy vs open surgery, with propensity-matched analysis. J. Minim. Invasive Gynecol. 2016, 23, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.; Jernigan, A.M.; Aljebori, Q.; Lockhart, D.; Moslemi-Kebria, M. The impact of obesity on the 30-day morbidity and mortality after surgery for endometrial cancer. J. Minim. Invasive Gynecol. 2015, 22, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.; Peijnenburg, E.; Stone, R.L.; Wethington, S.L.; Levinson, K.L.; Beavis, A.; Yen, T.-T.; Tanner, E.J.; Fader, A.N. Laparoscopic surgical access in morbidly obese women undergoing endometrial cancer surgery: Repurposing the left upper quadrant approach. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 56–59. [Google Scholar] [CrossRef]
- Hurd, W.W.; Bude, R.O.; DeLancey, J.O.; Pearl, M.L. The relationship of the umbilicus to the aortic bifurcation: Implications for laparoscopic technique. Obstet. Gynecol. 1992, 80, 48–51. [Google Scholar] [PubMed]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic oncology group LAP2 Study. J. Clin. Oncol. 2012, 30, 695–700. [Google Scholar] [CrossRef]
- Bouwman, F.; Smits, A.; Lopes, A.; Das, N.; Pollard, A.; Massuger, L.; Bekkers, R.; Galaal, K. The impact of BMI on surgical complications and outcomes in endometrial cancer surgery—An institutional study and systematic review of the literature. Gynecol. Oncol. 2015, 139, 369–376. [Google Scholar] [CrossRef]
- Yu, C.K.H.; Cutner, A.; Mould, T.; Olaitan, A. Total laparoscopic hysterectomy as a primary surgical treatment for endometrial cancer in morbidly obese women. BJOG Int. J. Obstet. Gynaecol. 2005, 112, 115–117. [Google Scholar] [CrossRef]
- Zullo, F.; Palomba, S.; Russo, T.; Falbo, A.; Costantino, M.; Tolino, A.; Zupi, E.; Tagliaferri, P.; Venuta, S. A prospective randomized comparison between laparoscopic and laparotomic approaches in women with early stage endometrial cancer: A focus on the quality of life. Am. J. Obstet. Gynecol. 2005, 193, 1344–1352. [Google Scholar] [CrossRef]
- Pellegrino, A.; Signorelli, M.; Fruscio, R.; Villa, A.; Buda, A.; Beretta, P.; Garbi, A.; Vitobello, D. Feasibility and morbidity of total laparoscopic radical hysterectomy with or without pelvic limphadenectomy in obese women with stage I endometrial cancer. Arch. Gynecol. Obstet. 2009, 279, 655–660. [Google Scholar] [CrossRef]
- Laparoscopy Versus Laparotomy for the Management of Presumed Early Stage Endometrial Cancer|COCHRANE. 2018. Available online: https://www.cochrane.org/CD006655/GYNAECA_laparoscopy-versus-laparotomy-management-presumed-early-stage-endometrial-cancer (accessed on 1 December 2020).
- Janda, M.; Gebski, V.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; McCartney, A.; Nascimento, M.; Neesham, D.; et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): A randomised trial. Lancet Oncol. 2010, 11, 772–780. [Google Scholar] [CrossRef]
- Ruan, X.C.; Wong, W.L.; Yeong, H.Q.; Lim, Y.K.T. Comparison of outcomes following laparoscopic and open hysterectomy with pelvic lymphadenectomy for early stage endometrial carcinoma. Singap. Med. J. 2018, 59, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Surgical Outcomes Among Elderly Women with Endometrial Cancer Treated by Laparoscopic Hysterectomy: A NRG/Gynecologic Oncology Group study. Abstract–Europe PMC. 2017. Available online: https://europepmc.org/article/pmc/pmc5756682 (accessed on 2 December 2020).
- Cakmak, Y.; Comert, D.K.; Sozen, I.; Oge, T. Comparison of laparoscopy and laparotomy in early-stage endometrial cancer: Early experiences from a developing country. J. Oncol. 2020, 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z. Laparoscopy versus laparotomy in the treatment of high-risk endometrial cancer: A propensity score matching analysis. Medicine 2015, 94, e1245. [Google Scholar] [CrossRef] [PubMed]
- Abdelazim, I.A.; Abu-Faza, M.; Zhurabekova, G.; Shikanova, S.; Karimova, B.; Sarsembayev, M.; Starchenko, T.; Mukhambetalyeva, G. Sentinel lymph nodes in endometrial cancer update 2018. Gynecol. Minim. Invasive Ther. 2019, 8, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.A.; Sozzi, G.; Gambino, G.; Cianciolo, A.; Riccò, M.; Monfardini, L.; Gaiano, M.; Chiantera, V.; Uccella, S.; Berretta, R. Laparoscopy versus laparotomy for surgical treatment of obese women with endometrial cancer: A cost-benefit comparative analysis. Mol. Clin. Oncol. 2019, 11, 335–342. [Google Scholar] [CrossRef]
- Mouraz, M.; Ferreira, C.S.; Gonçalves, S.; Martins, N.N.; Martins, F.N. Laparoscopic approach in surgical staging of endometrial cancer. Rev. Bras. Ginecol. Obstet. 2019, 41, 306–311. [Google Scholar] [CrossRef]

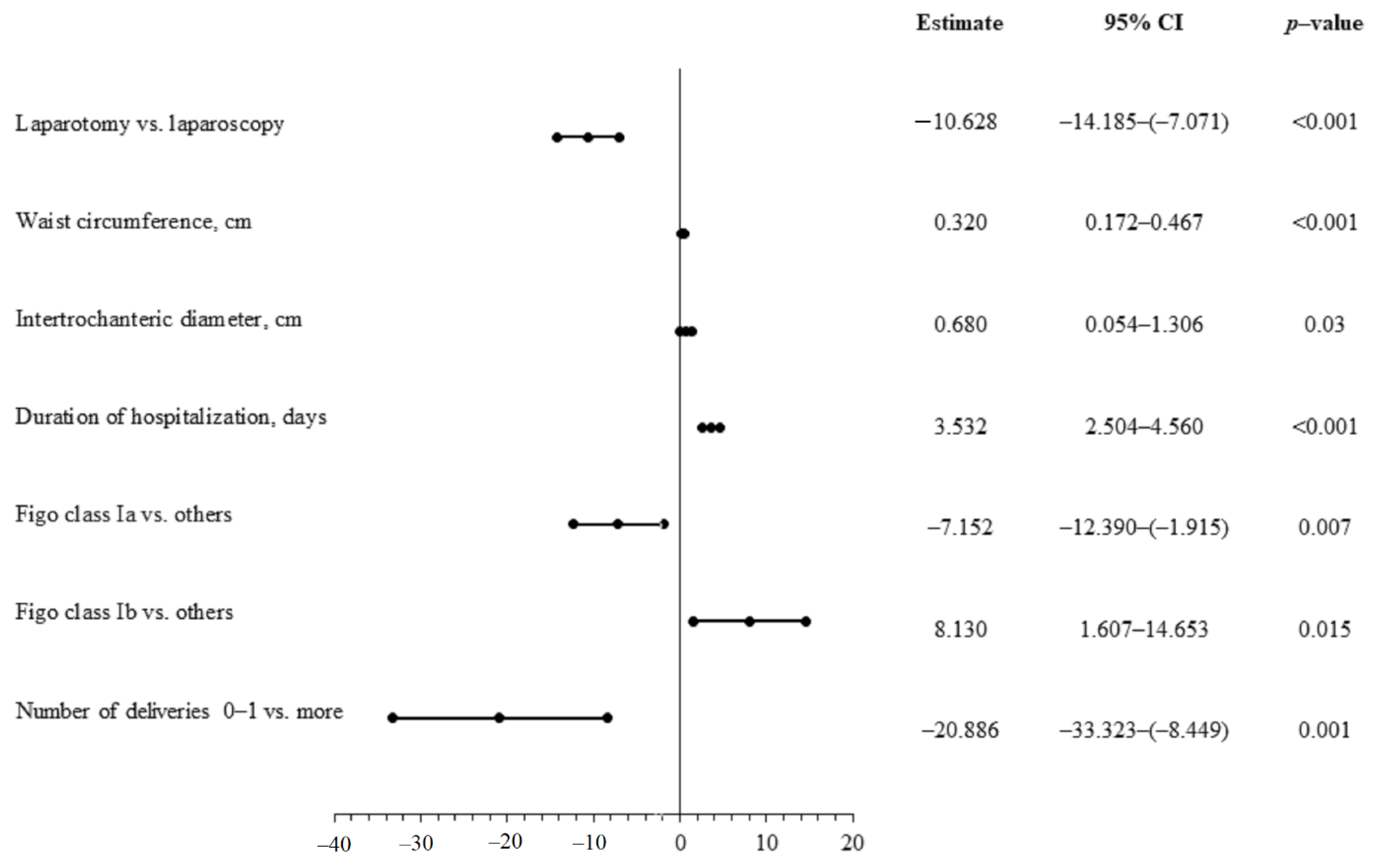

| Variables | Total n = 145 | Laparotomy n = 70 | Laparoscopy n = 75 | p-Value |
|---|---|---|---|---|
| Age, years | 61.8 ± 9.5 | 61.3 ± 10.4 | 62.2 ± 8.7 | 0.55 |
| Height, cm | 160.9 ± 5.3 161 (158 ÷ 164) | 160.1 ± 5.6 160 (157 ÷ 164) | 161.5 ± 4.9 162 (158 ÷ 164) | 0.22 |
| Weight, kg | 83.4 ± 19.8 | 82.6 ± 19.4 | 84.1 ± 20.3 | 0.65 |
| Body mass index, kg/m2 | 32.2 ± 7.6 31 (26.4 ÷ 36.2) | 32.2 ± 8.0 30.6 (26 ÷ 37.8) | 32.1 ± 7.3 31 (27.2 ÷ 34.8) | 0.73 |
| Waist circumstance, cm | 107.3 ± 18.6 | 105.3 ± 18.4 | 109.2 ± 18.8 | 0.24 |
| Hip circumstance, cm | 113.5 ± 18.0 111 (102 ÷ 125) | 112.5 ± 15.5 109.5 (100.5 ÷ 125) | 114.4 ± 20.2 112 (104 ÷ 125) | 0.24 |
| Waist-to-hip ratio | 0.93 ± 0.1 0.94 (0.9 ÷ 0.97) | 0.92 ± 0.1 0.92 (0.88 ÷ 0.97) | 0.94 ± 0.1 0.95 (0.91 ÷ 0.97) | 0.04 |
| Obesity, Body mass index >30 kg/m2 | 84 (57.9) | 37 (52.8) | 47 (62.7) | 0.24 |
| External conjugate diameter, cm | 22.0 ± 1.4 22 (21 ÷ 23) | 22.2 ± 1.5 22 (21 ÷ 23.2) | 21.9 ± 1.4 22 (21 ÷ 23) | 0.21 |
| Interspinosus diameter, cm | 27.1 ± 2.0 27 (26 ÷ 28) | 26.9 ± 2.1 27 (25 ÷ 28) | 27.3 ± 1.9 27 (26 ÷ 28) | 0.15 |
| Intercristal diameter, cm | 32.5 ± 2.8 33 (30 ÷ 34) | 32.3 ± 3.0 33 (30 ÷ 34.2) | 32.6 ± 2.6 33 (31 ÷ 34) | 0.56 |
| Intertrochanteric diameter, cm | 35.2 ± 3.5 35 (35 ÷ 37) | 35.3 ± 2.1 35 (34 ÷ 37) | 35.1 ± 4.4 35 (35 ÷ 37) | 0.15 |
| Variables | Total n = 145 | Laparotomy n = 70 | Laparoscopy n = 75 | p-Value |
|---|---|---|---|---|
| Prior abdominal surgery | 0.61 ± 0.7 | 0.65 ± 0.8 | 0.57 ± 0.6 | 0.54 |
| Number of prior abdominal surgeries: | ||||
| 0 | 71 (49.6) | 34 (50) | 37 (49.3) | 0.92 |
| 1 | 61 (42.7) | 28 (41.2) | 33 (44) | 0.62 |
| 2 | 8 (5.6) | 3 (4.4) | 5 (6.7) | 0.53 |
| 3 | 2 (1.4) | 2 (2.9) | 0 (0) | 0.14 |
| 4 | 1 (0.7) | 1 (1.5) | 0 (0) | 0.29 |
| Number of prior deliveries | 2.4 ± 1.2 | 2.6 ± 1.2 | 2.2 ± 1.2 | 0.13 |
| Most frequent concomitant diseases | ||||
| Diabetes mellitus | 57 (39.3) | 32 (45.7) | 25 (33.3) | 0.17 |
| Arterial hypertension | 67 (46.2) | 33 (47.1) | 34 (45.3) | 0.82 |
| Chronic obstructive pulmonary disease | 16 (11) | 8 (11.4) | 8 (10.7) | 0.88 |
| Bronchial asthma | 7 (4.8) | 1 (2,4) | 6(8) | 0.05 |
| Hypothyreosis | 27 (18.6) | 10 (4.3) | 17 (22.7) | 0.19 |
| Chronic pancreatitis | 11 (7.6) | 3 (4.3) | 8 (10.7) | 0.13 |
| Heart failure | 9 (6.2) | 5 (7.1) | 4 (5.3) | 0.65 |
| Coronary artery disease | 2 7 (18.6) | 17 (24.3) | 10 (13.3) | 0.09 |
| Number of concomitant diseases | 1.8 ± 1.6 | 1.8 ± 1.5 | 1.9 ± 1.6 | 0.53 |
| Variables | Total n = 145 | Laparotomy n = 70 | Laparoscopy n = 75 | p-Value |
|---|---|---|---|---|
| Duration of operation, min. | 88.8 ± 19.4 | 86.1 ± 20.4 | 91.4 ± 18.2 | 0.09 |
| Duration of hospitalization, days | 8.8 ± 3.4 8 (6 ÷ 12) | 11.3 ± 3.1 12 (8.7 ÷ 13) | 6.5 ± 1.7 7 (5 ÷ 8) | <0.001 |
| Class of lymphadenectomy | 1.4 ± 0.8 1 (1 ÷ 2) | 1.1 ± 0.9 1 (0 ÷ 2) | 1.6 ± 0.7 2 (1 ÷ 2) | 0.007 |
| Class of lymphadenectomy: | ||||
| 0 | 24 (16.5) | 23 (32.8) | 1 (1.3) | <0.001 |
| I | 50 (34.5) | 15 (21.4) | 35 (46.7) | 0.001 |
| II | 64 (44.1) | 31 (44.3) | 33 (44) | 0.97 |
| III | 7 (4.8) | 1 (1.4) | 6 (8) | 0.06 |
| Procedure-related complications | 10 (6.9) | 5 (7.1) | 5 (6.7) | 0.91 |
| Periprocedural hemoglobin loss, g/dL | 1.6 ± 0.9 | 2.25 ± 0.9 | 1.0 ± 0.2 | <0.001 |
| Staging according to FIGO classification: (Ia-1, IB-2, II-3, III-A-4, IIIB-5, IIIC1-6, IIIC2-7, IVA-8, IVB-9) | 2.1 ± 1.4 2 (1 ÷ 3) | 2.0 ± 1.5 1 (1 ÷ 2.25) | 2.3 ± 1.3 2 (2 ÷ 3) | 0.01 |
| Staging according to FIGO classification: | ||||
| IA | 54 (37.9) | 36 (51.4) | 18 (24) | 0.011 |
| IB | 52 (35.9) | 17 (24.3) | 35 (46.7) | 0.005 |
| II | 22 (15.2) | 9 (12.9) | 13 (17.3) | 0.45 |
| IIIA | 8 (5.5) | 5 (7.1) | 3 (4) | 0.4 |
| IIIB | 4 (2.8) | 0 (0) | 4 (5.3) | 0.05 |
| III C1 | 2 (1.4) | 1 (1.4) | 1 (1.3) | 0.96 |
| IIIC2 | 0 | 0 | 0 | 0 |
| IVA | 3 (2.1) | 2 (2.9) | 1 (1.3) | 0.6 |
| IVB | 0 | 0 | 0 | 0 |
| Histological grading; (G1-1, G2-2, G3-3) | 1.52 ± 0.7 2 (1 ÷ 3) | 1.56 ± 0.7 1 (1 ÷ 2.25) | 1.49 ± 0.6 2 (2 ÷ 3) | 0.53 |
| Histological grading: | ||||
| 1 | 81 (55.9) | 37 (52.9) | 44 (58.7) | 0.48 |
| 2 | 51 (35.2) | 26 (37.1) | 25 (33.3) | 0.63 |
| 3 | 13 (9) | 7 (10) | 6 (8) | 0.67 |
| Duration of Operation | Duration of Hospitalization | Periprocedural Hemoglobin Loss | ||||
|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | |
| Age, years | 0.11 | 0.16 | 0.01 | 0.86 | −0.03 | 0.68 |
| Height, cm | −0.07 | 0.37 | −0.13 | 0.11 | −0.11 | 0.15 |
| Weight, kg | 0.55 | <0.001 | 0.29 | <0.001 | 0.29 | <0.001 |
| BMI, kg/m2 | 0.58 | <0.001 | 0.31 | <0.001 | 0.32 | <0.001 |
| Waist circumference, cm | 0.65 | <0.001 | 0.29 | <0.001 | 0.26 | 0.001 |
| Hip circumference, cm | 0.57 | <0.001 | 0.22 | 0.007 | 0.23 | 0.005 |
| Waist-to-hip ratio | 0.44 | <0.001 | 0.13 | 0.1 | 0.1 | 0.19 |
| Intertrochanteric diameter, cm | 0.43 | <0.001 | 0.08 | 0.31 | 0.05 | 0.5 |
| Interspinosus diameter, cm | 0.35 | <0.001 | 0.08 | 0.28 | 0.02 | 0.77 |
| Intercristal diameter, cm | 0.31 | <0.001 | 0.08 | 0.31 | 0.13 | 0.11 |
| External conjugate diameter, cm | 0.2 | 0.01 | 0.3 | <0.001 | 0.23 | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Januszek, S.M.; Wita-Popow, B.; Kluz, M.; Janowska, M.; Januszek, R.; Wróbel, A.; Rogowski, A.; Malinowski, K.P.; Zuzak, T.; Kluz, T. Risk Factors for Surgical Treatment of Endometrial Cancer Using Traditional and Laparoscopic Methods. J. Clin. Med. 2021, 10, 429. https://doi.org/10.3390/jcm10030429
Januszek SM, Wita-Popow B, Kluz M, Janowska M, Januszek R, Wróbel A, Rogowski A, Malinowski KP, Zuzak T, Kluz T. Risk Factors for Surgical Treatment of Endometrial Cancer Using Traditional and Laparoscopic Methods. Journal of Clinical Medicine. 2021; 10(3):429. https://doi.org/10.3390/jcm10030429
Chicago/Turabian StyleJanuszek, Sławomir M., Barbara Wita-Popow, Marta Kluz, Magdalena Janowska, Rafał Januszek, Andrzej Wróbel, Artur Rogowski, Krzysztof P. Malinowski, Tomasz Zuzak, and Tomasz Kluz. 2021. "Risk Factors for Surgical Treatment of Endometrial Cancer Using Traditional and Laparoscopic Methods" Journal of Clinical Medicine 10, no. 3: 429. https://doi.org/10.3390/jcm10030429
APA StyleJanuszek, S. M., Wita-Popow, B., Kluz, M., Janowska, M., Januszek, R., Wróbel, A., Rogowski, A., Malinowski, K. P., Zuzak, T., & Kluz, T. (2021). Risk Factors for Surgical Treatment of Endometrial Cancer Using Traditional and Laparoscopic Methods. Journal of Clinical Medicine, 10(3), 429. https://doi.org/10.3390/jcm10030429







