Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers on Acute Kidney Injury in Emergency Medical Admissions
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Population and Acute Kidney Injury (AKI) Assessment
2.3. Treatment with RAS Blockers
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics and Risk Factors for AKI
3.2. RAS Blockers and AKI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.E.; Muntner, P.; Chertow, G.M.; Warnock, D.G. Acute kidney injury and mortality in hospitalized patients. Am. J. Nephrol. 2012, 35, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Bellomo, R.; Goldsmith, D.; Bates, S.; Ronco, C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit. Care Med. 2006, 34, 1913–1917. [Google Scholar] [CrossRef]
- Pannu, N.; James, M.; Hemmelgarn, B.R.; Dong, J.; Tonelli, M.; Klarenbach, S.; On Behalf of Alberta Kidney Disease Network. Modification of outcomes after acute kidney injury by the presence of CKD. Am. J. Kidney Dis. 2011, 58, 206–213. [Google Scholar] [CrossRef]
- Mansfield, K.E.; Douglas, I.J.; Nitsch, D.; Thomas, S.L.; Smeeth, L.; Tomlinson, L.A. Acute kidney injury and infections in patients taking antihypertensive drugs: A self-controlled case series analysis. Clin. Epidemiol. 2018, 10, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Savoia, C.; De Paolis, P.; Ostrowska, B.; Tarasi, D.; Rubattu, S. The Renin-Angiotensin System as a Risk Factor and Therapeutic Target for Cardiovascular and Renal Disease. J. Am. Soc. Nephrol. 2002, 13 (Suppl. 3), S173–S178. [Google Scholar] [CrossRef]
- Linde, C.; Bakhai, A.; Furuland, H.; Evans, M.; McEwan, P.; Ayoubkhani, D.; Qin, L. Real-World Associations of Renin–Angiotensin–Aldosterone System Inhibitor Dose, Hyperkalemia, and Adverse Clinical Outcomes in a Cohort of Patients With New-Onset Chronic Kidney Disease or Heart Failure in the United Kingdom. J. Am. Heart Assoc. 2019, 8, e012655. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Claggett, B.; Packer, M.; Zile, M.R.; Rouleau, J.; Swedberg, K.; Teerlink, J.R.; Desai, A.S.; Lefkowitz, M.; Shi, V.; et al. Efficacy of sacubitril/valsartan vs enalapril at lower than target doses in heart failure with reduced ejection fraction: The PARADIGM-HF trial. Eur. J. Heart Fail. 2016, 18, 1228–1234. [Google Scholar] [CrossRef]
- Suberviola, B.; Rodrigo, E.; González-castro, A.; Serrano, M.; Heras, M.; Castellanos-ortega, Á. Association between exposure to angiotensin-converting enzyme inhibitors and angiotensin receptor blockers prior to septic shock and acute kidney injury. Med. Intensiva 2017, 41, 21–27. [Google Scholar] [CrossRef]
- Weir, M.R. Acute changes in glomerular filtration rate with renin-angiotensin system (RAS) inhibition: Clinical implications. Kidney Int. 2017, 91, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Blakeman, T.; Harding, S.; O’Donoghue, D. Acute kidney injury in the community: Why primary care has an important role. Br. J. Gen. Pract. 2013, 63, 173–174. [Google Scholar] [CrossRef] [PubMed]
- Acedillo, R.R.; Wald, R.; McArthur, E.; Nash, D.M.; Silver, S.A.; James, M.T.; Schull, M.J.; Siew, E.D.; Matheny, M.E.; House, A.A.; et al. Characteristics and Outcomes of Patients Discharged Home from an Emergency Department with AKI. Clin. J. Am. Soc. Nephrol. 2017, 12, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Munar, M.Y.; Singh, H. Drug Dosing Adjustments in Patients with Chronic Kidney Disease. Am. Fam. Physician 2007, 75, 1487–1496. [Google Scholar]
- Scheuermeyer, F.X.; Grafstein, E.; Rowe, B.; Cheyne, J.; Grunau, B.; Bradford, A.; Levin, A. The Clinical Epidemiology and 30-Day Outcomes of Emergency Department Patients with Acute Kidney Injury. Can. J. Kidney Health Dis. 2017, 4, 205. [Google Scholar] [CrossRef]
- Palevsky, P.M.; Zhang, J.H.; Seliger, S.L.; Emanuele, N.; Fried, L.F. Incidence, severity, and outcomes of AKI associated with dual renin-angiotensin system blockade. Clin. J. Am. Soc. Nephrol. 2016, 11, 1944–1953. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.E.; Nitsch, D.; Smeeth, L.; Bhaskaran, K.; Tomlinson, L.A. Prescription of renin-angiotensin system blockers and risk of acute kidney injury: A population-based cohort study. BMJ Open. 2016, 6, e012690. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, L.A.; Gary, A.A.; Chaudhry, A.N.; Tomson, C.R.; Wilkinson, I.B.; Roland, M.O.; Payne, R.A. ACE Inhibitor and Angiotensin Receptor-II Antagonist Prescribing and Hospital Admissions with Acute Kidney Injury: A Longitudinal Ecological Study. PLoS ONE 2013, 8, e78465. [Google Scholar] [CrossRef] [PubMed]
- Lapi, F.; Azoulay, L.; Yin, H.; Nessim, S.J.; Suissa, S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: Nested case-control study. BMJ 2013, 346, e8525. [Google Scholar] [CrossRef]
- Yılmaz, R.; Erdem, Y. Acute kidney injury in the elderly population. Int. Urol. Nephrol. 2010, 42, 259–271. [Google Scholar] [CrossRef]
- SPRINT Research Group; Wright, J.T., Jr.; Williamson, J.D.; Whelton, P.K.; Snyder, J.K.; Sink, K.M.; Rocco, M.V.; Reboussin, D.M.; Rahman, M.; Oparil, S.; et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar]
- Lea-Henry, T.N.; Baird-Gunning, J.; Petzel, E.; Roberts, D.M. Medication management on sick days. Aust. Prescr. 2017, 40, 168–173. [Google Scholar] [CrossRef] [PubMed]
| Drug | Indication | Target Dose | Maximal Dose | Dose Adjustment | Excretion (Renal/Hepatic) |
|---|---|---|---|---|---|
| ACEi | |||||
| Enalapril | HF, HTN | 10–20 mg/day | 40mg/day |
| 100%/0% |
| Perindopril | HF | 2.5 mg/day | 5 mg/day |
| 100%/0% |
| HTN | 10mg/day | 10 mg/day | |||
| Ramipril | HF, HTN | 5 mg/day | 10 mg/day |
| 100%/0% |
| ARB | |||||
| Candesartan | HF, HTN | 32 mg/day | 32 mg/day |
| 33%/67% |
| Irbesartan | HF, HTN | 300 mg/day | 300 mg/day |
| 30%/70% |
| Losartan | HF | 150 mg/day | 150 mg/day |
| 10%/90% |
| HTN | 50 mg/day | 100 mg/day | |||
| Olmesartan | HF, HTN | 20 mg/day | 40 mg/day |
| 40%/60% |
| Valsartan | HF | 160 mg/day | 320 mg/day |
| 30%/70% |
| HTN | 80–160 mg/day | ||||
| Characteristic N (%) or Median (IQR) | Overall (n = 309) | AKI Group (n = 86) | Non-AKI Group (n = 223) | p Value |
|---|---|---|---|---|
| Age (years) | 75 (60–83) | 73.5 (61.5–81) | 76 (60–84) | 0.810 |
| Gender (Male) | 153 (50) | 50 (58) | 103 (46) | 0.060 |
| BMI (kg/m2) | 25.4 (21.7–28.7) | 26.2 (22.1–29.4) | 25.1(21.5–28.4) | 0.320 |
| Blood Pressure (mmHg) | ||||
| Systolic | 125 (110–137.5) | 120 (106.7–135) | 126 (113–140) | 0.007 |
| Diastolic | 70 (64–80.5) | 70 (60–79.3) | 70 (64–82) | 0.076 |
| Heart Rate (Beats/min) | 80 (69–90) | 81 (70–91) | 80 (68–90) | 0.650 |
| Saturation O2 (%) | 96 (94–98) | 97 (94–98) | 96 (93–98) | 0.166 |
| Chief Complaint | ||||
| CNS | 88 (28) | 23 (26.7) | 65 (29.1) | 0.675 |
| Cardiovascular | 12 (3.9) | 2 (2.3) | 10 (4.5) | 0.379 |
| Gastrointestinal | 70 (22.7) | 20 (23.3) | 50 (22.4) | 0.875 |
| Respiratory | 30 (9.7) | 4 (4.7) | 26 (11.7) | 0.062 |
| Musculoskeletal | 14 (4.5) | 5 (5.8) | 9 (4) | 0.501 |
| Metabolic/Electrolyte | 20 (6.5) | 7 (8.1) | 13 (5.8) | 0.460 |
| disturbances | ||||
| Hematopoietic | 17 (5.5) | 6 (7) | 11 (4.9) | 0.480 |
| Genitourinary | 28 (9.1) | 13 (15.1) | 15 (6.7) | 0.021 |
| Other | 30 (9.7) | 6 (7) | 24 (10.8) | 0.314 |
| Clinical Presentation | ||||
| Diarrhea | 28 (9.1) | 14 (16) | 14 (6) | 0.006 |
| Vomiting | 45 (14.6) | 15 (17.4) | 30 (13.5) | 0.370 |
| Edema | 27 (8.7) | 13 (15) | 14 (6) | 0.014 |
| Fever | 62 (20.1) | 21 (24.4) | 41 (18.4) | 0.396 |
| Respiratory infection | 27 (8.7) | 5 (5.8) | 22 (9.9) | 0.260 |
| Urinary tract infection | 20 (6.5) | 3 (3.5) | 17 (7.6) | 0.180 |
| Past Medical History | ||||
| Hypertension | 159 (51.5) | 55 (64) | 104 (46) | 0.006 |
| Diabetes Mellitus | 86 (27.8) | 26 (30.2) | 60 (26.9) | 0.560 |
| Dyslipidemia | 66 (21.4) | 27 (31) | 39 (17) | 0.008 |
| Hyperuricemia | 22 (7.1) | 9 (10.5) | 13 (5.8) | 0.160 |
| CAD | 36 (11.7) | 12 (14) | 24 (10.8) | 0.430 |
| Heart Failure | 27 (8.7) | 6 (6.9) | 21 (9.4) | 0.496 |
| CKD | 77 (24.9) | 27 (31.4) | 50 (22.4) | 0.100 |
| COPD | 28 (9.1) | 10 (11.6) | 18 (8.1) | 0.340 |
| Cancer | 55 (17.8) | 18 (20.9) | 37 (16.6) | 0.370 |
| Autoimmune Disorders | 8 (2.6) | 3 (3.5) | 5 (2.2) | 0.540 |
| Medications | ||||
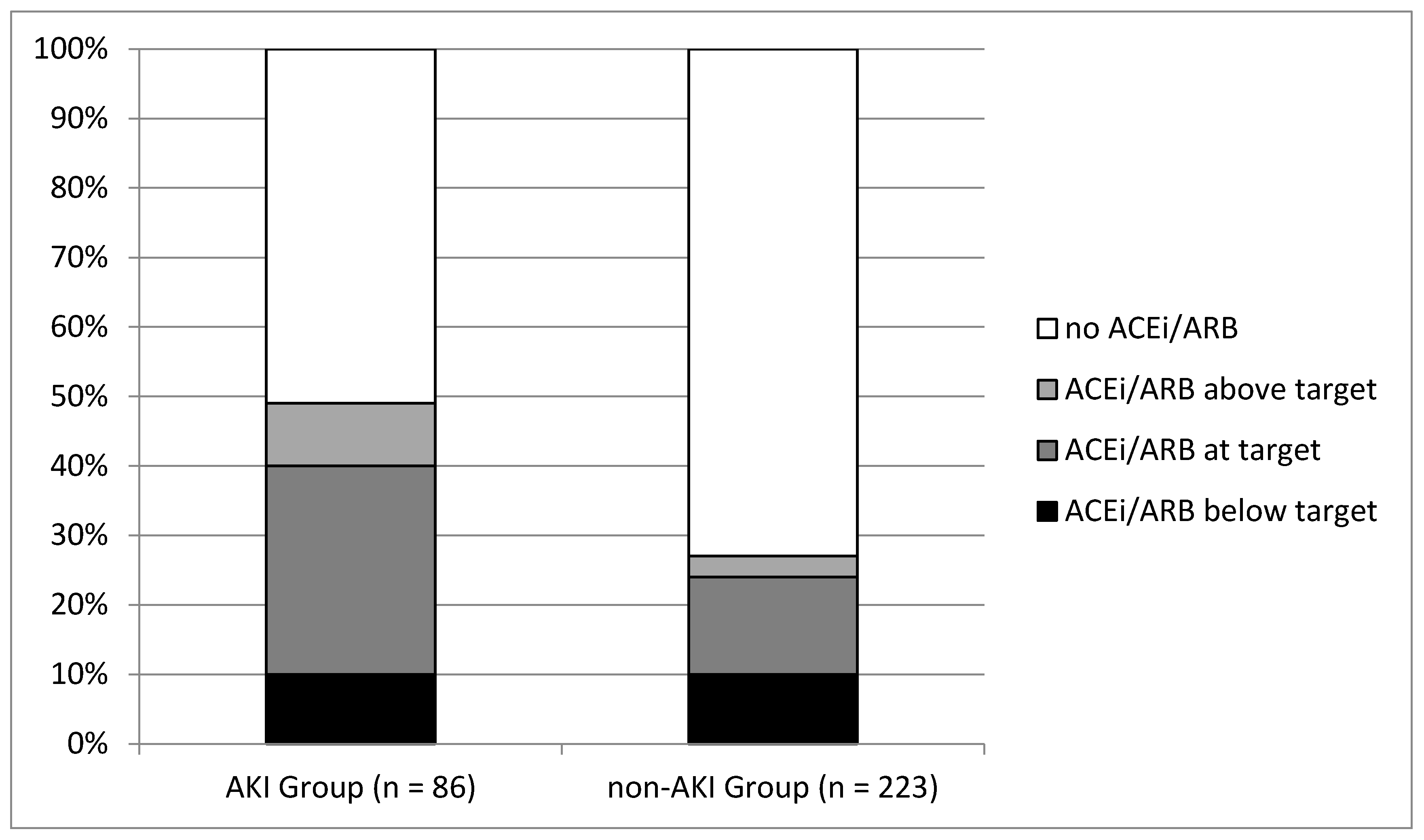
| ACEi/ARB | 104 (34) | 42 (49) | 62 (28) | <0.001 |
| -Above target dosage | 15 (4.8) | 8 (9) | 7 (3) | 0.024 |
| -Target dosage | 57 (18.4) | 25 (30) | 32 (14) | 0.003 |
| -Below target dosage | 32 (10) | 9 (10) | 23 (10) | 0.970 |
| β-Blocker | 103 (33) | 35 (40.7) | 68 (30.5) | 0.089 |
| Diuretic | 97 (31) | 30 (34.9) | 67 (30) | 0.410 |
| CCB | 56 (18.1) | 19 (22.1) | 37 (16.6) | 0.260 |
| Metformin | 39 (12.6) | 12 (14) | 27 (12.1) | 0.660 |
| Insulin | 26 (8.4) | 8 (9.3) | 18 (8.1) | 0.720 |
| Allopurinol | 31 (10) | 13 (15.1) | 18 (8.1) | 0.070 |
| Value, Median (IQR) | Overall (n = 309) | AKI Group (n = 86) | Non-AKI Group (n = 223) | p Value |
|---|---|---|---|---|
| Hematocrit (%) | 35.8 (30.95–40.35) | 34.65 (29.55–38.53) | 36.4 (31.4–40.7) | 0.107 |
| Hemoglobin (g/dL) | 12 (10–13.5) | 11.55 (9.68–13) | 12.1 (10.3–13.6) | 0.220 |
| MCV (%) | 86.7 (81.9–90.75) | 86.5 (80.55–89.75) | 86.8 (82–91) | 0.950 |
| Platelets (K/μL) | 236 (173–310) | 234 (168.8–309.3) | 236 (174–311) | 0.395 |
| WBCs (K/μL) | 9.08 (7.03–12.44) | 9.01 (7.13–13.41) | 9.1 (6.98–11.85) | 0.180 |
| CRP (mg/L) | 21.1 (3.71–92.4) | 34.7 (5.99–93.98) | 16.3 (3.35–91.8) | 0.130 |
| Glucose (mg/dL) | 115 (96–148) | 119.5 (98–163.3) | 112 (96–143) | 0.040 |
| Na (mg/dL) | 139 (135–141) | 137 (133.8–141) | 139 (136–141) | 0.070 |
| K (mg/dL) | 4.4 (4–5) | 4.5 (4.05–5.4) | 4.3 (4–4.9) | 0.250 |
| Ca (mg/dL) | 9 (8.55–9.4) | 8.9 (8.4–9.3) | 9.1 (8.7–9.4) | 0.020 |
| P (mg/dL) | 3.2 (2.7–3.7) | 3.5 (2.9–4.25) | 3.1 (2.6–3.5) | <0.001 |
| BUN (mg/dL) | 43.8 (30.15–65.55) | 67.4 (44.5–110.1) | 37.8 (26.1–52.5) | <0.001 |
| Uric acid (mg/dL) | 5.2 (3.95–7.25) | 7.15 (5.38–9.28) | 4.7 (3.7–6.3) | <0.001 |
| Creatinine (mg/dL) on admission | 0.9 (0.7–1.3) | 1.4 (1.1–2.63) | 0.8 (0.6–1.0) | <0.001 |
| Baseline Creatinine (mg/dL) | 0.7 (0.6–0.95) | 0.8 (0.6–1.0) | 0.7 (0.6–0.9) | 0.386 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Age (years) | 1.00 (0.99–1.02) | 0.816 | ||
| Gender (Male) | 1.61 (0.83–2.70) | 0.061 | 1.85 (1.01–3.37) | 0.520 |
| BMI (kg/m2) | 1.02 (0.98–1.07) | 0.329 | ||
| Blood Pressure (mmHg) | ||||
| Systolic | 0.98 (0.97–1.00) | 0.007 | 0.98 (0.96–1.00) | 0.010 |
| Diastolic | 0.99 (0.97–1.00) | 0.077 | 1.00 (0.97–1.03) | 0.931 |
| Heart Rate (Beats/min) | 1.00 (0.99–1.01) | 0.657 | ||
| Saturation O2 (%) | 1.00 (1.00–1.00) | 0.767 | ||
| Clinical Presentation | ||||
| Diarrhea | 2.90 (1.32–6.38) | 0.008 | 2.86 (0.13–0.91) | 0.034 |
| Vomiting | 1.36 (0.69–2.67) | 0.374 | ||
| Edema | 2.37 (1.14–4.92) | 0.021 | 2.86 (0.02–7.12) | 0.494 |
| Fever | 1.17 (0.88–1.55) | 0.278 | ||
| Respiratory infection | 0.56 (0.21–1.54) | 0.264 | ||
| Urinary tract infection | 0.44 (0.13–1.53) | 0.197 | ||
| Past Medical History | ||||
| Hypertension | 2.03 (1.22–3.39) | 0.007 | 1.31 (0.34–1.70) | 0.506 |
| Diabetes Mellitus | 1.12 (0.68–2.04) | 0.559 | ||
| Dyslipidemia | 2.16 (1.22–3.82) | 0.008 | 2.03 (0.24–1.01) | 0.054 |
| Hyperuricemia | 1.89 (0.78–4.60) | 0.161 | ||
| Coronary Artery Disease | 1.35 (0.64–2.83) | 0.434 | ||
| Heart Failure | 0.72 (0.28–1.85) | 0.498 | ||
| CKD | 1.58 (0.91–2.75) | 0.104 | 1.31 (0.35–1.66) | 0.490 |
| COPD | 1.50 (0.66–3.39) | 0.332 | ||
| Cancer | 1.33 (0.71–2.49) | 0.373 | ||
| Autoimmune Disorders | 1.58 (0.37–6.74) | 0.540 | ||
| Medications | ||||
| ACEi/ARB | 1.73 (1.30–2.29) | <0.001 | ||
| -Above target dosage | 4.18 (1.44–12.16) | 0.009 | 4.85 (1.22–19.33) | 0.025 |
| -Target dosage | 2.86 (1.54–5.32) | 0.001 | 2.94 (1.26–6.89) | 0.013 |
| -Below target dosage | 1.43 (0.62–3.32) | 0.402 | 1.42 (0.49–4.08) | 0.519 |
| β-Blocker | 1.56 (0.93–2.62) | 0.089 | 1.32 (0.38–1.52) | 0.438 |
| Diuretic | 1.25 (0.74–2.12) | 0.412 | 1.64 (0.79–3.38) | 0.185 |
| CCB | 0.51 (0.30–0.89) | 0.018 | 1.04 (0.42–2.17) | 0.917 |
| Metformin | 1.18 (0.57–2.44) | 0.662 | ||
| Insulin | 1.17 (0.49–2.80) | 0.727 | ||
| Allopurinol | 2.03 (0.95–4.34) | 0.069 | 1.07 (0.26–3.38) | 0.921 |
| Laboratory Data | ||||
| Hematocrit (%) | 0.97 (0.94–1.01) | 0.108 | ||
| Hemoglobin | 0.94 (0.85–1.04) | 0.220 | ||
| Na | 0.97 (0.93–1.00) | 0.072 | 0.99 (0.95–1.05) | 0.978 |
| K | 1.38 (1.04–1.83) | 0.028 | 1.02 (0.66–1.56) | 0.946 |
| Ca | 0.67 (0.48–0.94) | 0.019 | 0.62 (0.39–0.98) | 0.058 |
| P | 1.91 (1.44–2.52) | <0.001 | 1.29 (0.85–1.95) | 0.238 |
| BUN | 1.02 (1.01–1.03) | <0.001 | 1.01 (0.93–1.02) | 0.392 |
| Creatinine on admission | 2.64 (1.88–3.72) | <0.001 | 1.82 (1.05–3.15) | 0.033 |
| Uric acid | 1.44 (1.28–1.61) | <0.001 | 1.34 (1.14–1.56) | 0.067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feidakis, A.; Panagiotou, M.-R.; Tsoukakis, E.; Bacharaki, D.; Gounari, P.; Nikolopoulos, P.; Marathias, K.P.; Lionaki, S.; Vlahakos, D. Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers on Acute Kidney Injury in Emergency Medical Admissions. J. Clin. Med. 2021, 10, 412. https://doi.org/10.3390/jcm10030412
Feidakis A, Panagiotou M-R, Tsoukakis E, Bacharaki D, Gounari P, Nikolopoulos P, Marathias KP, Lionaki S, Vlahakos D. Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers on Acute Kidney Injury in Emergency Medical Admissions. Journal of Clinical Medicine. 2021; 10(3):412. https://doi.org/10.3390/jcm10030412
Chicago/Turabian StyleFeidakis, Athanasios, Maria-Rosa Panagiotou, Emmanouil Tsoukakis, Dimitra Bacharaki, Paraskevi Gounari, Petros Nikolopoulos, Katerina P. Marathias, Sophia Lionaki, and Demetrios Vlahakos. 2021. "Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers on Acute Kidney Injury in Emergency Medical Admissions" Journal of Clinical Medicine 10, no. 3: 412. https://doi.org/10.3390/jcm10030412
APA StyleFeidakis, A., Panagiotou, M.-R., Tsoukakis, E., Bacharaki, D., Gounari, P., Nikolopoulos, P., Marathias, K. P., Lionaki, S., & Vlahakos, D. (2021). Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin Receptor Blockers on Acute Kidney Injury in Emergency Medical Admissions. Journal of Clinical Medicine, 10(3), 412. https://doi.org/10.3390/jcm10030412






