Treatment of Hepatic Artery Stenosis in Liver Transplant Patients Using Drug-Eluting versus Bare-Metal Stents
Abstract
1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
3.1. Demographic Characteristics
3.2. Procedural Details
3.3. Post Procedure
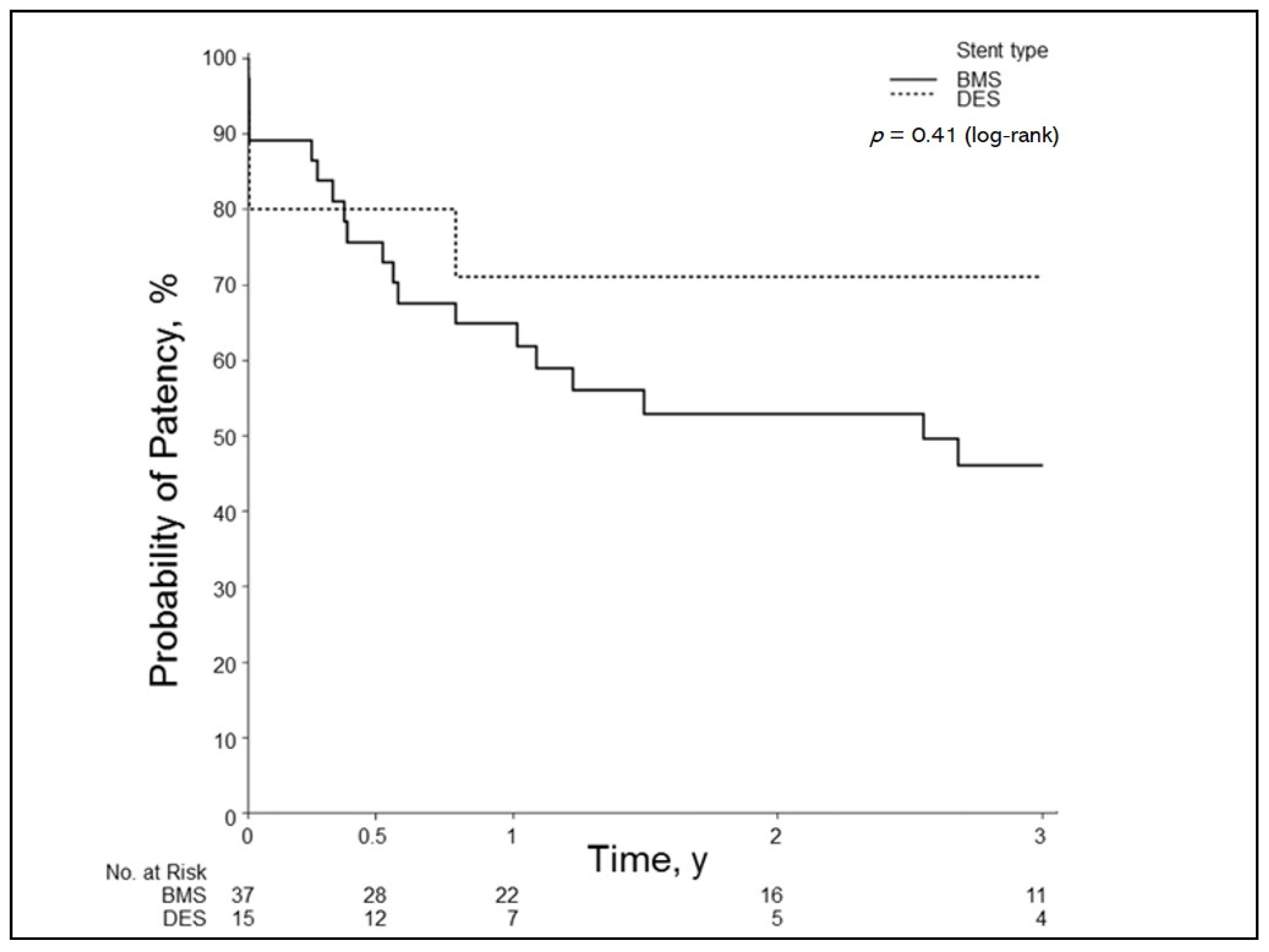
3.4. Primary Patency
3.5. Primary Patency of Small-Caliber Arteries
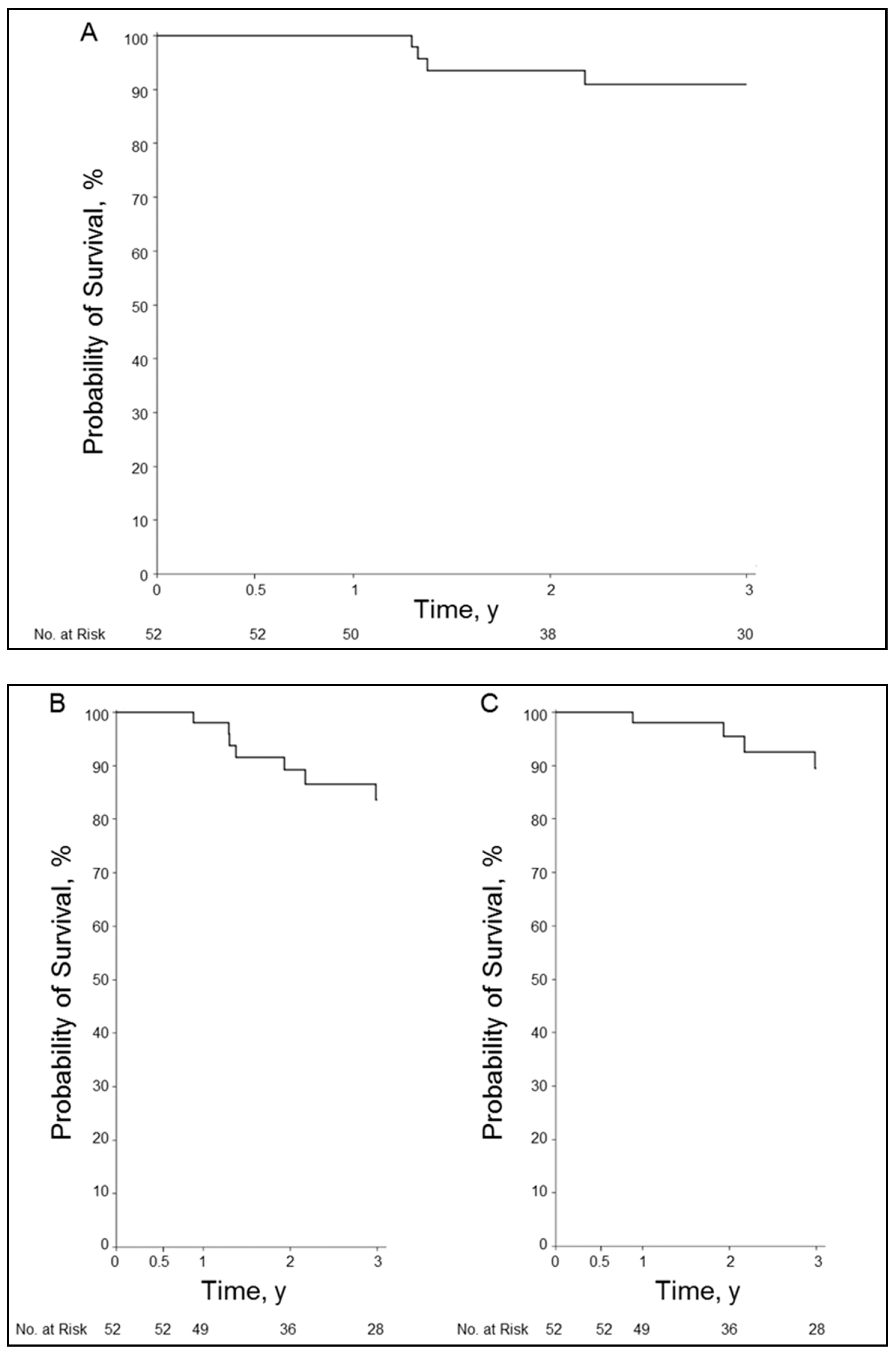
3.6. Overall Survival and Graft Survival
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da Silva, R.F.; Raphe, R.; Felício, H.; Rocha, M.; Duca, W.; Arroyo, P.; Palini, G.; Vasquez, A.; Miquelin, D.; Reis, L.; et al. Prevalence, treatment, and outcomes of the hepatic artery stenosis after liver transplantation. Transplant. Proc. 2008, 40, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Frongillo, F.; Grossi, U.; Lirosi, M.; Nure, E.; Sganga, G.; Avolio, A.; Inchingolo, R.; Di Stasi, C.; Rinaldi, P.; Agnes, S. Incidence, management, and results of hepatic artery stenosis after liver transplantation in the era of donor to recipient match. Transplant. Proc. 2013, 45, 2722–2725. [Google Scholar] [CrossRef] [PubMed]
- Saad, W.E.; Davies, M.G.; Sahler, L.; Lee, D.E.; Patel, N.C.; Kitanosono, T.; Sasson, T.; Waldman, D.L. Hepatic artery stenosis in liver transplant recipients: Primary treatment with percutaneous transluminal angioplasty. J. Vasc. Interv. Radiol. 2005, 16, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.P.; Hong, J.C.; Farmer, D.G.; Ghobrial, R.M.; Yersiz, H.; Hiatt, J.R.; Busuttil, R.W. Vascular complications of orthotopic liver transplantation: Experience in more than 4200 patients. J. Am. Coll. Surg. 2009, 208, 896–903; discussion 903-5. [Google Scholar] [CrossRef]
- Sarwar, A.; Chen, C.; Khwaja, K.; Malik, R.; Raven, K.E.; Weinstein, J.L.; Evenson, A.; Faintuch, S.; Fisher, R.; Curry, M.P.; et al. Primary Stent Placement for Hepatic Artery Stenosis After Liver Transplantation: Improving Primary Patency and Reintervention Rates. Liver Transpl. 2018, 24, 1377–1383. [Google Scholar] [CrossRef]
- Hamby, B.A.; Ramirez, D.E.; Loss, G.E.; Bazan, H.A.; Smith, T.A.; Bluth, E.; Sternbergh, W. Endovascular treatment of hepatic artery stenosis after liver transplantation. J. Vasc. Surg. 2013, 57, 1067–1072. [Google Scholar] [CrossRef]
- Sommacale, D.; Aoyagi, T.; Dondero, F.; Sibert, A.; Bruno, O.; Fteriche, S.; Francoz, C.; Durand, F.; Belghiti, J. Repeat endovascular treatment of recurring hepatic artery stenoses in orthotopic liver transplantation. Transpl. Int. 2013, 26, 608–615. [Google Scholar] [CrossRef]
- Bates, M.C.; Rashid, M.; Campbell, J.E.; Stone, P.A.; Broce, M.; Lavigne, P.S. Factors influencing the need for target vessel revascularization after renal artery stenting. J. Endovasc. Ther. 2006, 13, 569–577. [Google Scholar] [CrossRef]
- Miyagawa-Hayashino, A.; Tsuruyama, T.; Haga, H.; Oike, F.; Il-Deok, K.; Egawa, H.; Hiai, H.; Tanaka, K.; Manabe, T. Arteriopathy in chronic allograft rejection in liver transplantation. Liver Transpl. 2004, 10, 513–519. [Google Scholar] [CrossRef]
- Ardissino, D.; Cavallini, C.; Bramucci, E. Sirolimus-eluting vs uncoated stents for prevention of restenosis in small coronary arteries: A randomized trial. JAMA 2004, 292, 2727–2734. [Google Scholar] [CrossRef]
- Hanna, R.F.; Hao, F.; Kraus, C.; Mitsopoulos, G.; Goldstein, G.; Weintraub, J.; Sperling, D.; Susman, J.; Schlossberg, P.; Sheynzon, V. Renal Transplant Arterial Stenosis Treated With Bare-Metal Versus Drug-Eluting Stents: Comparison of Treatment Outcomes. Transplant. Proc. 2015, 47, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Estrada, C.C.; Musani, M.; Darras, F.; Suh, H.; Abate, M.T.; Mani, A.; Nord, E.P. 5 Years Experience With Drug Eluting and Bare Metal Stents as Primary Intervention in Transplant Renal Artery Stenosis. Transplant. Direct 2017, 3, e128. [Google Scholar] [CrossRef]
- Reyes-Corona, J.; Gonzalez-Huezo, M.S.; Zea-Medina, M.V.; Zamora-Valdés, D.; Victoria-Campos, J.L.; Mondragon-Sanchez, R.J. Paclitaxel coated-stent for early-onset thrombosis after liver transplantation. Ann. Hepatol. 2007, 6, 272–275. [Google Scholar] [CrossRef]
- Le, L.; Terral, W.; Zea, N.; Bazan, H.A.; Smith, T.A.; Loss, G.E.; Bluth, E.; Sternbergh, W. Primary stent placement for hepatic artery stenosis after liver transplantation. J. Vasc. Surg. 2015, 62, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, L.E.; Wiebke, K.; Seal, J.; Brinster, C.; Smith, T.A.; Bazan, H.A.; Sternbergh, W. Complications after endovascular treatment of hepatic artery stenosis after liver transplantation. J. Vasc. Surg. 2017, 66, 1488–1496. [Google Scholar] [CrossRef]
- Kirtane, A.J.; Gupta, A. Safety and efficacy of drug-eluting and bare metal stents: Comprehensive meta-analysis of randomized trials and observational studies. Circulation 2009, 119, 3198–3206. [Google Scholar] [CrossRef]
- Caixeta, A.; Leon, M.B.; Lansky, A.J.; Nikolsky, E.; Aoki, J.; Moses, J.W.; Schofer, J.; Morice, M.-C.; Schampaert, E.; Kirtane, A.J.; et al. 5-year clinical outcomes after sirolimus-eluting stent implantation insights from a patient-level pooled analysis of 4 randomized trials comparing sirolimus-eluting stents with bare-metal stents. J. Am. Coll. Cardiol. 2009, 54, 894–902. [Google Scholar] [CrossRef]
- Biederman, D.M.; Fischman, A.M.; Titano, J.J.; Kim, E.; Patel, R.S.; Nowakowski, F.S.; Florman, S.; Lookstein, R.A. Tailoring the endovascular management of transplant renal artery stenosis. Am. J. Transplant. 2015, 15, 1039–1049. [Google Scholar] [CrossRef]
- Rajakannu, M.; Awad, S.; Ciacio, O.; Pittau, G.; Adam, R.; Cunha, A.S.; Castaing, D.; Samuel, D.; Lewin, M.; Cherqui, D.; et al. Intention-to-treat analysis of percutaneous endovascular treatment of hepatic artery stenosis after orthotopic liver transplantation. Liver Transpl. 2016, 22, 923–933. [Google Scholar] [CrossRef]
- Dodd, G.D., 3rd; Memel, D.S.; Zajko, A.B.; Baron, R.L.; Santaguida, L.A. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology 1994, 192, 657–661. [Google Scholar] [CrossRef]
- Rostambeigi, N.; Hunter, D.J.; Duval, S.; Chinnakotla, S.; Golzarian, J. Stent placement versus angioplasty for hepatic artery stenosis after liver transplant: A meta-analysis of case series. Eur. Radiol. 2013, 23, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Fouzas, I.; Sklavos, A.; Bismpa, K.; Paxiadakis, I.; Antoniadis, N.; Giakoustidis, D.; Katsiki, E.; Tatsou, N.; Mouloudi, E.; Karapanagiotou, A.; et al. Hepatic artery thrombosis after orthotopic liver transplantation: 3 patients with collateral formation and conservative treatment. Transplant. Proc. 2012, 44, 2741–2744. [Google Scholar] [CrossRef] [PubMed]
- Garcia Bernardo, C.M.; García, B.A.; Buil, P.R.; De León, A.M.; Dieguez, L.G.; Rodrigo, V.C.; Arrillaga, I.G.-P.; Velasco, L. Collateral Development in Thrombosis of the Hepatic Artery After Transplantation. Transplant. Proc. 2016, 48, 3006–3009. [Google Scholar] [CrossRef] [PubMed]
- Pulitano, C.; Joseph, D.; Sandroussi, C.; Verran, D.; Strasser, S.I.; Shackel, N.A.; McCaughan, G.W.; Crawford, M. Hepatic artery stenosis after liver transplantation: Is endovascular treatment always necessary? Liver Transpl. 2015, 21, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, S.H.; Kennedy, L.; Dexter, D.F.; Veinot, J.P. A practical method to rapidly dissolve metallic stents. Cardiovasc. Pathol. 2009, 18, 127–133. [Google Scholar] [CrossRef] [PubMed]

| Stent Type | |||
|---|---|---|---|
| Characteristic | Drug-Eluting (n = 15) | Bare-Metal (n = 37) | Total (n = 52) |
| Age, mean (SD), y | 57 (11) | 57 (10) | 57 (10) |
| Range | 31–69 | 18–69 | 18–69 |
| Women, No. (%) | 6 (40) | 15 (41) | 21 (40) |
| BMI, mean (SD), kg/m2 | 23.9 (4.9) | 27.4 (5.1) | 26.2 (5.3) |
| Type of liver transplant | |||
| Whole | 13 | 32 | 45 |
| Split | 2 | 5 | 7 |
| Laboratory values before intervention, mean (SD) | |||
| AST | 55.1 (51.6) | 46.4 (37.9) | 48.2 (42.0) |
| ALT | 54.8 (55.0) | 69.8 (65.6) | 65.4 (62.6) |
| Alkaline phosphatase | 282.1 (344.1) | 234.5 (254.9) | 248.2 (280.8) |
| Total bilirubin | 0.97 (0.75) | 1.0 (1.2) | 1.0 (1.1) |
| Serum creatinine | 1.2 (0.4) | 1.4 (0.4) | 1.3 (0.4) |
| Lowest RI before intervention, mean (SD) | 0.37 (0.09) | 0.36 (0.12) | 0.36 (0.11) |
| Range | 0.25–0.55 | 0.0–0.49 | 0.0–0.55 |
| Stent Type | |||
|---|---|---|---|
| Variable | Drug-Eluting (n = 15) | Bare-Metal (n = 37) | Total (n = 52) |
| Days from transplant to intervention, mean (SD) | 263 (427) | 272 (784) | 269 (696) |
| Median (IQR) | 124 (108–208) | 123 (52–136) | 124 (58–138) |
| Range | 26–1752 | 19–4756 | 19–4756 |
| Angiographic findings | |||
| Single stenosis | 11 | 31 | 42 |
| Tandem or multiple stenoses | 4 | 6 | 10 |
| Occlusion | 0 | 0 | 0 |
| Stenosis type | |||
| Single straight | 11 | 22 | 33 |
| Single area of curvature, kinking a | 1 | 9 | 10 |
| Multiple straight | 2 | 4 | 6 |
| Multiple areas of kinking | 1 | 1 | 2 |
| Mix of straight and kinking | 0 | 1 | 1 |
| Stenosis location | |||
| Pre-anastomosis | 0 | 1 | 1 |
| Anastomosis | 11 | 30 | 41 |
| Post-anastomosis | 0 | 0 | 0 |
| Pre-anastomosis and anastomosis | 3 | 4 | 7 |
| Post-anastomosis and anastomosis | 1 | 2 | 3 |
| Wire system | |||
| 0.014″ (%) | 15 (100) | 28 (76) | 43 (83) |
| 0.035″ (%) | 0 (0) | 9 (24) | 9 (17) |
| Stent type | |||
| Balloon expandable, No. (%) | 15 (100) | 31 (84) | 46 (89) |
| Self-expandable, No. (%) | 0 (0) | 6 (16) | 6 (11) |
| Stent diameter, mean (SD), mm | 4.1 (0.5) | 5.1 (1.0) | 4.8 (1.0) |
| Range | 3.0–5.0 | 3.0–8.0 | 3.0–8.0 |
| Technical success, No. (%) | 15 (100) | 37 (100) | 52 (100) |
| Complications, No. (%) | 0 | 4 (11) | 4 (8) |
| Dissection, No. (%) | 0 | 3 (75) | 3 (75) |
| Pseudoaneurysm, No. (%) | 0 | 1 (25) | 1 (25) |
| Variable | Overall (n = 52) |
|---|---|
| Lowest pre-intervention RI in the left or right hepatic artery | |
| Mean (SD) | 0.36 (0.11) |
| Range | 0.0–0.55 |
| First post-intervention RI | |
| Mean (SD) | 0.55 (0.14) |
| Range | 0.0–0.74 |
| RI change, pre-intervention to post-intervention, No. (%) | |
| Higher | 45 (88) |
| Lower | 5 (10) |
| No change | 1 (2) |
| No follow-up study | 1 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidu, S.; Alzubaidi, S.; Knuttinen, G.; Patel, I.; Fleck, A.; Sweeney, J.; Aqel, B.; Larsen, B.; Buras, M.; Golafshar, M.; et al. Treatment of Hepatic Artery Stenosis in Liver Transplant Patients Using Drug-Eluting versus Bare-Metal Stents. J. Clin. Med. 2021, 10, 380. https://doi.org/10.3390/jcm10030380
Naidu S, Alzubaidi S, Knuttinen G, Patel I, Fleck A, Sweeney J, Aqel B, Larsen B, Buras M, Golafshar M, et al. Treatment of Hepatic Artery Stenosis in Liver Transplant Patients Using Drug-Eluting versus Bare-Metal Stents. Journal of Clinical Medicine. 2021; 10(3):380. https://doi.org/10.3390/jcm10030380
Chicago/Turabian StyleNaidu, Sailendra, Sadeer Alzubaidi, Grace Knuttinen, Indravadan Patel, Andrew Fleck, John Sweeney, Bashar Aqel, Brandon Larsen, Matthew Buras, Michael Golafshar, and et al. 2021. "Treatment of Hepatic Artery Stenosis in Liver Transplant Patients Using Drug-Eluting versus Bare-Metal Stents" Journal of Clinical Medicine 10, no. 3: 380. https://doi.org/10.3390/jcm10030380
APA StyleNaidu, S., Alzubaidi, S., Knuttinen, G., Patel, I., Fleck, A., Sweeney, J., Aqel, B., Larsen, B., Buras, M., Golafshar, M., & Oklu, R. (2021). Treatment of Hepatic Artery Stenosis in Liver Transplant Patients Using Drug-Eluting versus Bare-Metal Stents. Journal of Clinical Medicine, 10(3), 380. https://doi.org/10.3390/jcm10030380





