Remote Hospital Care for Recovering COVID-19 Patients Using Telemedicine: A Randomised Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Study Population
2.3. Care as Usual
2.4. Intervention
2.5. Follow Up
2.6. Endpoints and Data Collection
2.7. Sample Size
2.8. Statistical Analysis
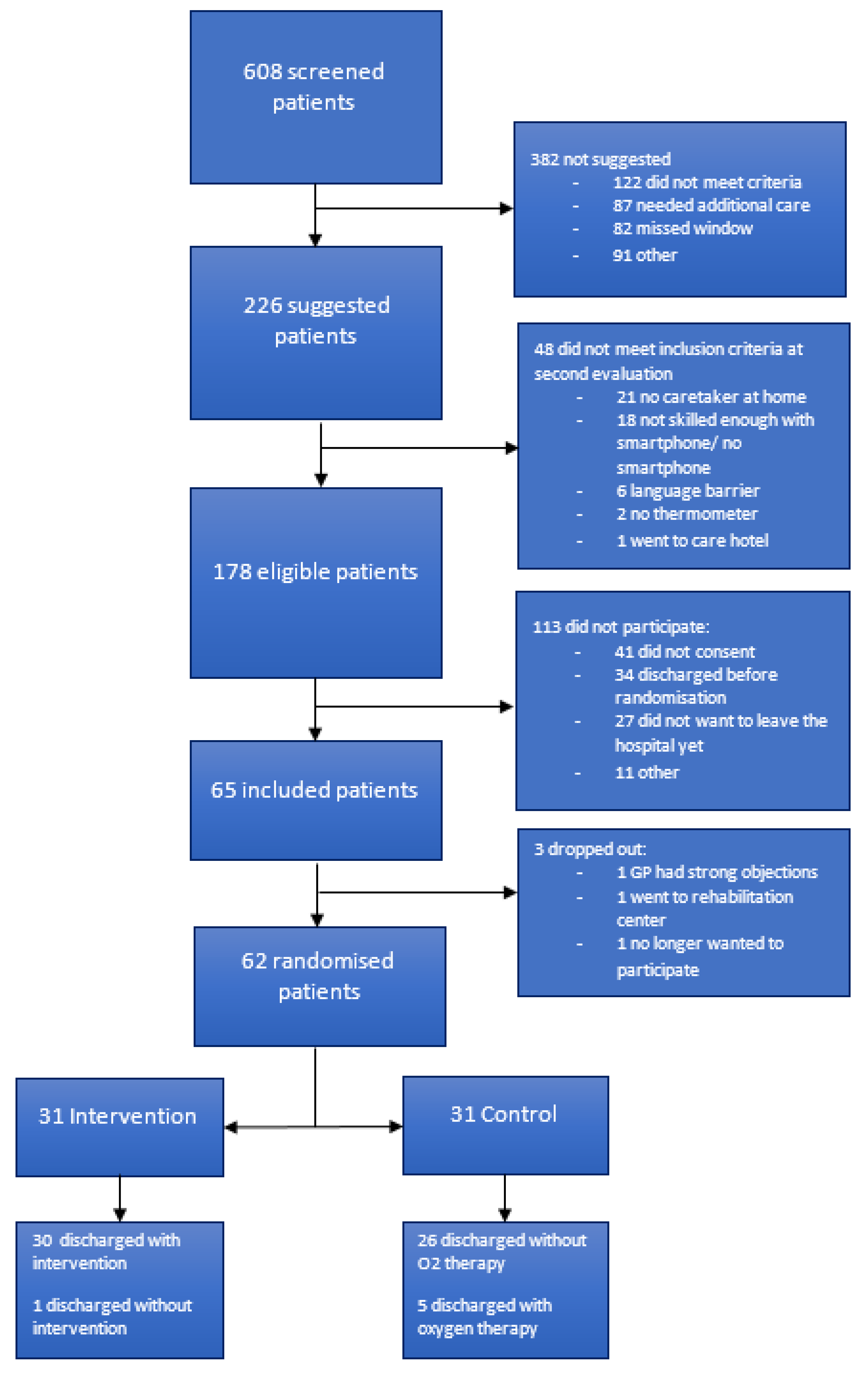
3. Results
4. Discussion
4.1. Comparison with Previous Research
4.2. Strengths and Limitations
4.3. Implications for Future Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saad-Roy, C.M.; Wagner, C.E.; Baker, R.E.; Morris, S.E.; Farrar, J.; Graham, A.L.; Levin, S.A.; Mina, M.J.; Metcalf, J.C.E.; Grenfell, B.T. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science 2020, 370, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.; Mafham, M.; Bell, J.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19–Preliminary Report. N. Engl. J. Med. 2020, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Fearday, A.; Safdar, N. Adverse effects of isolation in hospitalised patients: A systematic review. J. Hosp. Infect. 2010, 76, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, K.; Pieterse, M.E.; Pruyn, A.T. Physical environmental stimuli that turn healthcare facilities into healing environments through psychologically mediated effects: Systematic review. J. Adv. Nurs. 2006, 56, 166–181. [Google Scholar] [CrossRef]
- Hesselink, G.; Smits, M.; Doedens, M.; Nijenhuis, S.M.T.; van Bavel, D.; van Goor, H.; van de Belt, T.H. Environmental Needs, Barriers, and Facilitators for Optimal Healing in the Postoperative Process: A Qualitative Study of Patients’ Lived Experiences and Perceptions. Health Environ. Res. Des. J. 2020, 3, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Shepperd, S.; Iliffe, S.; Doll, H.A.; Clarke, M.J.; Kalra, L.; Wilson, A.D.; Gonçalves-Bradley, D.C. Admission avoidance hospital at home. Cochrane Database Syst. Rev. 2016, 9, 7491. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves-Bradley, D.C.; Iliffe, S.; Doll, H.A.; Broad, J.; Gladman, J.; Langhorne, P.; Richards, S.H.; Shepperd, S. Early discharge hospital at home. Cochrane Database Syst. Rev. 2017, 6, CD000356. [Google Scholar] [CrossRef] [Green Version]
- Hernández, C.; Aibar, J.; Seijas, N.; Puig, I.; Alonso, A.; Garcia-Aymerich, J.; Roca, J. Implementation of home hospitalization and early discharge as an integrated care service: A ten years pragmatic assessment. Int. J. Integr. Care 2018, 18, 12. [Google Scholar] [CrossRef]
- Palombo, D.; Mugnai, D.; Mambrini, S.; Robaldo, A.; Rousas, N.; Mazzei, R.; Bianca, P.; Spinella, G. Role of Interactive Home Telemedicine for Early and Protected Discharge 1 Day after Carotid Endarterectomy. Ann. Vasc. Surg. 2009, 23, 76–80. [Google Scholar] [CrossRef]
- Grutters, L.A.; Majoor, K.I.; Pol-Mattern, E.S.K.; Hardeman, J.A.; van Swol, C.F.P.; Vorselaars, A.D.M. Home-monitoring reduces hospital stay of COVID-19 patients. Eur. Respir. J. 2021, 58, 2101871. [Google Scholar] [CrossRef]
- van Herwerden, M.C.; van Steenkiste, J.; El Moussaoui, R.; den Hollander, J.G.; Helfrich, G.; Verberk, J.A.M. Home telemonitoring and oxygen therapy in COVID-19 patients: Safety, patient satisfaction, and cost-effectiveness. Ned. Tijdschr. Geneeskd. 2021, 165, D5740. [Google Scholar]
- Banerjee, J.; Canamar, C.P.; Voyageur, C.; Tangpraphaphorn, S.; Lemus, A.; Coffey, C.; Wald-Dickler, N.; Holtom, P.; Shoenberger, J.; Bowdish, M.; et al. Mortality and Readmission Rates among Patients with COVID-19 after Discharge from Acute Care Setting with Supplemental Oxygen. JAMA Netw. Open 2021, 4, 213990. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Mercier, J.C.; Jaulmes, L.; Artigou, J.Y.; Juillière, Y.; Yordanov, Y.; Jourdain, P. Safe Discharge Home With Telemedicine of Patients Requiring Nasal Oxygen Therapy After COVID-19. Front. Med. 2021, 8, 703017. [Google Scholar] [CrossRef]
- Gootenberg, D.B.; Kurtzman, N.; O’Mara, T.; Ge, J.Y.; Chiu, D.; Shapiro, N.I.; Mechanic, O.J.; Dagan, A. Developing a pulse oximetry home monitoring protocol for patients suspected with COVID-19 after emergency department discharge. BMJ Health Care Inform. 2021, 28, 100330. [Google Scholar] [CrossRef] [PubMed]
- Sitammagari, K.; Murphy, S.; Kowalkowski, M.; Chou, S.H.; Sullivan, M.; Taylor, S.; Kearns, J.; Batchelor, T.; Rivet, C.; Hole, C.; et al. Insights from rapid deployment of a “virtual hospital” as standard care during the covid-19 pandemic. Ann. Intern. Med. 2021, 174, 192–199. [Google Scholar] [CrossRef]
- Zilli, E.; Madia, A.; Giuriato, P.; Bonaldo, D.; Barbar, S.; Cassutti, F.; Bertoli, E.; Di Gregorio, G.; Cancian, L.; Bozzoli, C.; et al. Protected Discharge Model for Mild to Moderate Covid Patients in a North-East Italian Hospital; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 12940, ISBN 9783030881627. [Google Scholar]
- Auriemma, C.L.; Taylor, S.P.; Harhay, M.O.; Courtright, K.R.; Halpern, S.D. Hospital-free Days: A Pragmatic and Patient-centered Outcome for Trials Among Critically and Seriously Ill Patients. Am. J. Respir. Crit. Care Med. 2021, 204, 902–909. [Google Scholar] [CrossRef]
- Shah, M.R.; O’Connor, C.M.; Sopko, G.; Hasselblad, V.; Califf, R.M.; Stevenson, L.W. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness (ESCAPE): Design and rationale. Am. Heart J. 2001, 141, 528–535. [Google Scholar] [CrossRef]
- Jerath, A.; Austin, P.C.; Wijeysundera, D.N. Days Alive and out of Hospital: Validation of a Patient-centered Outcome for Perioperative Medicine. Anesthesiology 2019, 131, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Wasywich, C.A.; Gamble, G.D.; Whalley, G.A.; Doughty, R.N. Understanding changing patterns of survival and hospitalization for heart failure over two decades in New Zealand: Utility of “days alive and out of hospital” from epidemiological data. Eur. J. Heart Fail. 2010, 12, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Leijte, W.T.; Wagemaker, N.M.M.; van Kraaij, T.D.A.; de Kruif, M.D.; Mostard, G.J.M.; Leers, M.P.G.; Mostard, R.L.M.; Buijs, J.; van Twist, D.J.L. Sterfte en heropname na ziekenhuisopname met covid-19. Ned. Tijdschr. Geneeskd. 2020, 164, 5423. [Google Scholar]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. J. Am. Med. Assoc. 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- UyaroĞlu, O.A.; BaŞaran, N.Ç.; ÖziŞik, L.; Dİzman, G.T.; EroĞlu, İ.; Şahİn, T.K.; TaŞ, Z.; İnkaya, A.Ç.; TanriÖver, M.D.; Metan, G.; et al. Thirty-day readmission rate of COVID-19 patients discharged from a tertiary care university hospital in Turkey: An observational, single-center study. Int. J. Qual. Health Care J. Int. Soc. Qual. Health Care 2021, 33, 144. [Google Scholar] [CrossRef] [PubMed]
- Grutters, L.A.; Majoor, K.I.; Mattern, E.S.K.; Hardeman, J.A.; van Swol, C.F.P.; Vorselaars, A.D.M. Home telemonitoring makes early hospital discharge of COVID-19 patients possible. J. Am. Med. Inform. Assoc. 2020, 27, 1825–1827. [Google Scholar] [CrossRef] [PubMed]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap, 1st ed.; Chapman and Hall/CRC: Philadelphia, PA, USA, 1993; ISBN 0-412-04231-2. [Google Scholar]
- Annis, T.; Pleasants, S.; Hultman, G.; Lindemann, E.; Thompson, J.A.; Billecke, S.; Badlani, S.; Melton, G.B. Rapid implementation of a COVID-19 remote patient monitoring program. J. Am. Med. Inform. Assoc. 2020, 27, 1326–1330. [Google Scholar] [CrossRef]
- Shah, S.; Majmudar, K.; Stein, A.; Gupta, N.; Suppes, S.; Karamanis, M.; Capannari, J.; Sethi, S.; Patte, C. Novel Use of Home Pulse Oximetry Monitoring in COVID-19 Patients Discharged From the Emergency Department Identifies Need for Hospitalization. Acad. Emerg. Med. 2020, 27, 681–692. [Google Scholar] [CrossRef]
- McCullough, P.A.; Kelly, R.J.; Ruocco, G.; Lerma, E.; Tumlin, J.; Wheelan, K.R.; Katz, N.; Lepor, N.E.; Vijay, K.; Carter, H.; et al. Pathophysiological Basis and Rationale for Early Outpatient Treatment of SARS-CoV-2 (COVID-19) Infection. Am. J. Med. 2021, 134, 16–22. [Google Scholar] [CrossRef]
- Zhou, X.; Snoswell, C.L.; Harding, L.E.; Bambling, M.; Edirippulige, S.; Bai, X.; Smith, A.C. The Role of Telehealth in Reducing the Mental Health Burden from COVID-19. Telemed. J. Health 2020, 26, 377–379. [Google Scholar] [CrossRef] [Green Version]

| Control (n = 31) | Intervention (n = 31) | |
|---|---|---|
| Patient | ||
| Age (mean, sd) | 55.4 (13.2) | 55.1 (7.5) |
| Female (%) | 13 (41.9%) | 14 (45.1%) |
| CFS * (mean, sd) | 2.1 (1.3) | 2 (0.6) |
| Active smoker (%) | 0 (0%) | 1 (3.2%) |
| Hypertension (%) | 5 (16.1%) | 6 (19.4%) |
| Cardiovascular disease (%) | 3 (9.7%) | 2 (6.5%) |
| CCI ** (median, IQR) | 2 (0–3) | 1 (1–2) |
| Index admission | ||
| Transferred from a different hospital (%) | 26 (83.9%) | 28 (90.3%) |
| Admitted to ICU (%) | 3 (9.7%) | 4 (12.9%) |
| Length of hospital admission before randomisation (median, IQR) | 6 (4.5–9) | 6 (4–8.5) |
| Pulmonary embolism (%) | 3 (9.7%) | 2 (6.5%) |
| Bacterial superinfection (%) | 3 (9.7%) | 2 (6.5%) |
| Other (%) | 1 (3.2%) | 3 (10%) |
| Dexamethasone or prednisone treatment (%) | 31 (100%) | 31 (100%) |
| Highest delivered FiO2 at ward (median, IQR) | 0.44 (0.36–0.6) | 0.4 (0.36–0.6) |
| Oxygen therapy at randomisation (L/min) (mean, sd) | 2.0 (1.0) | 2.1 (0.9) |
| Discharged from hospital care with oxygen therapy (%) | 5 (16.1%) | 1 (3.2%) |
| Control (n = 31) | Intervention (n = 31) | Difference (95% CI) | p-Value | |
|---|---|---|---|---|
| Hospital-free days in 30 days following randomisation (mean, sd) | 26.7 (5.7) | 28.4 (3.8) | 1.7 (−0.5 to 4.2) | 0.112 * |
| Index hospital length of stay (mean, sd) | 10.0 (7.0) | 7.3 (4.3) | −2.7 (−5.7 to 0.0) | 0.045 * |
| Duration of index hospital stay after randomisation (mean, sd) | 2.3 (2.3) | 0.7 (0.9) | −1.6 (−2.4 to –0.8) | <0.001 * |
| Number of days in hospital or dead following index hospital stay (mean, sd) | 1.0 (3.7) | 0.9 (3.7) | −0.1 (−2.1 to 1.8) | 0.906 * |
| Duration of hospital responsibility (hospital stay + hospital care at home) (mean, sd) | 10.0 (7.0) | 14.1 (7.6) | 4.1 (0.5 to 7.7) | 0.028 * |
| Days of oxygen therapy following randomisation (mean, sd) | 3.4 (7.5) | 6.7 (7.5) | 3.3 (−0.5 to 6.8) | 0.101 * |
| ED visits (N, %) | 1 (3.2%) | 3 (9.7%) | – | – |
| COVID-19 | 1 | 3 | ||
| Other unplanned hospital visits (N, %) | 2 (6.5%) | 2 (6.5%) | – | – |
| - For COVID-19 | 2 | 2 | – | – |
| Readmission (N, %) | 1 (3.2%) | 2 (6.5%) | – | – |
| - For COVID-19 | 1 | 2 | ||
| GP visits (N, %) | 20 (64.5%) | 12 (38.7%) | – | 0.035 † |
| - For COVID-19 | 19 | 8 | ||
| Telephone contact with GP by patient (%) | 22 (71.0%) | 25 (80.6%) | – | 0.371 † |
| Mortality (%) | 1 (3.2%) | 0 (0%) | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Goor, H.M.R.; Breteler, M.J.M.; van Loon, K.; de Hond, T.A.P.; Reitsma, J.B.; Zwart, D.L.M.; Kalkman, C.J.; Kaasjager, K.A.H. Remote Hospital Care for Recovering COVID-19 Patients Using Telemedicine: A Randomised Controlled Trial. J. Clin. Med. 2021, 10, 5940. https://doi.org/10.3390/jcm10245940
van Goor HMR, Breteler MJM, van Loon K, de Hond TAP, Reitsma JB, Zwart DLM, Kalkman CJ, Kaasjager KAH. Remote Hospital Care for Recovering COVID-19 Patients Using Telemedicine: A Randomised Controlled Trial. Journal of Clinical Medicine. 2021; 10(24):5940. https://doi.org/10.3390/jcm10245940
Chicago/Turabian Stylevan Goor, Harriët M. R., Martine J. M. Breteler, Kim van Loon, Titus A. P. de Hond, Johannes B. Reitsma, Dorien L. M. Zwart, Cornelis J. Kalkman, and Karin A. H. Kaasjager. 2021. "Remote Hospital Care for Recovering COVID-19 Patients Using Telemedicine: A Randomised Controlled Trial" Journal of Clinical Medicine 10, no. 24: 5940. https://doi.org/10.3390/jcm10245940
APA Stylevan Goor, H. M. R., Breteler, M. J. M., van Loon, K., de Hond, T. A. P., Reitsma, J. B., Zwart, D. L. M., Kalkman, C. J., & Kaasjager, K. A. H. (2021). Remote Hospital Care for Recovering COVID-19 Patients Using Telemedicine: A Randomised Controlled Trial. Journal of Clinical Medicine, 10(24), 5940. https://doi.org/10.3390/jcm10245940






