Control of Low-Density Lipoprotein Cholesterol in Secondary Prevention of Coronary Artery Disease in Real-Life Practice: The DAUSSET Study in French Cardiologists
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Study Objectives
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participating Cardiologists
3.2. Patient Characteristics and Index Cardiac Event
3.3. Lipid Assessments
3.4. Follow-Up by Cardiologist after Index Cardiac Event and LDL-C Goal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boren, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: Pathophysiological, genetic, and therapeutic insights: A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Raygor, V.; Khera, A. New recommendations and revised concepts in recent guidelines on the management of dyslipidemias to prevent cardiovascular disease: The 2018 ACC/AHA and 2019 ESC/EAS guidelines. Curr. Cardiol. Rep. 2020, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur. Heart. J. 2016, 37, 2999–3058. [Google Scholar] [CrossRef] [Green Version]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019, 139, e1046–e1081. [Google Scholar] [PubMed]
- Kotseva, K.; Wood, D.; De Bacquer, D.; De Backer, G.; Ryden, L.; Jennings, C.; Gyberg, V.; Amouyel, P.; Bruthans, J.; Castro Conde, A.; et al. EUROASPIRE IV: A European Society of Cardiology survey on the lifestyle, risk factor and therapeutic management of coronary patients from 24 European countries. Eur. J. Prev. Cardiol. 2016, 23, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Gitt, A.K.; Lautsch, D.; Ferrieres, J.; Kastelein, J.; Drexel, H.; Horack, M.; Brudi, P.; Vanneste, B.; Bramlage, P.; Chazelle, F.; et al. Contemporary data on low-density lipoprotein cholesterol target value attainment and distance to target in a cohort of 57,885 statin-treated patients by country and region across the world. Data Brief 2016, 9, 616–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gitt, A.K.; Lautsch, D.; Ferrieres, J.; Kastelein, J.; Drexel, H.; Horack, M.; Brudi, P.; Vanneste, B.; Bramlage, P.; Chazelle, F.; et al. Low-density lipoprotein cholesterol in a global cohort of 57,885 statin-treated patients. Atherosclerosis 2016, 255, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Ferrieres, J.; De Ferrari, G.M.; Hermans, M.P.; Elisaf, M.; Toth, P.P.; Horack, M.; Brudi, P.; Lautsch, D.; Bash, L.D.; Baxter, C.A.; et al. Predictors of LDL-cholesterol target value attainment differ in acute and chronic coronary heart disease patients: Results from DYSIS II Europe. Eur. J. Prev. Cardiol. 2018, 25, 1966–1976. [Google Scholar] [CrossRef] [PubMed]
- Danchin, N.; Almahmeed, W.; Al-Rasadi, K.; Azuri, J.; Berrah, A.; Cuneo, C.A.; Karpov, Y.; Kaul, U.; Kayikcioglu, M.; Mitchenko, O.; et al. Achievement of low-density lipoprotein cholesterol goals in 18 countries outside Western Europe: The International ChoLesterol management Practice STUDY (ICLPS). Eur. J. Prev. Cardiol. 2018, 25, 1087–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrieres, J.; Rouyer, M.V.; Lautsch, D.; Ashton, V.; Ambegaonkar, B.M.; Brudi, P.; Gitt, A.K. Dyslipidemia international study, I.I.F.S.G. suboptimal achievement of low-density lipoprotein cholesterol targets in French patients with coronary heart disease. Contemporary data from the DYSIS II ACS/CHD study. Arch. Cardiovasc. Dis. 2017, 110, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Ferrieres, J.; Rouyer, M.V.; Lautsch, D.; Ambegaonkar, B.M.; Horack, M.; Brudi, P.; Gitt, A.K.; Dyslipidemia International Study (DYSIS) and DYSIS II Study Group. Improvement in achievement of lipid targets in France: Comparison of data from coronary patients in the DYSIS and DYSIS II studies. Int. J. Cardiol. 2016, 222, 793–794. [Google Scholar] [CrossRef] [PubMed]
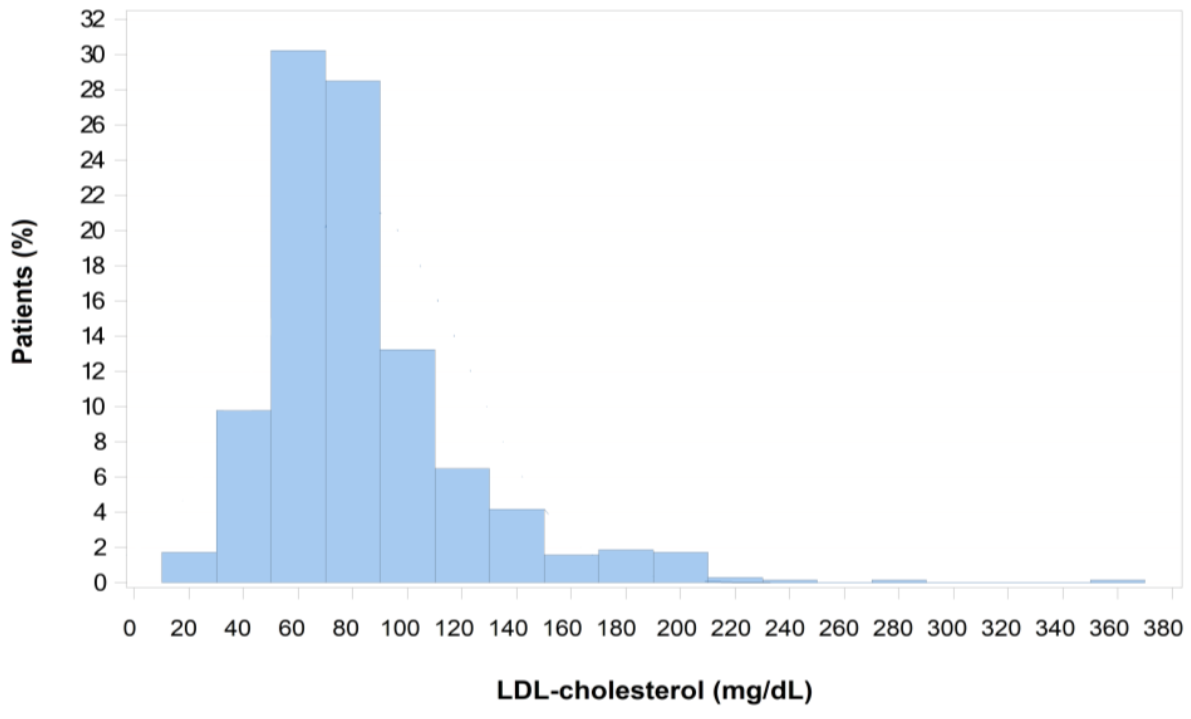
| Number of Patients Evaluated | Analysis Population (n = 912) | |
|---|---|---|
| Age, years, mean (SD) | 912 | 65.4 (11.8) |
| Gender, n (%) | ||
| Male | 912 | 694 (76.1) |
| Female | 912 | 218 (23.9) |
| Body mass index, kg/m2, mean (SD) | 889 | 27.1 (4.4) |
| Systolic blood pressure, mmHg, mean (SD) | 898 | 133.1 (17.4) |
| Diastolic blood pressure, mmHg, mean (SD) | 898 | 76.4 (10.2) |
| Heart rate, beats/min, mean (SD) | 889 | 66.1 (11.1) |
| Clinical signs of familial hypercholesterolemia, n (%) | 857 | 8 (0.9) |
| Risk factors, n (%) | ||
| Treated hypertension | 912 | 447 (49.0) |
| Known hypercholesterolemia before cardiac event | 912 | 426 (46.7) |
| Smoking | 887 | 391 (44.1) |
| Sedentary lifestyle | 897 | 338 (37.7) |
| Type 2 diabetes | 912 | 196 (21.5) |
| Depressive disorder | 909 | 56 (6.2) |
| Microalbuminuria >30 mg/24 h | 422 | 15 (3.6) |
| Untreated hypertension | 912 | 24 (2.6) |
| HIV infection | 863 | 15 (1.7) |
| Rheumatoid arthritis | 906 | 5 (0.6) |
| Patient cardiovascular history, n (%) | ||
| Coronary revascularization | 912 | 162 (17.8) |
| Myocardial infarction | 912 | 114 (12.5) |
| Unstable angina | 912 | 67 (7.3) |
| Peripheral artery disease | 909 | 58 (6.4) |
| Ischemic stroke | 909 | 52 (5.7) |
| Stable angina | 912 | 50 (5.5) |
| Heart failure | 910 | 38 (4.2) |
| Silent myocardial infarction | 912 | 11 (1.2) |
| Hemorrhagic stroke | 910 | 2 (0.2) |
| Family history of premature cardiovascular disease, n (%) | ||
| Male | 781 | 112 (14.3) |
| Female | 781 | 48 (6.1) |
| Number of Patients Evaluated | Analysis Population (n = 912) | |
|---|---|---|
| Age at the index event, years, mean (SD) | 912 | 64.1 (11.8) |
| Time between index event and inclusion, months, mean (SD) | 912 | 16.8 (9.2) |
| Type of occurrence, n (%) | ||
| First episode | 911 | 730 (80.1) |
| Recurrence | 911 | 181 (19.9) |
| Type of event, n (%) | ||
| ST-segment elevation myocardial infarction | 912 | 361 (39.6) |
| non-ST-segment elevation myocardial infarction | 912 | 272 (29.8) |
| Coronary disease diagnosis | 912 | 147 (16.1) |
| Unstable angina | 912 | 132 (14.5) |
| Main locations of the index event, n (%) a | ||
| Anterior | 898 | 380 (42.3) |
| Inferior | 898 | 268 (29.8) |
| Lateral | 898 | 103 (11.5) |
| Previous lipid-lowering therapy, n (%) | 884 | 348 (39.4) |
| Results of coronary angiography, n (%) | ||
| Single-vessel disease | 909 | 365 (40.2) |
| Two-vessel disease | 909 | 324 (35.6) |
| Three-vessel disease | 909 | 198 (21.8) |
| Left main artery | 909 | 4 (0.4) |
| Single-vessel disease and left main artery | 909 | 1 (0.1) |
| Two-vessel disease and left main artery | 909 | 6 (0.7) |
| Three-vessel disease and left main artery | 909 | 11 (1.2) |
| Revascularization procedure, n (%) | ||
| None | 912 | 68 (7.5) |
| Angioplasty with stent | 912 | 762 (83.6) |
| Angioplasty without stent | 912 | 21 (2.3) |
| Angioplasty (no information on stent) | 912 | 2 (0.2) |
| Coronary bypass surgery | 912 | 59 (6.5) |
| Acute heart failure during acute phase, n (%) | 904 | 79 (8.7) |
| Lipid-lowering therapy at discharge, n (%) a | 912 | 893 (97.9) |
| Low intensity | 893 | 38 (4.3) |
| Moderate intensity | 893 | 168 (18.8) |
| High intensity | 893 | 687 (76.9) |
| Associated treatment at discharge, n (%) | 912 | 911 (99.9) |
| Beta blockers | 910 | 802 (88.1) |
| Renin-angiotensin system blockers | 910 | 717 (78.8) |
| Calcium channel inhibitors | 907 | 121 (13.3) |
| Nitroglycerin | 902 | 103 (11.4) |
| Number of Patients Evaluated | Analysis Population (n = 912) | |
|---|---|---|
| Before the index event | ||
| Lipid-lowering treatment, n (%) | 296 | 143 (48.3) |
| Total cholesterol, mg/dL, mean (SD) | 134 | 196 (55) |
| LDL-cholesterol, mg/dL, mean (SD) | 143 | 121 (48) |
| HDL-cholesterol, mg/dL, mean (SD) | 136 | 49 (19) |
| Triglycerides, mg/dL, mean (SD) | 139 | 144 (82) |
| After the index cardiac event (within 7 days) | ||
| Lipid-lowering treatment at discharge, n (%) | 912 | 893 (97.9) |
| Total cholesterol, mg/dL, mean (SD) | 901 | 191 (55) |
| LDL-cholesterol, mg/dL, mean (SD) | 895 | 118 (047) |
| HDL-cholesterol, mg/dL, mean (SD) | 910 | 45 (16) |
| Triglycerides, mg/dL, mean (SD) | 908 | 147 (95) |
| At study inclusion (within 3 months) | ||
| Lipid-lowering treatment, n (%) | 912 | 894 (98.0) |
| Total cholesterol, mg/dL, mean (SD) | 689 | 157 (45) |
| LDL-cholesterol, mg/dL, mean (SD) | 695 | 83 (37) |
| LDL-cholesterol >100 mg/dL, n (%) | 695 | 153 (22.0) |
| HDL-cholesterol, mg/dL, mean (SD) | 695 | 48 (16) |
| Triglycerides, mg/dL, mean (SD) | 689 | 131 (96) |
| Number of Patients Evaluated | Analysis Population (n = 912) | |
|---|---|---|
| Follow-up by the investigator before index cardiac event, n (%) | 911 | 609 (66.8) |
| Duration of follow-up, years, mean (SD) | 302 | 8.1 (7.0) |
| Referral to participating cardiologist for the first time, n (%) | ||
| Hospital or clinic | 912 | 222 (24.3) |
| Directly after cardiac event | 912 | 360 (39.5) |
| Attending physician | 912 | 329 (36.1) |
| Other | 912 | 35 (3.8) |
| Cardiac rehabilitation program, n (%) | 900 | 407 (45.2) |
| In hospital | 407 | 87 (21.4) |
| In rehabilitation center | 407 | 327 (80.3) |
| In hearth and health club | 407 | 1 (0.2) |
| Compliance with healthy lifestyle, n (%) | 911 | 646 (70.9) |
| Compliance with treatment, n (%) | 898 | 840 (93.5) |
| Target LDL-C, mg/dL, n (%) | ||
| 50–70 | 912 | 6 (0.7) |
| 70 | 912 | 774 (84.9) |
| 70–100 | 912 | 55 (6.0) |
| 100 | 912 | 77 (8.4) |
| Target LDL-C communicated to patient, n (%) | 908 | 799 (88.0) |
| Target LDL-C communicated to attending physician, n (%) | 895 | 721 (80.6) |
| Satisfaction of cardiologist for treatment response, n (%) | ||
| Very satisfied | 890 | 381 (42.8) |
| Satisfied | 890 | 265 (29.8) |
| Moderately satisfied | 890 | 160 (18.0) |
| Not at all satisfied | 890 | 84 (9.4) |
| Reasons for moderate satisfaction/dissatisfaction, n (%) a | ||
| Objective not reached | 244 | 244 (100) |
| Treatment inefficacy | 244 | 131 (53.7) |
| Treatment intolerance | 244 | 57 (23.4) |
| Poor treatment compliance | 244 | 30 (12.3) |
| Treatment refusal | 244 | 6 (2.5) |
| Rare dyslipidemia | 244 | 1 (0.4) |
| Other reason | 244 | 48 (19.7) |
| Number of Patients Evaluated | Analysis Population (n = 912) | |
|---|---|---|
| LDL-C target achieved (<70 mg/dL), n (%) | 695 | 290 (41.7) |
| Patients with lipid-lowering treatment, n (%) a | 912 | 894 (98.0) |
| Therapy intensification | 894 | 726 (81.2) |
| Decrease in therapy | 891 | 241 (27.0) |
| Lipid-lowering treatment maintained | 894 | 117 (13.1) |
| Patients with no lipid-lowering treatment, n (%) | 912 | 18 (2.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrières, J.; Roubille, F.; Farnier, M.; Jourdain, P.; Angoulvant, D.; Boccara, F.; Danchin, N. Control of Low-Density Lipoprotein Cholesterol in Secondary Prevention of Coronary Artery Disease in Real-Life Practice: The DAUSSET Study in French Cardiologists. J. Clin. Med. 2021, 10, 5938. https://doi.org/10.3390/jcm10245938
Ferrières J, Roubille F, Farnier M, Jourdain P, Angoulvant D, Boccara F, Danchin N. Control of Low-Density Lipoprotein Cholesterol in Secondary Prevention of Coronary Artery Disease in Real-Life Practice: The DAUSSET Study in French Cardiologists. Journal of Clinical Medicine. 2021; 10(24):5938. https://doi.org/10.3390/jcm10245938
Chicago/Turabian StyleFerrières, Jean, François Roubille, Michel Farnier, Patrick Jourdain, Denis Angoulvant, Franck Boccara, and Nicolas Danchin. 2021. "Control of Low-Density Lipoprotein Cholesterol in Secondary Prevention of Coronary Artery Disease in Real-Life Practice: The DAUSSET Study in French Cardiologists" Journal of Clinical Medicine 10, no. 24: 5938. https://doi.org/10.3390/jcm10245938
APA StyleFerrières, J., Roubille, F., Farnier, M., Jourdain, P., Angoulvant, D., Boccara, F., & Danchin, N. (2021). Control of Low-Density Lipoprotein Cholesterol in Secondary Prevention of Coronary Artery Disease in Real-Life Practice: The DAUSSET Study in French Cardiologists. Journal of Clinical Medicine, 10(24), 5938. https://doi.org/10.3390/jcm10245938






