Analgesic Efficacy and Safety of Local Infiltration Following Lumbar Decompression Surgery: A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Outcome Measures
2.4. Quality and Risk of Bias Assessment
3. Results
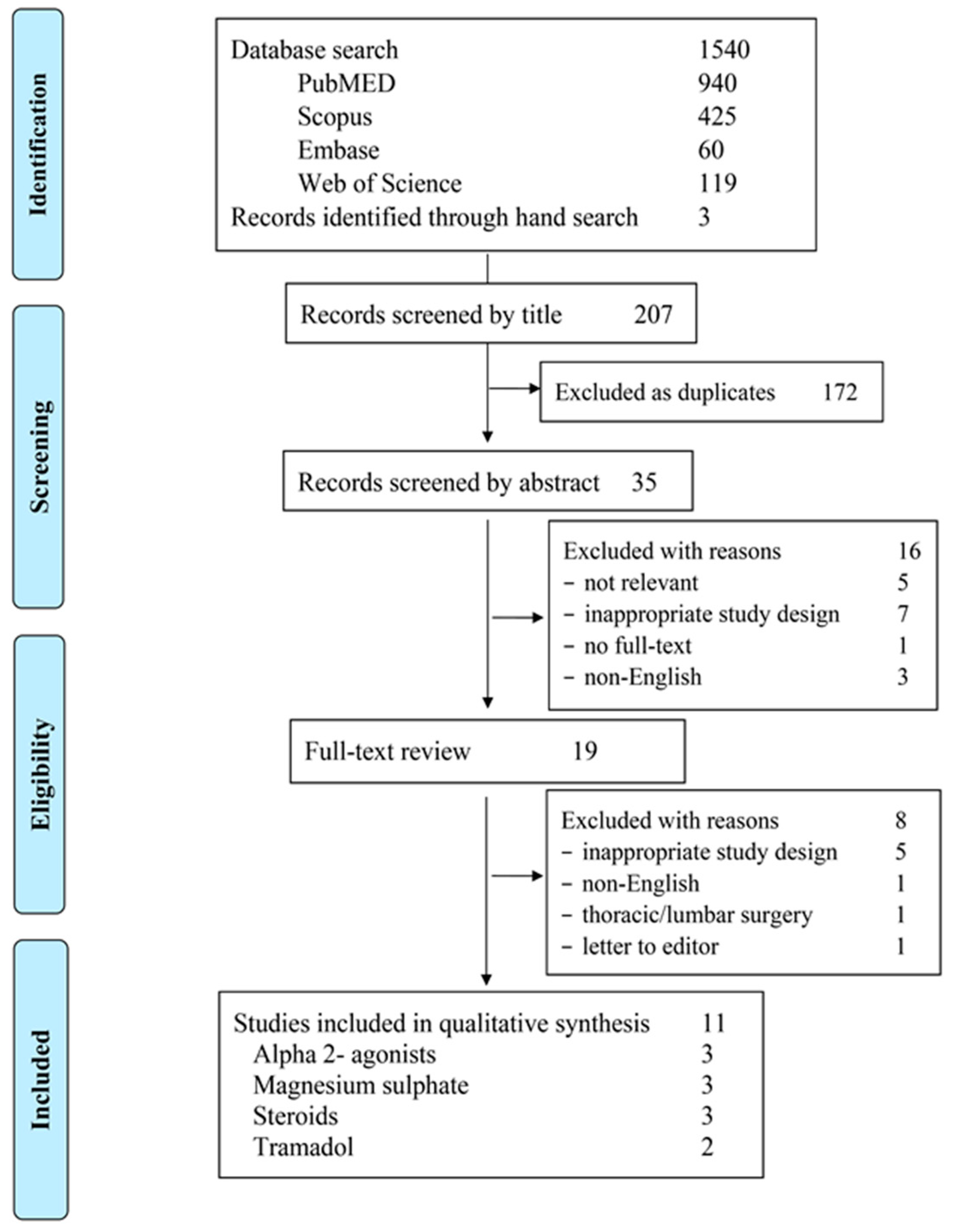
3.1. Studies Selection
3.2. Quality Assessment and Risk-of-Bias Estimation
3.3. Description of Included Trials
3.4. Analgesic Efficacy
3.4.1. Alpha 2-Agonists
3.4.2. Magnesium
3.4.3. Steroids
3.4.4. Tramadol
3.5. Other Effects
4. Discussion
4.1. Alpha 2-Adrenergic Agonists
4.2. Magnesium
4.3. Corticosteroids
4.4. Tramadol
4.5. Study Limitations
5. Conclusions
6. Implications for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsaousi, G.; Nikopoulou, A.; Pezikoglou, I.; Birba, V.; Grosomanidis, V. Implementation of magnesium sulphate as an adjunct to multimodal analgesic approach for perioperative pain control in lumbar laminectomy surgery: A randomized placebo-controlled clinical trial. Clin. Neurol. Neurosurg. 2020, 197, 106091. [Google Scholar] [CrossRef]
- Kraiwattanapong, C.; Arnuntasupakul, V.; Kantawan, R.; Woratanarat, P.; Keorochana, G.; Langsanam, N. Effect of multimodal drugs infiltration on postoperative pain in split laminectomy of lumbar spine: A randomized controlled trial. Spine 2020, 45, 1687–1695. [Google Scholar] [CrossRef]
- Kurd, M.F.; Kreitz, T.; Schroeder, G.; Vaccaro, A.R. The Role of Multimodal Analgesia in Spine Surgery. J. Am. Acad. Orthop. Surg. 2017, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Peene, L.; Le Cacheux, P.; Sauter, A.R.; Joshi, G.P.; Beloeil, H.; PROSPECT Working Group Collaborators; European Society of Regional Anaesthesia. Pain management after laminectomy: A systematic review and procedure-specific post-operative pain management (prospect) recommendations. Eur. Spine J. 2020, 30, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.W.; An, D.; Perlas, A.; Chan, V. Adjuncts to local anesthetic wound infiltration for postoperative analgesia: A systematic review. Reg. Anesth. Pain Med. 2020, 45, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.P.; Kehlet, H. Postoperative pain management in the era of ERAS: An overview. Best Pract. Res. Clin. Anaesthesiol. 2019, 33, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Cassinelli, E.H.; Messerschmitt, P.J.; Furey, C.G.; Bohlman, H.H. A multimodal approach for postoperative pain management after lumbar decompression surgery: A prospective, randomized study. J. Spinal Disord. Tech. 2013, 26, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Wound infiltration with local anaesthetics in ambulatory surgery. Curr. Opin. Anaesthesiol. 2010, 23, 708–713. [Google Scholar] [CrossRef]
- Raines, S.; Hedlund, C.; Franzon, M.; Lillieborg, S.; Kelleher, G.; Ahlén, K. Ropivacaine for continuous wound infusion for postoperative pain management: A systematic review and meta-analysis of randomized controlled trials. Eur. Surg. Res. 2014, 53, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Oremus, M.; Wolfson, C.; Perrault, A.; Demers, L.; Momoli, F.; Moride, Y. Interrater Reliability of the Modified Jadad Quality Scale for Systematic Reviews of Alzheimer’s Disease Drug Trials. Dement. Geriatr. Cogn. Disord. 2001, 12, 232–236. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Abdel Hay, J.; Kobaiter-Maarrawi, S.; Tabet, P.; Moussa, R.; Rizk, T.; Nohra, G.; Okais, N.; Samaha, E.; Maarrawi, J. Bupivacaine Field Block with Clonidine for Postoperative Pain Control in Posterior Spine Approaches: A Randomized Double-Blind Trial. Neurosurgery 2017, 82, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Daiki, M.; Najar, M.; Chkili, R.; Rafrafi, A.; Ben Gabsia, A.; Labbène, I.; Ferjani, M. Postoperative analgesia after wound infiltration with Dexmedetomidine and Ropivacaine versus Ropivacaine alone for lumbar discectomies: A randomized-controlled trial. Tunis Med. 2019, 97, 1375–1382. [Google Scholar] [PubMed]
- Deshwal, R.; Kumar, N.; Sharma, J.P.; Kumar, R. Efficacy of dexmedetomidine added to ropivacaine infilteration on postoperative pain following spine surgeries: A randomized controlled study. Anesth. Essays Res. 2018, 12, 700–704. [Google Scholar] [PubMed]
- Hazarika, R.; Parua, S.; Choudhury, D.; Barooah, R.K. Comparison of bupivacaine plus magnesium sulfate and ropivacaine plus mag-nesium sulfate infiltration for postoperative analgesia in patients undergoing lumbar laminectomy: A randomized double-blinded study. Anesth. Essays Res. 2017, 11, 686–691. [Google Scholar] [CrossRef]
- Sane, S.; Mahdkhah, A.; Golabi, P.; Hesami, S.A.; Kazemi, H.B. Comparison the effect of bupivacaine plus magnesium sulfate with ropivacaine plus magnesium sulfate infiltration on postoperative pain in patients undergoing lumbar laminectomy with general anes-thesia. Br. J. Neurosurg. 2020, 17, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Donadi, P.; Moningi, S.; Gopinath, R.; Donadi, P. Comparison of bupivacaine and bupivacaine plus magnesium sulphate infiltration for postoperative analgesia in patients undergoing lumbar laminectomy: A prospective randomised double-blinded controlled study. J. Neuroanaesth. Crit. Care 2014, 1, 183–187. [Google Scholar] [CrossRef][Green Version]
- Mitra, S.; Purohit, S.; Sharma, M. Postoperative Analgesia after Wound Infiltration with Tramadol and Dexmedetomidine as an Adjuvant to Ropivacaine for Lumbar Discectomies: A Randomized-controlled Clinical Trial. J. Neurosurg. Anesthesiol. 2017, 29, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Gurbet, A.; Bekar, A.; Bilgin, H.; Ozdemir, N.; Kuytu, T. Preemptive wound infiltration in lumbar laminectomy for postoperative pain: Comparison of bupivacaine and levobupivacaine. Turk. Neurosurg. 2014, 24, 48–53. [Google Scholar] [PubMed]
- Ozyilmaz, K.; Ayoglu, H.; Okyay, R.D.; Yurtlu, S.; Koksal, B.; Hancı, V.; Erdogan, G.; Turan, I.O. Postoperative Analgesic Effects of Wound Infiltration with Tramadol and Levobupivacaine in Lumbar Disk Surgeries. J. Neurosurg. Anesthesiol. 2012, 24, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Gurbet, A.; Bekar, A.; Bilgin, H.; Korfali, G.; Yilmazlar, S.; Tercan, M. Pre-emptive infiltration of levobupivacaine is superior to at-closure administration in lumbar laminectomy patients. Eur. Spine J. 2008, 17, 1237–1241. [Google Scholar] [CrossRef]
- Ersayli, D.T.; Gurbet, A.; Bekar, A.; Uckunkaya, N.; Bilgin, H. Effects of perioperatively administered bupivacaine and bupiva-caine-methylprednisolone on pain after lumbar discectomy. Spine 2006, 31, 2221–2226. [Google Scholar] [CrossRef] [PubMed]
- De Boer, H.D.; Detriche, O.; Forget, P. Opioid-related side effects: Postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 499–504. [Google Scholar] [CrossRef]
- Lewis, S.R.; Nicholson, A.; Cardwell, M.E.; Siviter, G.; Smith, A.F. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst. Rev. 2013, 7, CD003591. [Google Scholar]
- Tsaousi, G.G.; Chatzistravou, A.; Papazisis, G.; Grosomanidis, V.; Kouvelas, D.; Pourzitaki, C. Analgesic Efficacy and Safety of Local Infiltration of Tramadol in Pediatric Tonsillectomy Pain: A Systematic Review and Meta-Analysis. Pain Pract. 2020, 20, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Ahn, J.; Buvanendran, A.; Singh, K. Multimodal analgesia in pain management after spine surgery. J. Spine Surg. 2019, 5, S154–S159. [Google Scholar] [CrossRef]
- Devin, C.J.; McGirt, M.J. Best evidence in multimodal pain management in spine surgery and means of assessing postoperative pain and functional outcomes. J. Clin. Neurosci. 2015, 22, 930–938. [Google Scholar] [CrossRef]
- Licina, A.; Silvers, A.; Laughlin, H.; Russell, J.; Wan, C. Pathway for enhanced recovery after spinal surgery-a systematic review of evidence for use of individual components. BMC Anesthesiol. 2021, 21, 74. [Google Scholar] [CrossRef]
- Morrison, I.; Perini, I.; Dunham, J. Facets and mechanisms of adaptive pain behavior: Predictive regulation and action. Front. Hum. Neurosci. 2013, 7, 755. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Liu, H.; Zhu, D.; Ruan, B.; Yu, H.; Xu, X.; Wang, Y. Wound infiltration of dexmedetomidine as an adjunct to local anesthesia in postoperative analgesia for lumbar surgery: A systematic review and meta-analysis. Minerva Anestesiol. 2021, 87, s0375–s9393. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shi, W.; Chen, C.; Li, H.; Zheng, X.; Zheng, X.; Niu, C. Efficacy of dexmedetomidine as an adjuvant to local wound infiltration anaesthesia in abdominal surgery: A meta-analysis of randomised controlled trials. Int. Wound J. 2019, 16, 1206–1213. [Google Scholar] [CrossRef]
- Sukegawa, S.; Higuchi, H.; Inoue, M.; Nagatsuka, H.; Maeda, S.; Miyawaki, T. Locally Injected Dexmedetomidine Inhibits Carrageenin-Induced Inflammatory Responses in the Injected Region. Anesth. Analg. 2014, 118, 473–480. [Google Scholar] [CrossRef]
- Talke, P.; Stapelfeldt, C.; Lobo, E.; Brown, R.; Scheinin, M.; Snapir, A. Effect of α2B-Adrenoceptor Polymorphism on Peripheral Vasoconstriction in Healthy Volunteers. Anesthesiology 2005, 102, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Dontukurthy, S.; Bala, I.; Singh, G. Postoperative analgesic effect of intravenous clonidine compared with clonidine admin-istration in wound infiltration for open cholecystectomy. Br. J. Anaesth. 2013, 111, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, S.K.; Chakravarty, N.; Asati, D.P.; Bankwar, V. Use of Clonidine as an Adjuvant to Infiltration Anaesthesia in Tympanoplasty: A Randomized Double Blind Study. Indian J. Otolaryngol. Head Neck Surg. 2014, 66, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.; Yap, J.L.L.; Izham, I.N.; Teoh, W.Y.; Kwok, P.E.; Koh, W.J. The effect of intravenous magnesium on postoperative morphine consumption in noncardiac surgery: A systematic review and meta-analysis with trial sequential analysis. Eur. J. Anaesthesiol. 2020, 37, 212–223. [Google Scholar] [CrossRef]
- De Oliveira, G.S., Jr.; Castro-Alves, L.J.; Khan, J.H.; McCarthy, R.J. Perioperative systemic magnesium to minimize postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology 2013, 119, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.L.; Lin, Y.; Hu, W.; Zhen, C.X.; Bao-Cheng, Z.; Wu, H.H.; Kaye, A.D.; Duan, J.H.; Qu, Y. Effects of systemic magnesium on post-operative analgesia: Is the current evidence strong enough? Pain. Physician 2015, 18, 405–418. [Google Scholar]
- Petrenko, A.B.; Yamakura, T.; Baba, H.; Shimoji, K. The Role of N-Methyl-d-Aspartate (NMDA) Receptors in Pain: A Review. Anesth. Analg. 2003, 97, 1108–1116. [Google Scholar] [CrossRef]
- Iseri, L.T.; French, J.H. Magnesium: Nature’s physiologic calcium blocker. Am. Heart J. 1984, 108, 188–193. [Google Scholar] [CrossRef]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Cairns, B.E.; Gambarota, G.; Dunning, P.S.; Mulkern, R.V.; Berde, C.B. Activation of Peripheral Excitatory Amino Acid Receptors Decreases the Duration of Local Anesthesia. Anesthesiology 2003, 98, 521–529. [Google Scholar] [CrossRef]
- Pathak, A.; Yadav, N.; Mohanty, S.N.; Ratnani, E.; Sanjeev, O.P. Comparison of three different concentrations 0.2%, 0.5%, and 0.75% epidural ropivacaine for postoperative analgesia in lower limb orthopedic surgery. Anesth. Essays Res. 2017, 11, 1022–1025. [Google Scholar] [CrossRef]
- Liang, M.; Chen, Y.; Zhu, W.; Zhou, D. Efficacy and safety of different doses of ropivacaine for laparoscopy-assisted infiltration analgesia in patients undergoing laparoscopic cholecystectomy: A prospective randomized control trial. Medicine 2020, 99, e22540. [Google Scholar] [CrossRef]
- Huang, L.; Hu, H.; Zhong, Z.; Teng, C.; He, B.; Yan, S. Should corticosteroids be administered for local infiltration analgesia in knee arthroplasty? A meta-analysis and systematic review. J. Clin. Pharm. Ther. 2021, 46, 1441–1458. [Google Scholar] [CrossRef] [PubMed]
- Evaristo-Méndez, G.; De Alba-García, J.E.G.; Sahagún-Flores, J.E.; Ventura-Sauceda, F.A.; Méndez-Ibarra, J.U.; Sepúlveda-Castro, R.R. Analgesic efficacy of the incisional infiltration of ropivacaine vs ropivacaine with dexamethasone in the elective laparoscopic cholecystectomy. Cir. Cir. 2013, 81, 383–393. [Google Scholar]
- Jabalameli, M.; Saryazdi, H.; Massahipour, O. The effect of subcutaneous dexamethasone added to bupivacaine on postcesarean pain: A randomized controlled trial. Iran. J. Med. Sci. 2010, 35, 21–26. [Google Scholar]
- Sousa, Â.M.; Ashmawi, H.A. Local analgesic effect of tramadol is not mediated by opioid receptors in early postoperative pain in rats. Braz. J. Anesthesiol. 2015, 65, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Mico, J.A.; Berrocoso, E. Discovery and development of tramadol for the treatment of pain. Expert Opin. Drug Discov. 2017, 12, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Brummett, C.; Williams, B.A. Additives to Local Anesthetics for Peripheral Nerve Blockade. Int. Anesthesiol. Clin. 2011, 49, 104–116. [Google Scholar] [CrossRef] [PubMed]
| Author | Design | Jadad Score | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Randomization | Blinding | Attrition Info | Inclusion/ Exclusion Criteria | Adverse Effects Method | Statistical Analysis Info | ||
| Abdel Hay et al., 2017 [13] | Double-blind, RCT | 7 | 2 | 2 | 1 | 1 | 0 | 1 |
| Daiki et al., 2019 [14] | Double-blind, RCT | 8 | 2 | 2 | 1 | 1 | 1 | 1 |
| Deshwal et al., 2018 [15] | Double-blind, RCT | 7 | 2 | 2 | 1 | 1 | 1 | 0 |
| Hazarika et al., 2017 [16] | Double-blind, RCT | 8 | 2 | 2 | 1 | 1 | 1 | 1 |
| Sane et al., 2020 [17] | Double-blind, PBO-controlled, RCT | 6 | 2 | 2 | 0 | 1 | 0 | 1 |
| Donadi et al., 2014 [18] | Double-blind, RCT | 6 | 2 | 1 | 0 | 1 | 1 | 1 |
| Mitra et al., 2017 [19] | Double-blind, RCT | 8 | 2 | 2 | 1 | 1 | 1 | 1 |
| Gurbet et al., 2014 [20] | Double-blind, PBO-controlled, RCT | 6 | 0 | 2 | 1 | 1 | 1 | 1 |
| Ozyilmaz et al., 2012 [21] | Double-blind, RCT | 5 | 0 | 2 | 0 | 1 | 1 | 1 |
| Gurbet et al., 2008 [22] | Double-blind, PBO-controlled, RCT | 5 | 1 | 1 | 0 | 1 | 1 | 1 |
| Ersayli et al., 2006 [23] | Double-blind, PBO-controlled, RCT | 4 | 0 | 1 | 0 | 1 | 1 | 1 |
| Author | Random Sequence Generation | Allocation Concealment | Personnel and Participants | Outcome Assessors Blinded | Incomplete Outcome Data | Selective Reporting | Other Bias | Final Estimation |
|---|---|---|---|---|---|---|---|---|
| Abdel Hay et al., 2017 [13] | Low | Unclear | Low | Low | Low | Low | High | Low |
| Daiki et al., 2019 [14] | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Deshwal et al., 2018 [15] | Low | Low | Low | Low | Moderate | Moderate | Low | Low |
| Hazarika et al., 2017 [16] | Low | Unclear | Low | Low | High | Moderate | Low | Moderate |
| Sane et al., 2020 [17] | Low | Low | Low | Unclear | Moderate | Low | Moderate | Moderate |
| Donadi et al., 2014 [18] | Low | Unclear | Low | Unclear | High | Unclear | High | High |
| Mitra et al., 2017 [19] | Low | Unclear | Low | Low | Low | Low | Low | Low |
| Gurbet et al., 2014 [20] | High | Low | Low | Unclear | Moderate | High | Moderate | Moderate |
| Ozyilmaz et al., 2012 [21] | High | Low | Low | Low | Low | Low | Low | Low |
| Gurbet et al., 2008 [22] | High | High | High | Unclear | High | High | High | High |
| Ersayli et al., 2006 [23] | High | High | High | Unclear | Low | Low | High | High |
| Study ID | Study Arms | No Pts | Type of Surgery | Anesthesia Protocol | Basic Analgesia/Rescue Analgesia | Follow-Up | Primary Outcome | Secondary Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Analgesic Requirements | Pain Intensity | Time (h) to Rescue Analgesic | Other Effects | |||||||
| Abdel Hay et al., 2017 [13] | Bupi 0.25% 19 mL + CLON 150 μg 1 mL vs. Bupi 0.25% 20 mL Pre-incisional | 225 (Bupi + CLON (116)/Bupi (109)) | Laminectomy/Discectomy | PROP 3 mg/kg + SUF 10 mcg and SEVO (+N2O) + SUF 5 mcg boluses (iv) | APAP 1gr + Ketoprofen 50 mg (iv) every 6 h/Morphine 5 mg (sc) up to Day 3 | NRS/2 h up to day 2 and /8 h from day 3 to day 8 and morphine up to day 3 | Lower in Bupi + CLON vs. Bupi group (p < 0.001) in lumbar stenosis surgery | AUC of NRS lower in Bupi + CLON vs. Bupi group (p < 0.05) in all lumbar discectomy and stenosis surgery | N/A | Hemodynamics (0) Atelectasis (ns) Superficial wound infection (ns) |
| Daiki et al., 2019 [14] | Ropi 2 mg/kg + DEX 0.5 mcg/kg (30 mL) vs. Ropi 2 mg/kg (30 mL) End of surgery | 63 (Ropi + DEX (33)/Ropi (30)) | Discectomy | PROP 2.5 mg/kg + Remi 015 μg/kg and PRO (6 mg/kg/h) + Remi 0.05–2 mcg/h (iv) | APAP 1 gr or Tram or Ketoprofen 50 mg upon request (iv) | VAS at 0, 2, 4, 6, 12, 18, and 24 h and total analgesics up to 24 h | Lower in Ropi + DEX (median 0 mg) vs. Ropi (median 3 mg) in morphine equivalents (p < 0.001) | VAS lower in Ropi + DEX vs. Ropi group up to 24 h (p < 0.001) | Longer in Ropi + DEX (median 21 h) vs. Ropi (median 8 h) group (p < 0.001) | PONV (ns) Sedation (ns) HR higher in Ropi vs. Ropi + DEX (p = 0.002) MAP (ns) Urinary retention (ns) |
| Deshwal et al., 2018 [15] | Ropi 0.2% 30 mL + DEX 1 mcg/kg vs. Ropi 0.2% 30 mL End of surgery | 60 (30 per group) | Discectomy | PROP 2 mg/kg + FNT 2 μg/kg and SEVO (+N2O) + FNT 1 mcg/h (iv) | PCA FNT 25 mcg/dose 4-h limit 400 mcg | VAS and PPS (static and dynamic) at 0, 0.5,1, 2,4,6,12, and 24 and FNT up to 24 h | Lower in Ropi + DEX (294 ± 39 mcg) vs. Ropi (470 ±3 0 mcg) group (p < 0.001) | VAS and PPS (dynamic) lower in Ropi + DEX vs. Ropi group up to 24 h (p < 0.001) | N/A | Hemodynamics (0) PONV (0) Wound infection (0) |
| Hazarika et al., 2017 [16] | Bupi 50 mg + Mg 500 mg (20 mL) vs. Ropi 50 mg + Mg 500 mg (20 mL) End of surgery | 60 (Bupi + Mg(30)/Ropi + Mg (31)) | Laminectomy | PROP 2 mg/kg + FNT 2 μg/kg and ISO + FNT 1 mcg/kg/h (iv) | Nalbuphine 5 mg/3 h on demand | VAS hourly up to 24 h | Lower in Bupi + Mg (12 ± 4) vs. Ropi + Mg (15 ± 5) (p < 0.01) | VAS lower in Bupi + Mg vs. Ropi + Mg from 4 h to 8 h | Longer in Bupi + Mg (7.3 ± 0.4) vs. Ropi + Mg (6.6 ± 0.7) (p < 0.001) | Agitation, enhanced hemodynamics in Bupi + Mg at 7 h and 8 h/Ropi + Mg at 6 h and 7 h Urinary retention (ns) |
| Sane et al., 2020 [17] | Ropi 70 mg + Mg 500 mg (20 mL) vs. Bupi 70 mg + Mg 500 mg (20 mL) End of surgery | 60 (30 per group) | Laminectomy | PROP 2 mg/kg + FNT 1 mcg/kg (iv) and ISO + REMI 1 mc/kg/min | PCA morphine 2 mg/bolus | VAS at 6,12, and 24 h Analgesics up to 24 h | Lower in Ropi + Mg (mean 185 mg) vs. Bupi + Mg (mean 220 mg) groups (p = 0.03) | VAS lower at 6 and 12 h in Ropi + Mg (mean 2.8 and 2.9) vs. Bupi + Mg (mean 3.7 and 4) (p < 0.05) | N/A | Hemodynamics (ns) |
| Donadi et al., 2014 [18] | Bupi 0.25% 20 mL + Mg 500 mg vs. Bupi 0.25% 20 mL End of surgery | 60 (30 per group) | Laminectomy | THIOP 4–7 mg/kg + FNT 2 mcg/kg and ISO+ FNT 1–5 mcg/kg/h (iv) | Tram 100–150 mg (im) | VAS at 0, 1, 2, 4, 8, 12, and 24 h Analgesics up to 24 h | Lower tramadol in Bupi + Mg (117 ± 63.4 mg) vs. Bupi (202 ± 76 mg) group (p < 0.0001) | VAS lower in Bupi + Mg vs. Bupi group up to 4 h (p < 0.05) | Longer in Bupi + Mg (7.8 ± 1.3 h) vs. Bupi (4.6 ± 0.9 h) group (p < 0.0001) | Satisfaction higher in Bupi + Mg (2.7 ± 0.6) vs. Bupi (2 ± 0.5) group (p < 0.001) PONV, urinary retention, dry mouth, allergic reactions, respiratory depression (0) |
| Gurbet et al., 2014 [20] | LevoBupi 0.25% 20 mL + MP 40 mg vs. Bupi 0.25% 20 mL + MP 40 mg vs. PBO End of surgery | 60 (30 per group) | Laminectomy | PROP 3 mg/kg + FNT 2 μg/kg and SEVO (+N2O) + FNT boluses (iv) | PCA morphine 2 mg/bolus (iv) /Diclofenac 20 mg (im) | VAS (static and dynamic) up to 24 h/morphine up to 24 h | Lower in LevoBupi + MP (9.9 ± 2.1 mg) and Bupi+MP (9.4 ± 1.9 mg) vs. PBO (30 ± 5.6 mg) (p < 0.001) | VAS in LevoBupi + MP and Bupi + MP (ns) VAS lower in treatment groups vs. PBO up to 4 h (p < 0.001) | Longer in LevoBupi + MP (53 ± 16 min) and Bupi+MP (56 ± 17 min) vs. PBO (32 ± 14 min) (p < 0.001) | Sedation, nausea (ns) |
| Gurbet et al., 2008 [22] | LevoBupi 0.25% 30 mL + MP 40 mg vs. LevoBupi 0.25% 30 mL end of surgery vs. LevoBupi 0.25% 30 mL + MP 40 mg vs. LevoBupi 0.25% 30 mL (preemptive) vs. PBO End of surgery | 80 (20 per group) | Discectomy | PROP 2–2.5 mg/kg + FNT 1–1.5 μg/kg and SEVO (+N2O) + FNT boluses (iv) | PCA morphine 2 mg/bolus and 4-h limit 0.4 mg/kg (iv)/Diclofenac 75 mg (im) | VAS at 1,4,8,16,20, and 24 h Analgesics up to 24 h | Similar in all tested groups vs. PBO (ns) | VAS lower in LevoBupi + MP and LevoBupi (end of surgery) vs. other tested groups (p < 0.05) | Longer in all tested groups vs. PBO (p < 0.05) Longer in LevoBupi + MP and LevoBupi (end of surgery) vs. LevoBupi + MP and LevoBupi (preemptive) (p < 0.01) | Sedation (ns) Nausea higher in PBO vs. other tested groups (p < 0.05) Vomiting (ns) |
| Ersayli et al., 2006 [23] | Bupi 0.25% 30 mL + MP 40 mg vs. Bupi 0.25% preemptive Bupi 0.25% 30 mL + MP 40 mg vs. Bupi 0.25% vs. PBO End of surgery | 75 (15 per group) | Discectomy | PROP 2–2.5 mg/kg + FNT 1–1.5 μg/kg and SEVO (+N2O) + FNT boluses (iv) | PCA morphine 4-h limit 0.4 mg/kg (iv) | VAS and VER (static and dynamic) at 1, 4, 8, 16, 20, and 24 h and morphine up to 24 h | Lower in all tested groups vs. PBO (p < 0.001) Lower in preemptive Bupi + MP vs. other groups (p < 0.05) | VAS lower in preemptive Bupi + MP and Bupi groups vs. other groups up to 16 h (p < 0.05) | Longer in all tested groups vs. PBO (p < 0.05) Longer in preemptive Bupi + MP vs. other groups (p < 0.05) | PONV higher in PBO (p < 0.05) Sedation (ns) |
| Ozyilmaz et al., 2012 [21] | LevoBupi 0.5% 20 mL + Tram 2 mg/kg vs. Tram 2 mg/kg vs. LevoBupi 0.5% 20 mL vs. PBO End of surgery | 80 (20 per group) | Discectomy | PROP 2 mg/kg + FNT 1 μg/kg and SEVO (+N2O) + FNT 50 mcg boluses (iv) | PCA pethidine 10 mg/bolus (iv)4-h limit 100 mg/Diclofenac 75 mg/12 h (iv) | VAS at 0, 1, 2, 4, 8, 12, and 24 h Analgesics up to 24 h | No patient in LevoBupi + Tram required analgesia Lower in Tram (37 ± 35 mg) vs. LevoBupi (129 ± 78 mg) vs. PBO (196 ± 71 mg) group (p < 0.001) | VAS lower in all tested groups vs. PBO up to 1 h (p < 0.001) VAS lower in LevoBupi + Tram and Tram vs. LevoBupi and PBO up to 4 h and 12 h (p < 0.05) VAS similar in LevoBupi and Tram up to 24 h (ns) | Longer in LevoBupi + Tram (803 ± 268 min) vs. LevoBupi (163 ± 216 min) vs. PBO (11 ± 2 min) group (p < 0.001) | PONV lower in LevoBupi + Tram group Itching (0) |
| Mitra et al., 2017 [19] | Ropi 0.5% 20 mL + Tram 2 mg/kg vs. Ropi 0.5% 20 mL + DEX 0.5 mcg/kg vs. Ropi 0.5% 20 mL End of surgery | 45 (15 per group) | Discectomy | PROP 2 mg/kg + FNT 2 μg/kg and SEVO (+N2O) + FNT 1 mcg/kg boluses (iv) | Diclofenac 75 mg (im) | VAS at 0, 2, 4, 6, 12, 18, and 24 h Analgesics up to 24 h | Lower in Ropi + DEX (median 75 mg) vs. Ropi + Tram and Ropi (median 150 mg for both) groups (p = 0.008) | VAS lower in Ropi + DEX vs. Ropi group up to 24 h (p < 0.05)VAS lower in Ropi + DEX vs. Ropi + Tram group from 2 to 6 h (p < 0.01) | Longer median time in Ropi + DEX (930 min) vs. Ropi + Tram (420 min) and Ropi (270 min) group (p < 0.001) | Hemodynamics (ns) Sedation (ns) Nausea (ns) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsaousi, G.; Tsitsopoulos, P.P.; Pourzitaki, C.; Palaska, E.; Badenes, R.; Bilotta, F. Analgesic Efficacy and Safety of Local Infiltration Following Lumbar Decompression Surgery: A Systematic Review of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 5936. https://doi.org/10.3390/jcm10245936
Tsaousi G, Tsitsopoulos PP, Pourzitaki C, Palaska E, Badenes R, Bilotta F. Analgesic Efficacy and Safety of Local Infiltration Following Lumbar Decompression Surgery: A Systematic Review of Randomized Controlled Trials. Journal of Clinical Medicine. 2021; 10(24):5936. https://doi.org/10.3390/jcm10245936
Chicago/Turabian StyleTsaousi, Georgia, Parmenion P. Tsitsopoulos, Chryssa Pourzitaki, Eleftheria Palaska, Rafael Badenes, and Federico Bilotta. 2021. "Analgesic Efficacy and Safety of Local Infiltration Following Lumbar Decompression Surgery: A Systematic Review of Randomized Controlled Trials" Journal of Clinical Medicine 10, no. 24: 5936. https://doi.org/10.3390/jcm10245936
APA StyleTsaousi, G., Tsitsopoulos, P. P., Pourzitaki, C., Palaska, E., Badenes, R., & Bilotta, F. (2021). Analgesic Efficacy and Safety of Local Infiltration Following Lumbar Decompression Surgery: A Systematic Review of Randomized Controlled Trials. Journal of Clinical Medicine, 10(24), 5936. https://doi.org/10.3390/jcm10245936







