The Bonebridge BCI 602 Active Transcutaneous Bone Conduction Implant in Children: Objective and Subjective Benefits
Abstract
:1. Introduction
1.1. Bone Conduction Hearing Systems
1.2. BonebridgeDescription
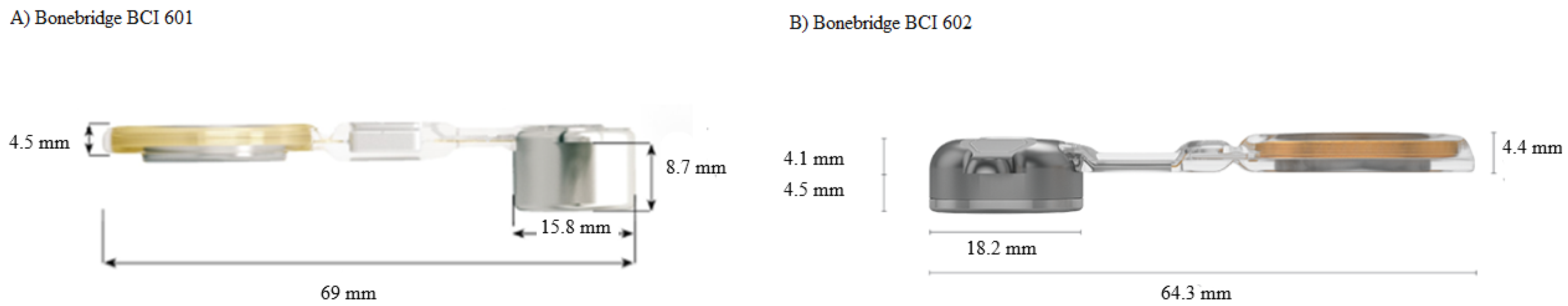
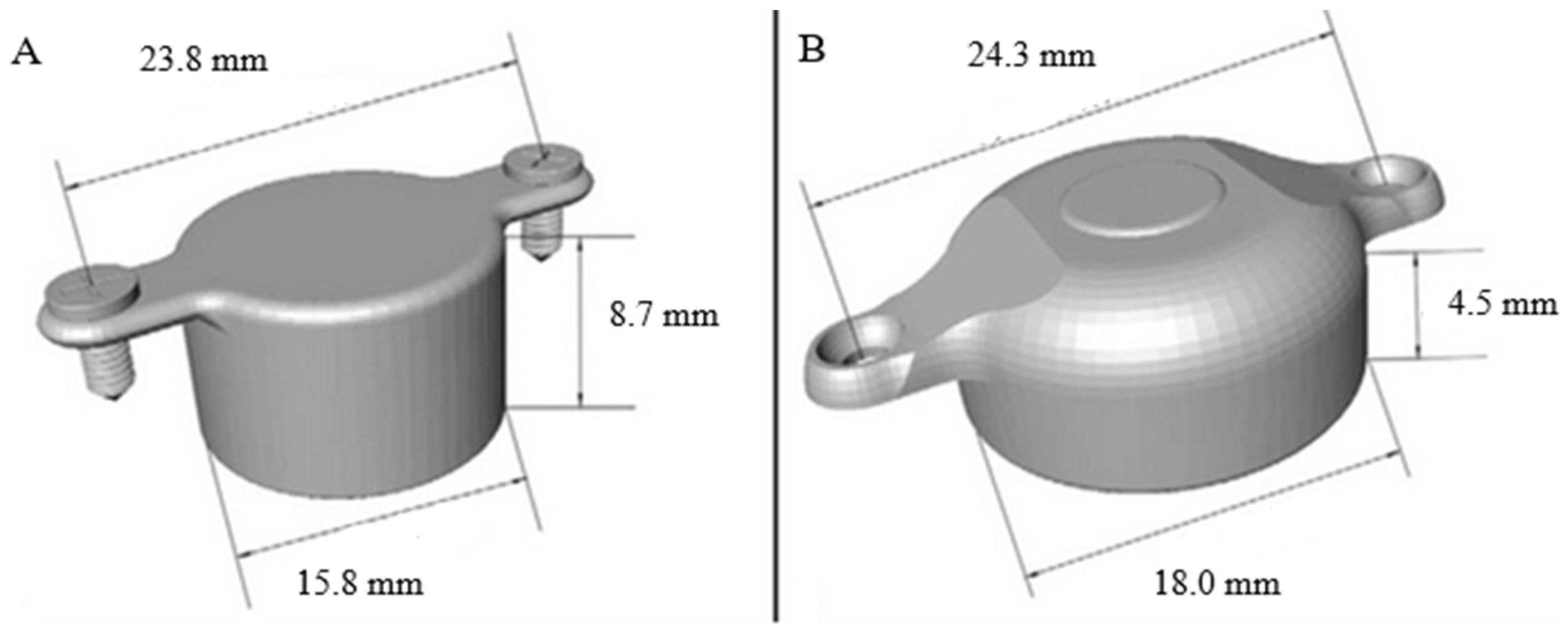
1.3. The New BonebridgeBCI 602
2. Materials and Methods
2.1. Study Design and Participants
2.2. Audiological Evaluation
2.3. Survey Assessment
2.4. Statistical Analysis
2.5. Surgery
3. Results
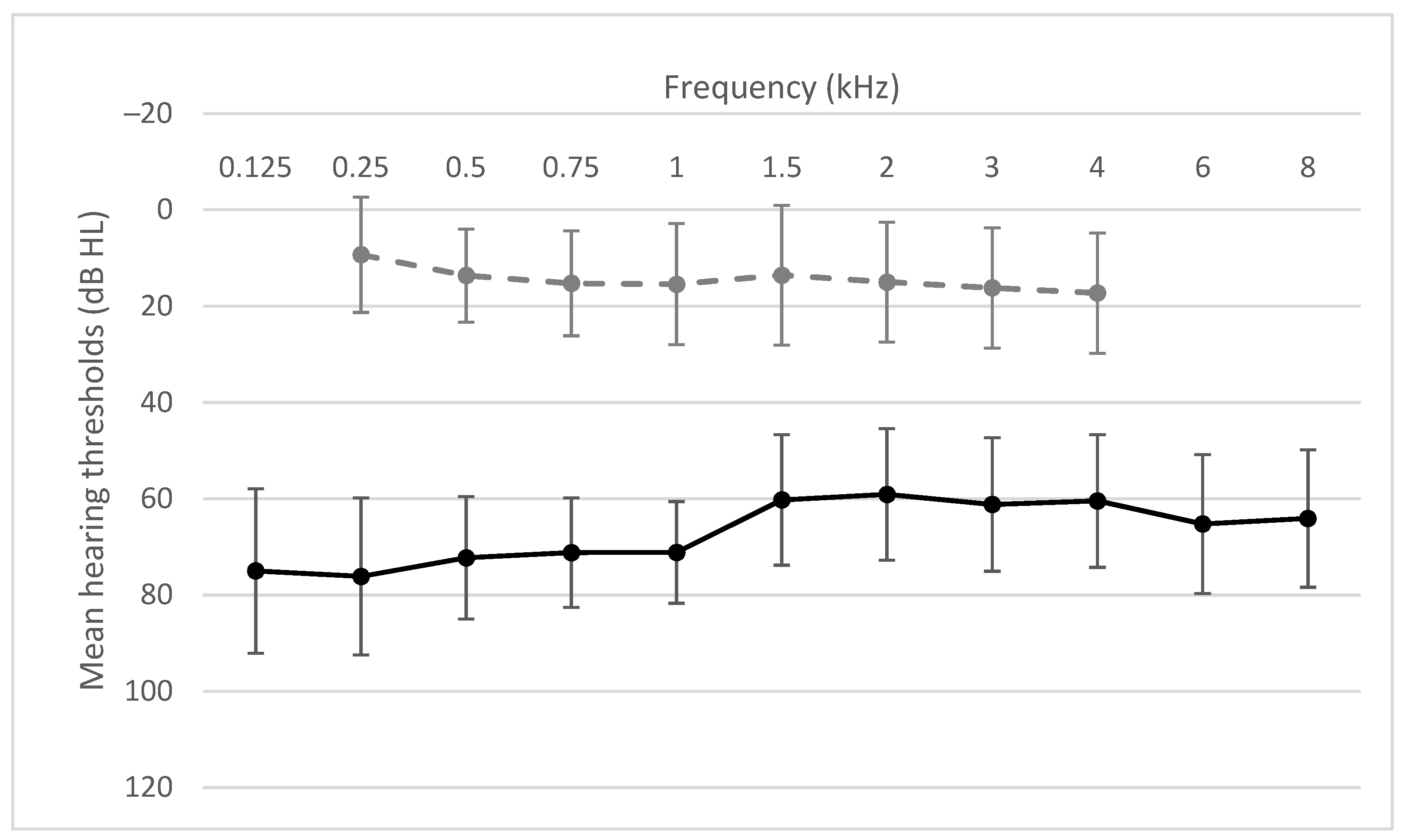
3.1. Pure Tone Audiometry
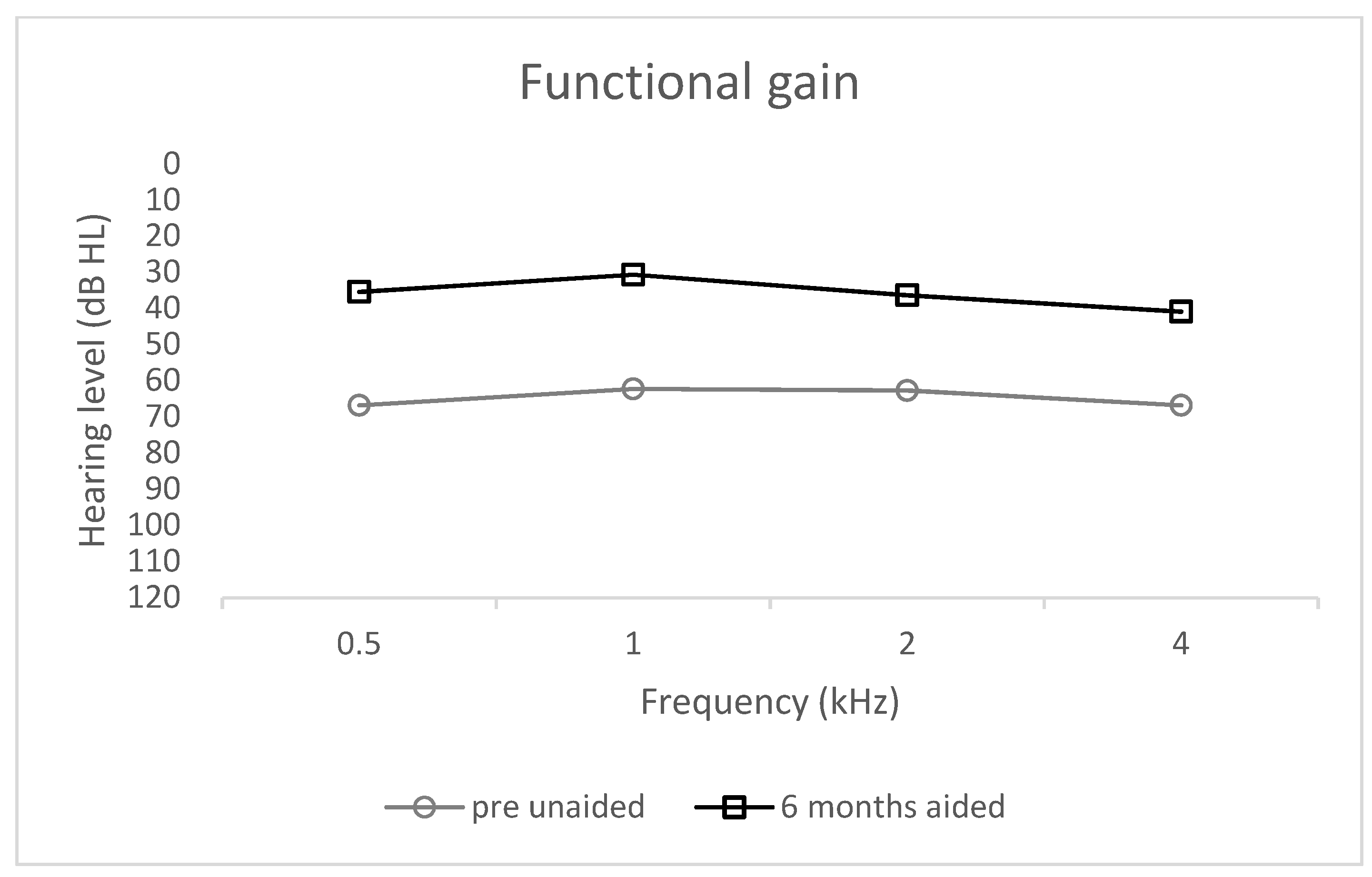
3.2. Functional Gain
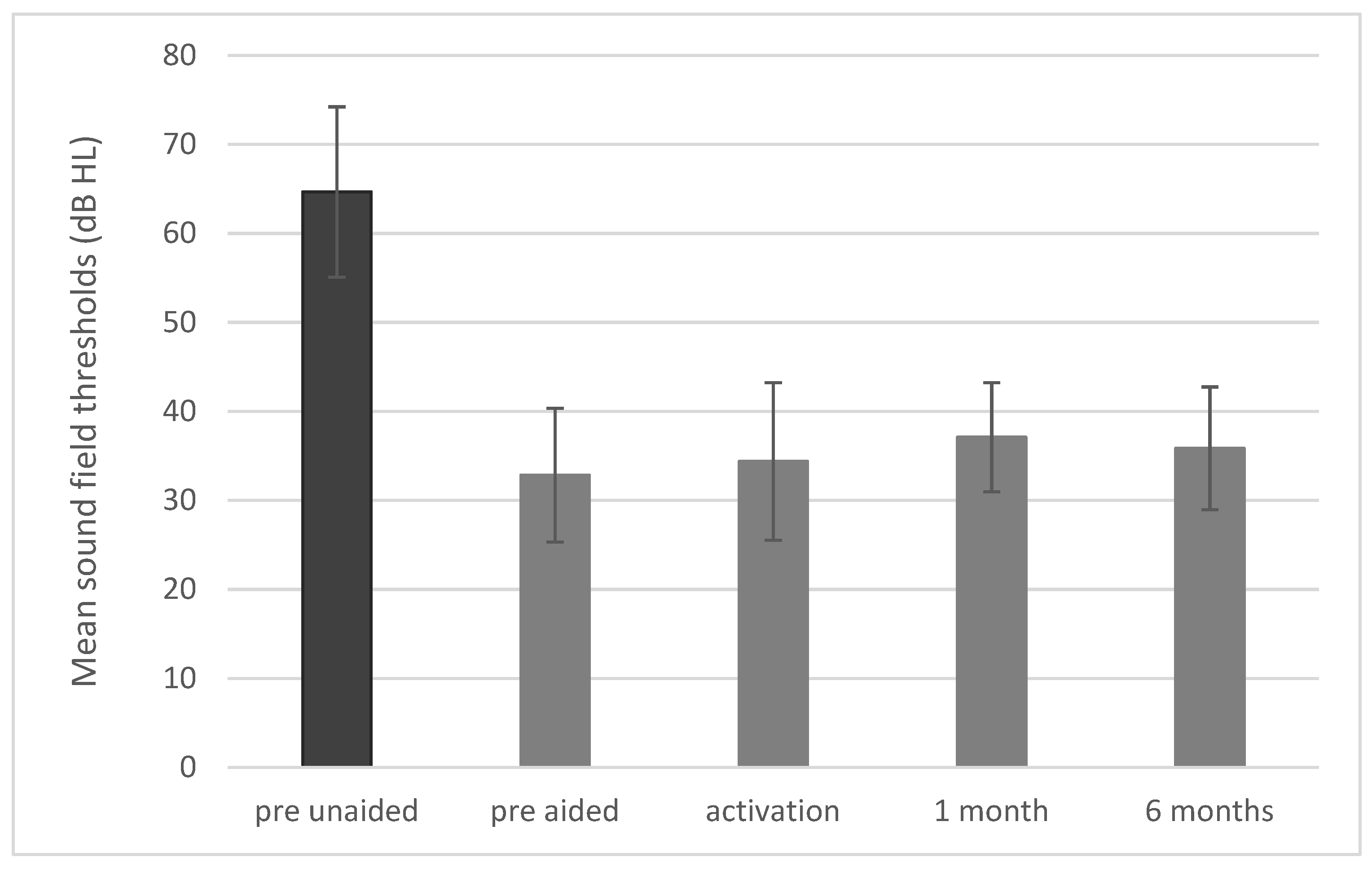
3.3. Sound Field Thresholds
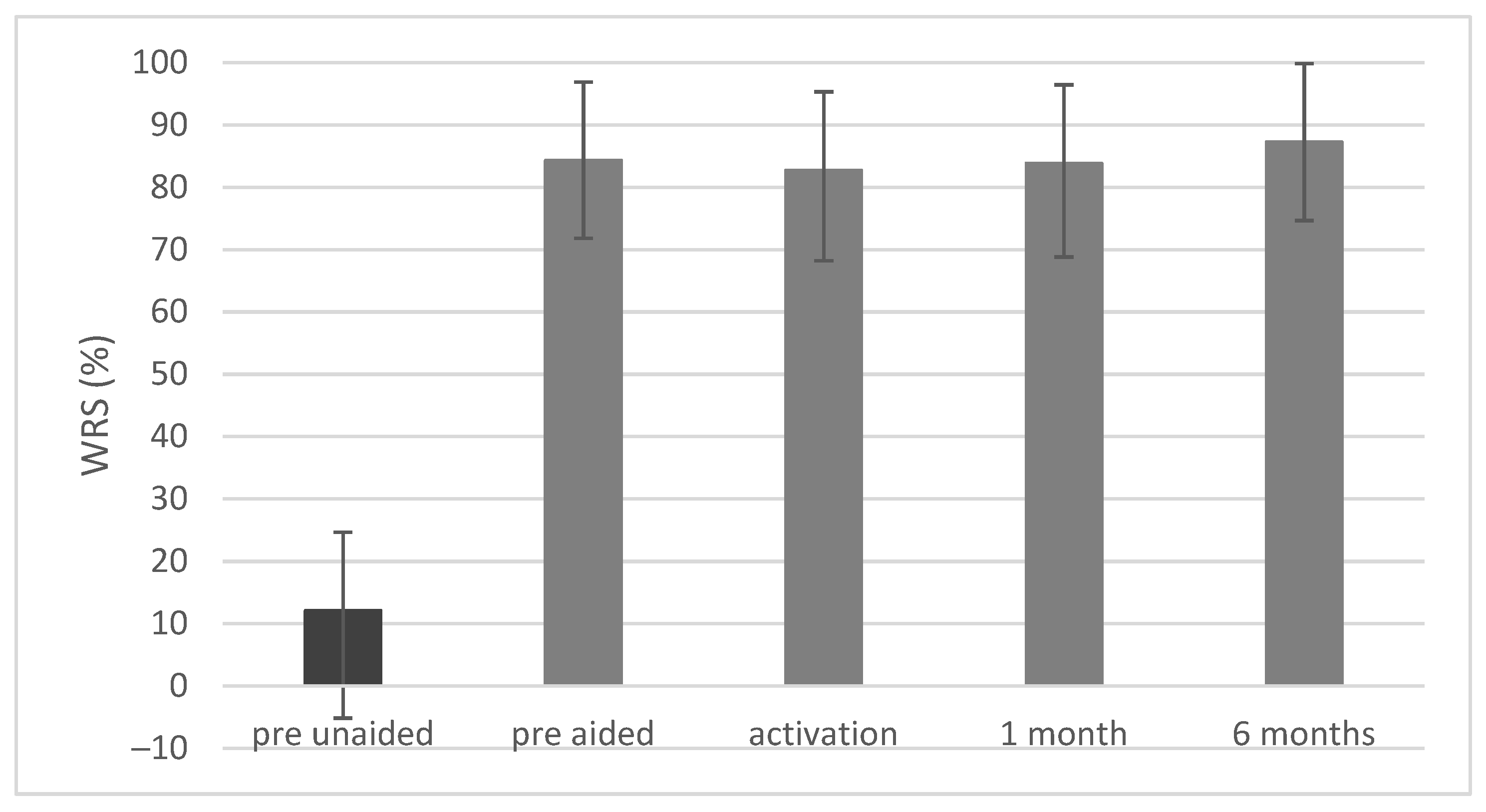
3.4. Free-Field Word Recognition in Quiet
3.5. Intelligibility of Speech in Noise
3.6. Patient-Reported Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moeller, M.P. Early Intervention and Language Development in Children Who Are Deaf and Hard of Hearing. Pediatrics 2006, 106, e43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tharpe, A.M. Unilateral and Mild Bilateral Hearing Loss in Children: Past and Current Perspectives. Trends Amplif. 2008, 12, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.; Bly, R.A.; Sie, K.C.Y. Management of Conductive Hearing Loss Associated with Aural Atresia and Microtia. In Modern Microtia Reconstruction: Art, Science, and New Clinical Techniques; Reinisch, J.F., Tahiri, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 143–166. [Google Scholar]
- Dougherty, W.; Kesser, B.W. Management of Conductive Hearing Loss in Children. Otolaryngol. Clin. N. Am. 2015, 48, 955–974. [Google Scholar] [CrossRef]
- Kruyt, I.J.; Monksfield, P.; Skarzynski, P.H.; Green, K.; Runge, C.; Bosman, A.; Blechert, J.I.; Wigren, S.; Mylanus, E.A.M.; Hol, M.K.S. Results of a 2-Year Prospective Multicenter Study Evaluating Long-Term Audiological and Clinical Outcomes of a Transcutaneous Implant for Bone Conduction Hearing. Otol. Neurotol. 2020, 41, 901–911. [Google Scholar] [CrossRef]
- Jones, S.; Spielmann, P. Device Profile of the Bonebridge Bone Conduction Implant System in Hearing Loss: An Overview of Its Safety and Efficacy. Expert Rev. Med. Devices 2020, 17, 983–992. [Google Scholar] [CrossRef]
- Zernotti, M.E.; Sarasty, A.B. Active Bone Conduction Prosthesis: Bonebridge(TM). Int. Arch. Otorhinolaryngol. 2015, 19, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.E. Osseointegrated Auditory Devices: Bonebridge. Otolaryngol. Clin. N. Am. 2019, 52, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, P.H.; Ratuszniak, A.; Osinska, K.; Koziel, M.; Krol, B.; Cywka, K.B.; Skarzynski, H. A Comparative Study of a Novel Adhesive Bone Conduction Device and Conventional Treatment Options for Conductive Hearing Loss. Otol. Neurotol. 2019, 40, 858–864. [Google Scholar] [CrossRef]
- Kruyt, I.J.; Bakkum, K.H.E.; Caspers, C.J.I.; Hol, M.K.S. The Efficacy of Bone-Anchored Hearing Implant Surgery in Children: A Systematic Review. Int. J. Pediatr.Otorhinolaryngol. 2020, 132, 109906. [Google Scholar] [CrossRef] [PubMed]
- Oberlies, N.R.; Castaño, J.E.; Freiser, M.E.; McCoy, J.L.; Shaffer, A.D.; Jabbour, N. Outcomes of BAHA Connect vs BAHA Attract in Pediatric Patients. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110125. [Google Scholar] [CrossRef] [PubMed]
- Pfiffner, F.; Caversaccio, M.-D.; Kompis, M. Audiological Results with Baha in Conductive and Mixed Hearing Loss. In Implantable Bone Conduction Hearing Aids; Karger Publishers: Basel, Switzerland, 2011; Volume 71, pp. 73–83. [Google Scholar] [CrossRef]
- Dun, C.A.J.; Faber, H.T.; de Wolf, M.J.F.; Mylanus, E.A.M.; Cremers, C.W.R.J.; Hol, M.K.S. Assessment of More than 1000 Implanted Percutaneous Bone Conduction Devices: Skin Reactions and Implant Survival. Otol. Neurotol. 2012, 33, 192–198. [Google Scholar] [CrossRef]
- Hougaard, D.D.; Boldsen, S.K.; Jensen, A.M.; Hansen, S.; Thomassen, P.C. A Multicenter Study on Objective and Subjective Benefits with a Transcutaneous Bone-Anchored Hearing Aid Device: First Nordic Results. Eur. Arch. Oto-Rhino-laryngol. 2017, 274, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.; Khan, I.; Hey, S.Y.; Hussain, S.S.M. A Systematic Review on Skin Complications of Bone-Anchored Hearing Aids in Relation to Surgical Techniques. Eur. Arch. Oto-Rhino-Laryngol. 2014, 273, 559–565. [Google Scholar] [CrossRef]
- Schmerber, S.; Deguine, O.; Marx, M.; Van de Heyning, P.; Sterkers, O.; Mosnier, I.; Garin, P.; Godey, B.; Vincent, C.; Venail, F.; et al. Safety and Effectiveness of the Bonebridge Transcutaneous Active Direct-Drive Bone-Conduction Hearing Implant at 1-Year Device Use. Eur. Arch. Oto-Rhino-laryngol. 2017, 274, 1835–1851. [Google Scholar] [CrossRef]
- den Besten, C.; Monksfield, P.; Bosman, A.; Skarzynski, P.; Green, K.; Runge, C.; Wigren, S.; Blechert, J.; Flynn, M.; Mylanus, E.; et al. Audiological and Clinical Outcomes of a Transcutaneous Bone Conduction Hearing Implant: Six-Month Results from a Multicentre Study. Clin. Otolaryngol. 2019, 44, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Magele, A.; Schoerg, P.; Stanek, B.; Gradl, B.; Sprinzl, G.M. Active Transcutaneous Bone Conduction Hearing Implants: Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0221484. [Google Scholar] [CrossRef] [Green Version]
- Król, B.; Cywka, K.B.; Skarżyńska, M.B.; Skarżyński, P.H. Implantation of the Bonebridge BCI 602 after Mastoid Obliteration with S53P4 Bioactive Glass: A Safe Method of Treating Difficult Anatomical Conditions-Preliminary Results. Life 2021, 11, 374. [Google Scholar] [CrossRef]
- Król, B.; Porowski, M.; Cywka, K.B.; Skarżyńska, M.B.; Skarżyński, P.H. Mastoid Obliteration with S53P4 Bioactive Glass Can Make Bonebridge Implantation Feasible: A Case Report. Am. J. Case Rep. 2020, 21, e925914. [Google Scholar] [CrossRef] [PubMed]
- Skarżyński, P.H.; Ratuszniak, A.; Król, B.; Kozieł, M.; Osińska, K.; Cywka, K.B.; Sztabnicka, A.; Skarżyński, H. The Bonebridge in Adults with Mixed and Conductive Hearing Loss: Audiological and Quality of Life Outcomes. Audiol. Neurootol. 2019, 24, 90–99. [Google Scholar] [CrossRef]
- Plontke, S.K.; Götze, G.; Wenzel, C.; Rahne, T.; Mlynski, R. Implantation of a New Active Bone Conduction Hearing Device with Optimized Geometry. HNO 2020, 68, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Utrilla, C.; Gavilán, J.; García-Raya, P.; Calvino, M.; Lassaletta, L. MRI after Bonebridge Implantation: A Comparison of Two Implant Generations. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3203–3209. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Siegel, L.H.; Kassam, Z.; Hebb, M.; Parnes, L.; Ladak, H.M.; Agrawal, S.K. The Middle Fossa Approach with Self-Drilling Screws: A Novel Technique for BONEBRIDGE Implantation. J. Otolaryngol. Head Neck Surg. 2019, 48, 1–10. [Google Scholar] [CrossRef] [Green Version]
- MED-EL Medical Electronics White Paper on Safety Outcomes of Bone Conduction and Middle Ear Implants: A Systemic Review. Available online: https://www.medel.pro/products/bci602 (accessed on 9 December 2021).
- Almuhawas, F.A.; Dhanasingh, A.E.; Mitrovic, D.; Abdelsamad, Y.; Alzhrani, F.; Hagr, A.; Al Sanosi, A. Age as a Factor of Growth in Mastoid Thickness and Skull Width. Otol. Neurotol. 2020, 41, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.A.W.; Bergman, M.; Keith, J.P.; Powell, K.A.; Hittle, B.; Malhotra, P.; Wiet, G.J. Segmentation of Temporal Bone Anatomy for Patient-Specific Virtual Reality Simulation. Ann. Otol. Rhinol. Laryngol. 2021, 130, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.E.; Small, S.A. Effective Masking Levels for Bone Conduction Auditory Steady State Responses in Infants and Adults with Normal Hearing. Ear Hear 2012, 33, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Aithal, V.; Yonovitz, A.; Aithal, S.; Dold, N. Tonal Masking Level Difference in Children. Aust. N. Z. J. Audiol. 2006, 28, 11–17. [Google Scholar] [CrossRef]
- Hall, J.W.; Grose, J.H. The Masking-Level Difference in Children. J. Am. Acad. Audiol. 1990, 1, 81–88. [Google Scholar] [PubMed]
- Håkansson, B.; Reinfeldt, S.; Persson, A.-C.; Jansson, K.-J.F.; Rigato, C.; Hultcrantz, M.; Eeg-Olofsson, M. The Bone Conduction Implant—A Review and 1-Year Follow-Up. Int. J. Audiol. 2019, 58, 945–955. [Google Scholar] [CrossRef]
- Löhler, J.; Gräbner, F.; Wollenberg, B.; Schlattmann, P.; Schönweiler, R. Sensitivity and Specificity of the Abbreviated Profile of Hearing Aid Benefit (APHAB). Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3593–3598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopun, J.G.; Stelmachowicz, P.G. Perceived Communication Difficulties of Children With Hearing Loss. Am. J. Audiol. 1998, 7, 30–38. [Google Scholar] [CrossRef]
- Ratuszniak, A.; Skarzynski, P.H.; Gos, E.; Skarzynski, H. The Bonebridge Implant in Older Children and Adolescents with Mixed or Conductive Hearing Loss: Audiological Outcomes. Int. J. Pediatr. Otorhinolaryngol. 2019, 118, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Zernotti, M.E.; Chiaraviglio, M.M.; Mauricio, S.B.; Tabernero, P.A.; Zernotti, M.; Di Gregorio, M.F. Audiological Outcomes in Patients with Congenital Aural Atresia Implanted with Transcutaneous Active Bone Conduction Hearing Implant. Int. J. Pediatr. Otorhinolaryngol. 2019, 119, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, G.M.; Wolf-Magele, A. The Bonebridge Bone Conduction Hearing Implant: Indication Criteria, Surgery and a Systematic Review of the Literature. Clin. Otolaryngol. 2016, 41, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Torres, S.; Der-Mussa, C.; Fuentes-López, E. Active Transcutaneous Bone Conduction Implant: Audiological Results in Paediatric Patients with Bilateral Microtia Associated with External Auditory Canal Atresia. Int. J. Audiol. 2017, 57, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ngui, L.X.; Tang, I.P. Bonebridge Transcutaneous Bone Conduction Implant in Children with Congenital Aural Atresia: Surgical and Audiological Outcomes. J. Laryngol. Otol. 2018, 132, 693–697. [Google Scholar] [CrossRef]
- Carnevale, C.; Til-Pérez, G.; Arancibia-Tagle, D.J.; Tomás-Barberán, M.D.; Sarría-Echegaray, P.L. Hearing Outcomes of the Active Bone Conduction System Bonebridge® in Conductive or Mixed Hearing Loss. Acta Otorrinolaringol. 2019, 70, 80–88. [Google Scholar] [CrossRef]
- Weiss, R.; Leinung, M.; Baumann, U.; Weissgerber, T.; Rader, T.; Stöver, T. Improvement of Speech Perception in Quiet and in Noise without Decreasing Localization Abilities with the Bone Conduction Device Bonebridge. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 2107–2115. [Google Scholar] [CrossRef]
- Baumgartner, W.-D.; Hamzavi, J.-S.; Böheim, K.; Wolf-Magele, A.; Schlögel, M.; Riechelmann, H.; Zorowka, P.; Koci, V.; Keck, T.; Potzinger, P.; et al. A New Transcutaneous Bone Conduction Hearing Implant: Short-Term Safety and Efficacy in Children. Otol. Neurotol. 2016, 37, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, K.; Olsen, S.; Miyazaki, H.; Bille, M.; Cayé-Thomasen, P. Objective and Subjective Outcome of a New Transcutaneous Bone Conduction Hearing Device. Otol. Neurotol. 2016, 37, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Kulasegarah, J.; Burgess, H.; Neeff, M.; Brown, C.R.S. Comparing Audiological Outcomes between the Bonebridge and Bone Conduction Hearing Aid on a Hard Test Band: Our Experience in Children with Atresia and Microtia. Int. J. Pediatr. Otorhinolaryngol. 2018, 107, 176–182. [Google Scholar] [CrossRef]
- Ren, R.; Zhao, S.; Wang, D.; Li, Y.; Ma, X.; Li, Y.; Fu, X.; Chen, P.; Dou, J. Audiological Effectiveness of Bonebridge Implantation for Bilateral Congenital Malformation of the External and Middle Ear. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, C.; Schilde, S.; Plontke, S.K.; Rahne, T. Changes in Bone Conduction Implant Geometry Improve the Bone Fit in Mastoids of Children and Young Adults. Otol. Neurotol. 2020, 41, 1406–1412. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cywka, K.B.; Skarżyński, H.; Król, B.; Skarżyński, P.H. The Bonebridge BCI 602 Active Transcutaneous Bone Conduction Implant in Children: Objective and Subjective Benefits. J. Clin. Med. 2021, 10, 5916. https://doi.org/10.3390/jcm10245916
Cywka KB, Skarżyński H, Król B, Skarżyński PH. The Bonebridge BCI 602 Active Transcutaneous Bone Conduction Implant in Children: Objective and Subjective Benefits. Journal of Clinical Medicine. 2021; 10(24):5916. https://doi.org/10.3390/jcm10245916
Chicago/Turabian StyleCywka, Katarzyna B., Henryk Skarżyński, Bartłomiej Król, and Piotr H. Skarżyński. 2021. "The Bonebridge BCI 602 Active Transcutaneous Bone Conduction Implant in Children: Objective and Subjective Benefits" Journal of Clinical Medicine 10, no. 24: 5916. https://doi.org/10.3390/jcm10245916
APA StyleCywka, K. B., Skarżyński, H., Król, B., & Skarżyński, P. H. (2021). The Bonebridge BCI 602 Active Transcutaneous Bone Conduction Implant in Children: Objective and Subjective Benefits. Journal of Clinical Medicine, 10(24), 5916. https://doi.org/10.3390/jcm10245916







