The Impact of the FilmArray-Based Detection of Microbial Pathogens from Positive Blood Culture Vials on the Time to Optimal Antimicrobial Regimen in Intensive Care Units of the Helios University Clinic Wuppertal, Germany
Abstract
:1. Introduction
2. Methods and Materials
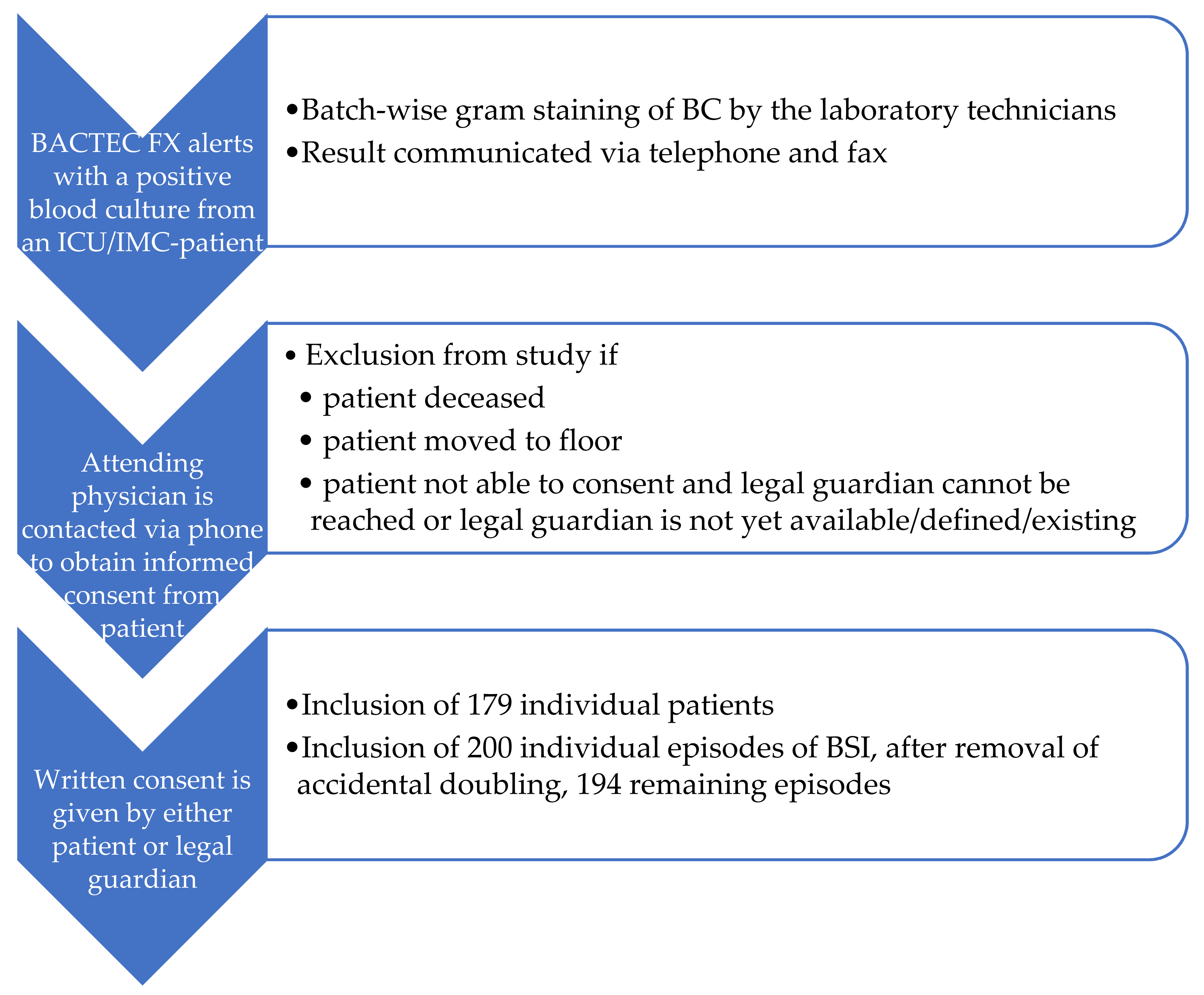
2.1. Objectives of the Study and Inclusion Criteria
2.2. Control Group Population and Conventional Identification Methods
2.3. Intervention Group Population and Multiplex PCR FilmArray BCID Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Primary Endpoints
3.3. Secondary Endpoints
4. Discussion
4.1. Subgroup of Coagulase Negative Staphylococci
4.2. Subgroup of Staphylococcus aureus
4.3. Further Findings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Adhikari, N.K.J.; Fowler, R.A.; Bhagwanjee, S.; Rubenfeld, G.D. Critical care and the global burden of critical illness in adults. Lancet 2010, 376, 1339–1346. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Ferrer, R.; Martin-Loeches, I.; Phillips, G.; Osborn, T.M.; Townsend, S.; Dellinger, R.P.; Artigas, A.; Schorr, C.; Levy, M.M. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: Results from a guideline-based performance improvement program. Crit. Care Med. 2014, 42, 1749–1755. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Kumar, A.; Ellis, P.; Arabi, Y.; Roberts, D.; Light, B.; Parrillo, J.E.; Dodek, P.; Wood, G.; Kumar, A.; Simon, D.; et al. Initiation of inappropriate antimicrobial therapy results in a fivefold reduction of survival in human septic shock. Chest 2009, 136, 1237–1248. [Google Scholar] [CrossRef]
- Paul, M.; Dickstein, Y.; Raz-Pasteur, A. Antibiotic de-escalation for bloodstream infections and pneumonia: Systematic review and meta-analysis. Clin. Microbiol. Infect. 2016, 22, 960–967. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Gutiérrez-Pizarraya, A.; Escoresca-Ortega, A.; Corcia-Palomo, Y.; Fernández-Delgado, E.; Herrera-Melero, I.; Ortiz-Leyba, C.; Márquez-Vácaro, J.A. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014, 40, 32–40. [Google Scholar] [CrossRef]
- Florio, W.; Morici, P.; Ghelardi, E.; Barnini, S.; Lupetti, A. Recent advances in the microbiological diagnosis of bloodstream infections. Crit. Rev. Microbiol. 2018, 44, 351–370. [Google Scholar] [CrossRef]
- Peker, N.; Couto, N.; Sinha, B.; Rossen, J.W. Diagnosis of bloodstream infections from positive blood cultures and directly from blood samples: Recent developments in molecular approaches. Clin. Microbiol. Infect. 2018, 24, 944–955. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Teng, C.B.; Cunningham, S.A.; Ihde, S.M.; Steckelberg, J.M.; Moriarty, J.P.; Shah, N.D.; Mandrekar, J.N.; Patel, R. Randomized Trial of Rapid Multiplex Polymerase Chain Reaction-Based Blood Culture Identification and Susceptibility Testing. Clin. Infect. Dis. 2015, 61, 1071–1080. [Google Scholar] [CrossRef] [Green Version]
- Bookstaver, P.B.; Nimmich, E.B.; Smith, T.J.; Justo, J.A.; Kohn, J.; Hammer, K.L.; Troficanto, C.; Albrecht, H.A.; Al-Hasan, M.N. Cumulative Effect of an Antimicrobial Stewardship and Rapid Diagnostic Testing Bundle on Early Streamlining of Antimicrobial Therapy in Gram-Negative Bloodstream Infections. Antimicrob. Agents Chemother. 2017, 61, e00189-17. [Google Scholar] [CrossRef] [Green Version]
- MacVane, S.H.; Nolte, F.S. Benefits of Adding a Rapid PCR-Based Blood Culture Identification Panel to an Established Antimicrobial Stewardship Program. J. Clin. Microbiol. 2016, 54, 2455–2463. [Google Scholar] [CrossRef] [Green Version]
- Messacar, K.; Hurst, A.L.; Child, J.; Campbell, K.; Palmer, C.; Hamilton, S.; Dowell, E.; Robinson, C.C.; Parker, S.K.; Dominguez, S.R. Clinical Impact and Provider Acceptability of Real-Time Antimicrobial Stewardship Decision Support for Rapid Diagnostics in Children with Positive Blood Culture Results. J. Pediatr. Infect. Dis. Soc. 2017, 6, 267–274. [Google Scholar] [CrossRef]
- Pardo, J.; Klinker, K.P.; Borgert, S.J.; Butler, B.M.; Giglio, P.G.; Rand, K.H. Clinical and economic impact of antimicrobial stewardship interventions with the FilmArray blood culture identification panel. Diagn. Microbiol. Infect. Dis. 2016, 84, 159–164. [Google Scholar] [CrossRef]
- Ray, S.T.J.; Drew, R.J.; Hardiman, F.; Pizer, B.; Riordan, A. Rapid Identification of Microorganisms by FilmArray Blood Culture Identification Panel Improves Clinical Management in Children. Pediatr. Infect. Dis. J. 2016, 35, e134-8. [Google Scholar] [CrossRef]
- Vijayan, A.L.; Vanimaya; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R.; Manoj, G. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef]
- Buss, B.A.; Baures, T.J.; Yoo, M.; Hanson, K.E.; Alexander, D.P.; Benefield, R.J.; Spivak, E.S. Impact of a Multiplex PCR Assay for Bloodstream Infections with and without Antimicrobial Stewardship Intervention at a Cancer Hospital. Open Forum Infect. Dis. 2018, 5, ofy258. [Google Scholar] [CrossRef]
- Plettig, R.; Nowak, A.; Balau, V.; Hahnenkamp, K.; Usichenko, T. Prospective comparison of a PCR assay and a microbiological culture technique for identification of pathogens from blood and non-blood samples in septic patients. J. Intensive Care 2015, 3, 51. [Google Scholar] [CrossRef] [Green Version]
- MacVane, S.H.; Hurst, J.M.; Boger, M.S.; Gnann, J.W. Impact of a rapid multiplex polymerase chain reaction blood culture identification technology on outcomes in patients with vancomycin-resistant Enterococcal bacteremia. Infect. Dis. 2016, 48, 732–737. [Google Scholar] [CrossRef]
- Tseng, A.S.; Kasule, S.N.; Rice, F.; Mi, L.; Chan, L.; Seville, M.T.; Grys, T.E. Is It Actionable? An Evaluation of the Rapid PCR-Based Blood Culture Identification Panel on the Management of Gram-Positive and Gram-Negative Blood Stream Infections. Open Forum Infect. Dis. 2018, 5, ofy308. [Google Scholar] [CrossRef] [Green Version]
- Samuel, L. Direct Detection of Pathogens in Bloodstream During Sepsis: Are We There Yet? J. Appl. Lab. Med. 2019, 3, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Leitner, E.; Hoenigl, M.; Wagner, B.; Krause, R.; Feierl, G.; Grisold, A.J. Performance of the FilmArray Blood culture identification panel in positive blood culture bottles and cerebrospinal fluid for the diagnosis of sepsis and meningitis. GMS Infect. Dis. 2016, 4, Doc06. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.; Champagne, S.; Lowe, C.; Leung, V.; Hinch, M.; Romney, M.G. Evaluation of the FilmArray Blood Culture Identification Panel compared to direct MALDI-TOF MS identification for rapid identification of pathogens. J. Med. Microbiol. 2018, 67, 1253–1256. [Google Scholar] [CrossRef]
- Saito, K.; Endo, S.; Katsumi, M.; Ishizawa, C.; Fujikawa, Y.; Inomata, S.; Toyokawa, M.; Kaku, M. Evaluation of the FilmArray Blood Culture Identification Panel on Detection of Pathogenic Microorganisms in Positive Blood Cultures: The First Clinical Report in Japan. Jpn. J. Infect. Dis. 2018, 71, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Salimnia, H.; Fairfax, M.R.; Lephart, P.R.; Schreckenberger, P.; DesJarlais, S.M.; Johnson, J.K.; Robinson, G.; Carroll, K.C.; Greer, A.; Morgan, M.; et al. Evaluation of the FilmArray Blood Culture Identification Panel: Results of a Multicenter Controlled Trial. J. Clin. Microbiol. 2016, 54, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Southern, T.R.; VanSchooneveld, T.C.; Bannister, D.L.; Brown, T.L.; Crismon, A.S.; Buss, S.N.; Iwen, P.C.; Fey, P.D. Implementation and performance of the BioFire FilmArray® Blood Culture Identification panel with antimicrobial treatment recommendations for bloodstream infections at a midwestern academic tertiary hospital. Diagn. Microbiol. Infect. Dis. 2015, 81, 96–101. [Google Scholar] [CrossRef]
- Altun, O.; Almuhayawi, M.; Ullberg, M.; Ozenci, V. Clinical evaluation of the FilmArray blood culture identification panel in identification of bacteria and yeasts from positive blood culture bottles. J. Clin. Microbiol. 2013, 51, 4130–4136. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Polanco, W.; Carter, D.; Shulman, S. Rapid identification of pathogens from pediatric blood cultures by use of the FilmArray blood culture identification panel. J. Clin. Microbiol. 2014, 52, 4368–4371. [Google Scholar] [CrossRef] [Green Version]
- Eggimann, P.; Bille, J.; Marchetti, O. Diagnosis of invasive candidiasis in the ICU. Ann. Intensive Care 2011, 1, 37. [Google Scholar] [CrossRef] [Green Version]
- Charles, P.E.; Dalle, F.; Aho, S.; Quenot, J.-P.; Doise, J.-M.; Aube, H.; Olsson, N.-O.; Blettery, B. Serum procalcitonin measurement contribution to the early diagnosis of candidemia in critically ill patients. Intensive Care Med. 2006, 32, 1577–1583. [Google Scholar] [CrossRef]
- Martini, A.; Gottin, L.; Menestrina, N.; Schweiger, V.; Simion, D.; Vincent, J.-L. Procalcitonin levels in surgical patients at risk of candidemia. J. Infect. 2010, 60, 425–430. [Google Scholar] [CrossRef]
| Variable | C/I | n | Mean | SD | Min | 25th Perc. | Median | 75th Perc. | Max | p-Value * |
|---|---|---|---|---|---|---|---|---|---|---|
| Duration until antimicrobial change (h) 1 | C I | 71 66 | 167.6 92.9 | 447.2 181.4 | 0 0 | 34 15 | 60 36 | 120 94 | 3180 1227 | 0.029 |
| Time to optimal therapy (h) 1 | C I | 101 118 | 41.3 25.4 | 48.3 29.3 | 0 0 | 0 0 | 37 20 | 63 41 | 242 131 | 0.071 |
| Duration of antimicrobials (h) 2 | C I | 137 157 | 209.0 212.0 | 363.0 290.5 | 2 1 | 77 58 | 120 136 | 199 253 | 3300 2615 | 0.986 |
| Duration | C | 149 | 305.2 | 410.3 | 20 | 99 | 187 | 343 | 3329 | 0.241 |
| of ICU stay (h) 2 | I | 178 | 312.7 | 412.8 | 10 | 65 | 164 | 406 | 2871 | |
| Duration of ventilation (h) 2 | C I | 83 87 | 428.4 311.5 | 1938.6 453.2 | 6 2 | 30 52 | 104 154 | 236 406 | 17,616 2783 | 0.109 |
| Time to 80% PCT level reduction (h) 2 | C I | 49 44 | 124.1 190.4 | 76.9 138.8 | 15 50 | 70 113 | 113 147 | 168 211 | 345 716 | 0.003 |
| Time to optimal therapy (h) 3 | C I | 14 12 | 51.3 30.0 | 41.6 28.3 | 0 0 | 23 21 | 40 22 | 94 31 | 122 106 | 0.084 |
| Primary endpoint | Time to effective/appropriate antimicrobial therapy 1 In subgroup of Staphylococcus aureus BSI | rejected: p = 0.071 rejected: p = 0.084 |
| Secondary endpoints | ICU length of stay | rejected: p = 0.241 |
| Duration of mechanical ventilation | rejected: p = 0.109 | |
| Mortality | rejected: p = 0.135 | |
| PCT level reduction of ≥80% or <0.5 ng/mL | rejected, faster drop-in control group, p = 0.003 | |
| Duration of systemic antimicrobial therapy in CoNS-cases | rejected, shorter duration in control group, p = 0.041 | |
| Others | No effect in BSI cases caused by fungi |
| Variable | C/I | n | Mean | SD | Min | 25th Perc. | Median | 75th Perc. | Max | p-Value * |
|---|---|---|---|---|---|---|---|---|---|---|
| Duration | C | 59 | 160.9 | 151.3 | 2 | 65 | 117 | 199 | 765 | 0.041 |
| of antibiotics (h) | I | 48 | 256.0 | 259.6 | 3 | 80 | 166 | 362 | 1247 | |
| Duration | C | 50 | 206.2 | 327.9 | 6 | 23 | 94 | 236 | 2073 | 0.006 |
| of ventilation (h) | I | 39 | 340.9 | 319.1 | 6 | 119 | 268 | 519 | 1296 | |
| Duration until 80 % PCT level reduction (h) | C I | 20 12 | 120.9 204.7 | 83.6 128.0 | 44 68 | 70 116 | 107 179 | 134 259 | 345 505 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumann, J.; Johanns, U.; Ahmad-Nejad, P.; Ghebremedhin, B.; Woebker, G. The Impact of the FilmArray-Based Detection of Microbial Pathogens from Positive Blood Culture Vials on the Time to Optimal Antimicrobial Regimen in Intensive Care Units of the Helios University Clinic Wuppertal, Germany. J. Clin. Med. 2021, 10, 5880. https://doi.org/10.3390/jcm10245880
Schumann J, Johanns U, Ahmad-Nejad P, Ghebremedhin B, Woebker G. The Impact of the FilmArray-Based Detection of Microbial Pathogens from Positive Blood Culture Vials on the Time to Optimal Antimicrobial Regimen in Intensive Care Units of the Helios University Clinic Wuppertal, Germany. Journal of Clinical Medicine. 2021; 10(24):5880. https://doi.org/10.3390/jcm10245880
Chicago/Turabian StyleSchumann, Jannik, Ulrike Johanns, Parviz Ahmad-Nejad, Beniam Ghebremedhin, and Gabriele Woebker. 2021. "The Impact of the FilmArray-Based Detection of Microbial Pathogens from Positive Blood Culture Vials on the Time to Optimal Antimicrobial Regimen in Intensive Care Units of the Helios University Clinic Wuppertal, Germany" Journal of Clinical Medicine 10, no. 24: 5880. https://doi.org/10.3390/jcm10245880
APA StyleSchumann, J., Johanns, U., Ahmad-Nejad, P., Ghebremedhin, B., & Woebker, G. (2021). The Impact of the FilmArray-Based Detection of Microbial Pathogens from Positive Blood Culture Vials on the Time to Optimal Antimicrobial Regimen in Intensive Care Units of the Helios University Clinic Wuppertal, Germany. Journal of Clinical Medicine, 10(24), 5880. https://doi.org/10.3390/jcm10245880





