Rituximab Therapy for Adults with Nephrotic Syndromes: Standard Schedules or B Cell-Targeted Therapy?
Abstract
1. Introduction
2. Rational and Mechanisms of Action of Rituximab
3. Rituximab Therapy in Nephrotic Syndrome
4. Pitfalls and Open Issues of Rituximab Therapy in Nephrotic Syndrome
5. The Nephrotic Syndrome and Rituximab Plasma Levels
6. The Correlation between CD19+ Depletion and Treatment Response
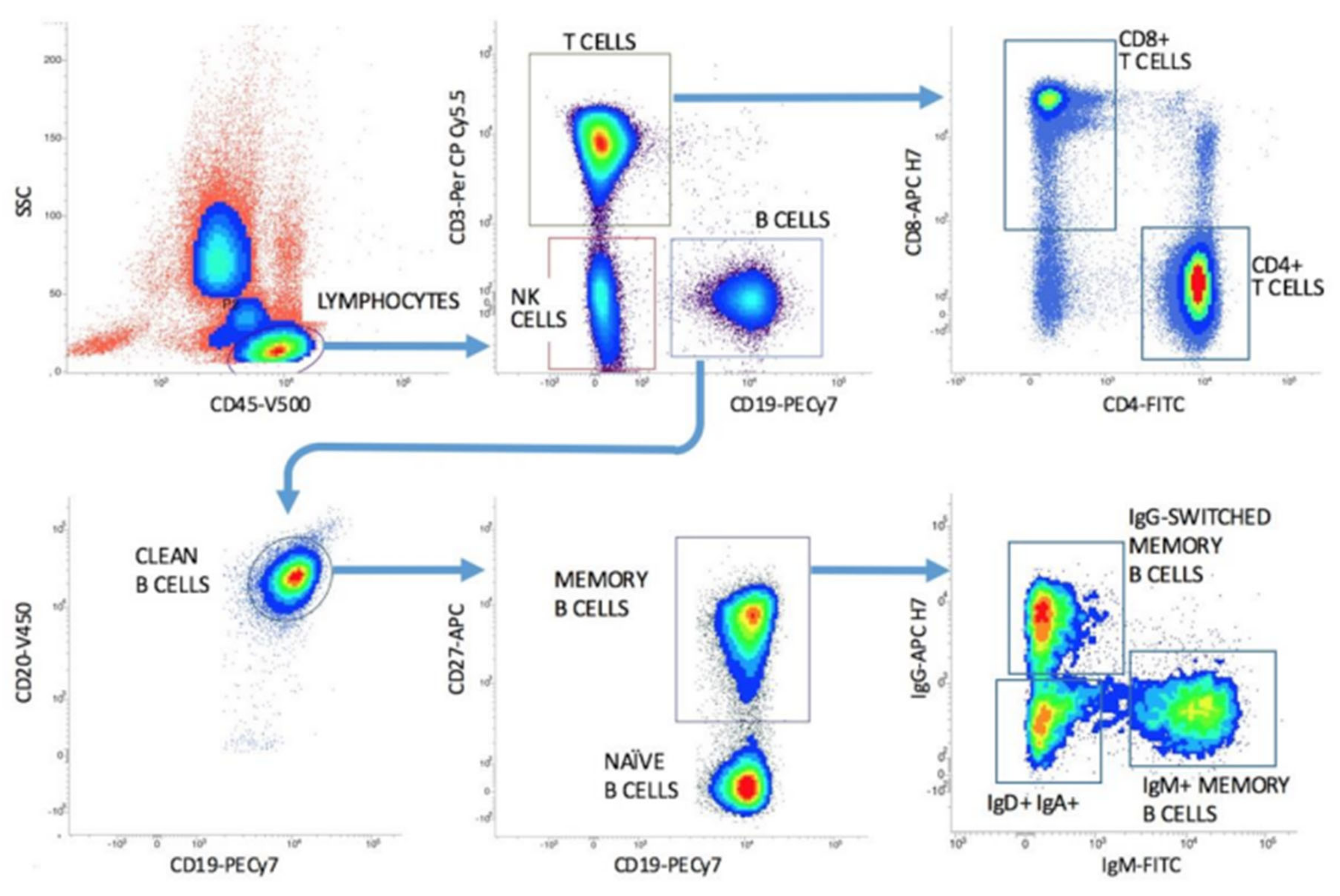
7. High-Sensitivity Flow Cytometric Cell Counting for Immune Monitoring of Anti-CD20 Therapies
8. High-Sensitivity Flow Cytometric Cell Counting in Clinical Practice
9. New Anti-CD20 Targeted Therapies for Nephrotic Syndrome
10. Future Developments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojciechowski, W.; Harris, D.P.; Sprague, F.; Mousseau, B.; Makris, M.; Kusser, K.; Honjo, T.; Mohrs, K.; Mohrs, M.; Randall, T.; et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity 2009, 30, 421–433. [Google Scholar] [CrossRef]
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B Cells, Antibodies, and More. Clin. J. Am. Soc. Nephrol. 2016, 11, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.H.; Bonegio, R.G.B.; Lambeau, G. M-type phospholipase A2 receptor as target antigen in primary membranous nephropathy. N. Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, J.M.; Beck, L.H.; Beck, D.M. Anti-phospholipase A2receptor antibodies correlate with clinical status in primary membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, E.; Thiele, I.; Zahner, G. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1357–1366. [Google Scholar] [CrossRef]
- Tomas, N.M.; Beck, L.H.; Meyer-Schwesinger, C. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 2014, 371, 2277–2287. [Google Scholar] [CrossRef]
- Ronco, P.; Debiec, H. Membranous nephropathy: Current understanding of various causes in light of new target antigens. Curr. Opin. Nephrol. Hypertens. 2021, 30, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ghiggeri, G.M.; Seitz-Polski, B.; Justino, J.; Zaghrini, C.; Payré, C.; Brglez, V.; Dolla, G.; Sinico, A.; Scolari, F.; Vaglio, A.; et al. Multi-autoantibody signature and clinical outcome in membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2020, 15, 1762–1776. [Google Scholar] [CrossRef]
- Maas, R.J.; Deegens, J.K.; Smeets, B.; Moeller, M.J.; Wetzels, J.F. Minimal change disease and idiopathic FSGS: Manifestations of the same disease. Nat. Rev. Nephrol. 2016, 12, 768–776. [Google Scholar] [CrossRef]
- Podestà, M.A.; Ponticelli, C. Autoimmunity in focal segmental glomerulosclerosis: A long-standing yet elusive association. Front. Med. 2020, 7, 604–961. [Google Scholar] [CrossRef]
- Gauckler, P.; Shin, J.; Alberici, F.; Audard, V.; Bruchfeld, A.; Busch, M.; Cheung, C.K.; Crnogorac, M.; Delbarba, E.; Eller, K.; et al. Rituximab in adult minimal change disease and focal segmental glomerulosclerosis—What is known and what is still unknown? Autoimmun. Rev. 2020, 19, 102671. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.A.; Asmar, A.; Alsabbagh, M.M.; Ahsan, N. Rituximab in immunologic glomerular diseases. MAbs 2012, 4, 198–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beers, S.A.; Chan, C.H.T.; French, R.R.; Cragg, M.S.; Glennie, M.J. CD20 as a target for therapeutic Type I and II monoclonal antibodies. Semin. Hematol. 2010, 47, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Brando, B.; Gatti, A.; Lurati, A.M.; Faggioli, P.M.L. Monitoring anti-B cell immunotherapies in autoimmune diseases: Go with the flow. A position paper of the Italian Society for Clinical Cell Analysis (ISCCA). Beyond Rheumatol. 2019, 1, 26. [Google Scholar] [CrossRef]
- Sacco, K.A.; Abraham, R.S. Consequences of B-cell-depleting therapy: Hypogammaglobulinemia and impaired B-cell reconstitution. Immunotherapy 2018, 10, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Weiner, G.J. Rituximab: Mechanism of action. Semin. Hematol. 2010, 47, 115–123. [Google Scholar] [CrossRef]
- Rougé, L.; Chiang, N.; Steffek, M.; Kugel, C.; Croll, T.I.; Tam, C.; Estevez, A.; Arthur, C.P.; Koth, C.M.; Ciferri, C.; et al. Structure of CD20 in complex with the therapeutic monoclonal antibody rituximab. Science 2020, 367, 1224–1230. [Google Scholar] [CrossRef]
- Van der Kolk, L.E.; Grillo-Lopez, A.J.; Baars, J.W.; Hack, C.E.; van Oers, M.H. Complement activation plays a key role in the side-effects of rituximab treatment. Br. J. Haematol. 2001, 115, 807–811. [Google Scholar] [CrossRef]
- Merkt, W.; Lorenz, H.M.; Watzl, C. Rituximab induces phenotypical and functional changes of NK cells in a non-malignant experimental setting. Arthritis Res. Ther. 2016, 18, 206. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, M.; Shimizu, A.; Hanekamp, I.; Torabi, R.; Villani, V.; Yamada, K. Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J. Am. Soc. Nephrol. 2014, 25, 737–744. [Google Scholar] [CrossRef]
- Fornoni, A.; Sageshima, J.; Wei, C.; Merscher-Gomez, S.; Aguillon-Prada, R.; Jauregui, A.N.; Li, J.; Mattiazzi, A.; Ciancio, G.; Chen, L.; et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci. Transl. Med. 2011, 3, 85ra46. [Google Scholar] [CrossRef] [PubMed]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Floege, J. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S3–S276. [Google Scholar]
- Dahan, K.; Debiec, H.; Plaisier, E.; Cachanado, M.; Rousseau, A.; Wakselman, L.; Michel, P.A.; Mihout, F.; Dussol, B.; Matignon, M.; et al. Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J. Am. Soc. Nephrol. 2017, 28, 348–358. [Google Scholar] [CrossRef]
- Fervenza, F.C.; Appel, G.B.; Barbour, S.J.; Rovin, B.H.; Lafayette, R.A.; Aslam, N.; Jefferson, J.A.; Gipson, P.E.; Rizk, D.V.; Sedor, J.R.; et al. MENTOR Investigators. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N. Engl. J. Med. 2019, 381, 36–46. [Google Scholar] [CrossRef]
- Ponticelli, C.; Passerini, P.; Del Vecchio, L.; Locatelli, F. The evolution of the therapeutic approach to membranous nephropathy. Nephrol. Dial. Transpl. 2021, 36, 768–773. [Google Scholar] [CrossRef]
- Huang, L.; Dong, Q.R.; Zhao, Y.J.; Hu, G.C. Rituximab for the management of idiopathic membranous nephropathy: A meta-analysis. Int. Urol. Nephrol. 2021, 53, 111–119. [Google Scholar] [CrossRef]
- Fernández-Juárez, G.; Rojas-Rivera, J.; Logt, A.V.; Justino, J.; Sevillano, A.; Caravaca-Fontán, F.; Ávila, A.; Rabasco, C.; Cabello, V.; Varela, A.; et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021, 99, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Delbarba, E.; Santoro, D.; Gesualdo, L.; Pani, A.; Dallera, N.; Mani, L.Y.; Santostefano, M.; Feriozzi, S.; Quaglia, M.; et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: The RI-CYCLO Randomized Trial. J. Am. Soc. Nephrol. 2021, 32, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, R.; Baldovino, S.; Sciascia, S.; De Simone, E.; Del Vecchio, G.; Ferro, M.; Quattrocchio, G.; Naretto, C.; Roccatello, D. Efficacy of low or standard rituximab-based protocols and comparison to Ponticelli’s regimen in membranous nephropathy. J. Nephrol. 2021, 34, 565–571. [Google Scholar] [CrossRef]
- Ren, H.; Lin, L.; Shen, P.; Li, X.; Xie, J.; Pan, X.; Zhang, W.; Chen, N. Rituximab treatment in adults with refractory minimal change disease or focal segmental glomerulosclerosis. Oncotarget 2017, 8, 93438–93443. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Rosenthal, J.; Laliberte, K.; Niles, J.L. Continuous B-cell depletion in frequently relapsing, steroid-dependent and steroid-resistant nephrotic syndrome. Clin. Kidney. J. 2018, 12, 224–231. [Google Scholar] [CrossRef]
- Kamei, K.; Ishikura, K.; Sako, M.; Ito, S.; Nozu, K.; Iijima, K. Rituximab therapy for refractory steroid-resistant nephrotic syndrome in children. Pediatric Nephrol. 2020, 35, 17–24. [Google Scholar] [CrossRef]
- Allinovi, M.; Trivioli, G.; Lugli, G.; Villanti, M.; Gianassi, I.; Antognoli, G.; Romagnani, P.; Vaglio, A.; Caroti, L.; Cirami, C.L. Proteinuria selectivity index predicts response to rituximab in adults with minimal change disease and focal segmental glomerulosclerosis. Nephrol. Dial. Transpl. 2021, 12, gfab323. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Ruggiero, B.; Cravedi, P.; Vivarelli, M.; Massella, L.; Marasà, M.; Chianca, A.; Rubis, N.; Ene-Iordache, B.; Rudnicki, M.; et al. Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J. Am. Soc. Nephrol. 2014, 25, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Roccatello, D.; Sciascia, S.; Rossi, D.; Alpa, M.; Naretto, C.; Radin, M.; Barreca, A.; Fenoglio, R.; Baldovino, S.; Menegatti, E. High-dose rituximab ineffective for focal segmental glomerulosclerosis: A long-term observation study. Am. J. Nephrol. 2017, 46, 108–113. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Sethi, S.; Nath, K.A.; Glassock, R.J.; Fervenza, F.C. Differentiating primary, genetic, and secondary FSGS in adults: A clinicopathologic approach. J. Am. Soc. Nephrol. 2018, 29, 759–774. [Google Scholar] [CrossRef]
- Hansrivijit, P.; Cheungpasitporn, W.; Thongprayoon, C.; Ghahramani, N. Rituximab therapy for focal segmental glomerulosclerosis and minimal change disease in adults: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 134. [Google Scholar] [CrossRef]
- Iijima, K.; Sako, M.; Nozu, K.; Mori, R.; Tuchida, N.; Kamei, K.; Miura, K.; Aya, K.; Nakanishi, K.; Ohtomo, Y.; et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2014, 384, 1273–1281. [Google Scholar] [CrossRef]
- Ravani, P.; Rossi, R.; Bonanni, A.; Quinn, R.R.; Sica, F.; Bodria, M.; Pasini, A.; Montini, G.; Edefonti, A.; Belingheri, M.; et al. Rituximab in Children with Steroid-Dependent Nephrotic Syndrome: A Multicenter, Open-Label, Noninferiority, Randomized Controlled Trial. J. Am. Soc. Nephrol. 2015, 26, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.H.; Kim, S.H.; Han, K.H.; Choi, H.J.; Cho, H.; Lee, J.W.; Shin, J.I.; Cho, M.H.; Lee, J.H.; Park, Y.S.; et al. Efficacy and safety of rituximab in childhood-onset, difficult-to-treat nephrotic syndrome: A multicenter open-label trial in Korea. Medicine 2018, 97, e13157. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Sander, A.; Roy, B.; Preussler, S.; Barua, S.; Mahapatra, T.K.S.; Schaefer, F. Efficacy of Rituximab vs Tacrolimus in Pediatric Corticosteroid-Dependent Nephrotic Syndrome: A Randomized Clinical Trial. JAMA Pediatric 2018, 172, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Van de Logt, A.E.; Dahan, K.; Rousseau, A.; van der Molen, R.; Debiec, H.; Ronco, P.; Wetzels, J. Immunological remission in PLA2R-antibody-associated membranous nephropathy: Cyclophosphamide versus rituximab. Kidney Int. 2018, 93, 1016–1017. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Z.; Zhang, Y.M.; Qu, Z.; Wang, F.; Meng, L.Q.; Cheng, X.Y.; Liu, G.; Zhou, F.D.; Zhao, M.H. Rituximab for non-responsive idiopathic membranous nephropathy in a Chinese cohort. Nephrol. Dial. Transpl. 2018, 33, 1558–1563. [Google Scholar] [CrossRef]
- Pourcine, F.; Dahan, K.; Mihout, F.; Cachanado, M.; Brocheriou, I.; Debiec, A.; Ronco, P. Prognostic value of PLA2R autoimmunity detected by measurement of anti-PLA2R antibodies combined with detection of PLA2R antigen in membranous nephropathy: A single-centre study over 14 years. PLoS ONE 2017, 12, e0173201. [Google Scholar] [CrossRef] [PubMed]
- Dahan, K.; Johannet, C.; Esteve, E.; Plaisier, E.; Debiec, H.; Ronco, P. Retreatment with rituximab for membranous nephropathy with persistently elevated titers of anti-phospholipase A2 receptor antibody. Kidney Int. 2019, 95, 233–234. [Google Scholar] [CrossRef]
- Seitz-Polski, B.; Debiec, H.; Rousseau, A.; Dahan, K.; Zaghrini, C.; Payré, C.; Esnault, V.L.M.; Lambeau, G.; Ronco, P. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J. Am. Soc. Nephrol. 2018, 29, 401–408. [Google Scholar] [CrossRef]
- Brglez, V.; Boyer-Suavet, S.; Zorzi, K.; Fernandez, C.; Fontas, E.; Esnault, V.; Seitz-Polski, B. Personalized medicine for PLA2R1-related membranous nephropathy: A multicenter randomized control trial. Front. Med. 2020, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Boyer-Suavet, S.; Andreani, M.; Cremoni, M.; Brglez, V.; Benzaken, S.; Bernard, G.; Nachman, P.; Esnault, V.; Seitz-Polski, B. Rituximab bioavailability in primary membranous nephropathy. Nephrol. Dial. Transpl. 2019, 34, 1423–1425. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C. Is there a role for rituximab in the treatment of idiopathic childhood nephrotic syndrome? Pediatric Nephrol. 2007, 22, 893–898. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kumano, Y.; Kagaya, S.; Fujii, A.; Tsuruyama, Y.; Matsuura, T.; Yamazaki, K.; Nomura, K.; Okada, K.; Okino, K.; et al. Retrospective single-arm cohort study of steroid-dependent minimal change nephrotic syndrome treated with very low-dose rituximab. Clin. Nephrol. 2021, 95, 29–36. [Google Scholar] [CrossRef]
- Prytuła, A.; Iijima, K.; Kamei, K.; Fujii, A.; Tsuruyama, Y.; Matsuura, T.; Yamazaki, K.; Nomura, K.; Okada, K.; Okino, K.; et al. Rituximab in refractory nephrotic syndrome. Pediatric Nephrol. 2010, 25, 461–468. [Google Scholar] [CrossRef]
- Chan, E.; Webb, H.; Yu, E.; Ghiggeri, G.M.; Kemper, M.J.; Lap-Tak Ma, A.; Yamamura, T.; Sinha, A.; Bagga, A.; Hogan, J.; et al. Both the rituximab dose and maintenance immunosuppression in steroid-dependent/frequently-relapsing nephrotic syndrome have important effects on outcomes. Kidney Int. 2020, 97, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Hogan, J.; Dossier, C.; Kwon, T.; Macher, M.A.; Maisin, A.; Couderc, A.; Niel, O.; Baudouin, V.; Deschênes, G. Effect of different rituximab regimens on B cell depletion and time to relapse in children with steroid-dependent nephrotic syndrome. Pediatric Nephrol. 2019, 34, 253–259. [Google Scholar] [CrossRef]
- Maxted, A.; Dalrymple, R.; Chisholm, D.; McColl, J.; Tse, Y.; Christian, M.; Reynolds, B.C. Low-dose rituximab is no less effective for nephrotic syndrome measured by 12-month outcome. Pediatric Nephrol. 2019, 34, 855–863. [Google Scholar] [CrossRef]
- Fervenza, F.C.; Abraham, R.S.; Erickson, S.B.; Irazabal, M.V.; Eirin, A.; Specks, U.; Nachman, P.H.; Bergstralh, E.J.; Leung, N.; Cosio, F.G.; et al. Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin. J. Am. Soc. Nephrol. 2010, 5, 2188–2198. [Google Scholar] [CrossRef]
- Fogueri, U.; Cheungapasitporn, W.; Bourne, D.; Fervenza, F.C.; Joy, M.S. Rituximab exhibits altered pharmacokinetics in patients with membranous nephropathy. Ann. Pharmacother. 2019, 53, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Stahl, K.; Duong, M.; Schwarz, A.; Wagner, A.D.; Haller, H.; Schiffer, M.; Jacobs, R. Kinetics of rituximab excretion into urine and peritoneal fluid in two patients with nephrotic syndrome. Case Rep. Nephrol. 2017, 2017, 1372859. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Langer-Jacobus, T.; Stahl, K.; Haller, H.; Schmidt, R.E.; Schiffer, M. Detection and quantification of rituximab in the human urine. J. Immunol. Methods 2017, 451, 118–121. [Google Scholar] [CrossRef]
- Counsilman, C.E.; Jol-van der Zijde, C.M.; Stevens, J.; Cransberg, K.; Bredius, R.G.; Sukhai, R.N. Pharmacokinetics of rituximab in a pediatric patient with therapy-resistant nephrotic syndrome. Pediatric Nephrol. 2015, 30, 1367–1370. [Google Scholar] [CrossRef][Green Version]
- Seitz-Polski, B.; Dahan, K.; Debiec, H.; Rousseau, A.; Andreani, M.; Zaghrini, C.; Ticchioni, M.; Rosenthal, A.; Benzaken, S.; Bernard, G.; et al. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2019, 14, 1173–1182. [Google Scholar] [CrossRef]
- Ellrichmann, G.; Bolz, J.; Peschke, M.; Duscha, A.; Hellwig, K.; Lee, D.H.; Linker, R.A.; Gold, R.; Haghikia, A. Peripheral CD19(+) B-cell counts and infusion intervals as a surrogate for long-term B-cell depleting therapy in multiple sclerosis and neuromyelitis optica/neuromyelitis optica spectrum disorders. J. Neurol. 2019, 266, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Crayne, C.B.; Cron, R.P. Patterns of B Cell Repletion Following Rituximab Therapy in a Pediatric Rheumatology Cohort. ACR Open Rheumatol. 2019, 1, 527–532. [Google Scholar] [CrossRef]
- Venhoff, N.; Niessen, L.; Kreuzaler, M.; Rolink, A.G.; Hässler, F.; Rizzi, M.; Voll, R.E.; Thiel, J. Reconstitution of the peripheral B lymphocyte compartment in patients with ANCA-associated vasculitides treated with rituximab for relapsing or refractory disease. Autoimmunity 2014, 47, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Trouvin, A.P.; Jacquot, S.; Grigioni, S.; Curis, E.; Dedreux, I.; Roucheux, E.; Boulard, H.; Vittecoq, O.; Le Loët, X.; Boyer, O.; et al. Usefulness of monitoring of B cell depletion in rituximab-treated rheumatoid arthritis patients in order to predict clinical relapse: A prospective observational study. Clin. Exp. Immunol. 2015, 180, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.R.; Pepper, R.J.; Shah, K.; Cambridge, G.; Henderson, S.R.; Klein, C.; Kell, L.; Taylor, S.J.; Isenberg, D.A.; Cragg, M.S.; et al. Disparity in Peripheral and Renal B-cell Depletion with Rituximab in Systemic Lupus Erythematosus: An Opportunity for Obinutuzumab? Rheumatology 2021. ahead of print. [Google Scholar]
- Kemper, M.; Gellermann, J.; Habbig, S.; Krmar, R.T.; Dittrich, K.; Jungraithmayr, T.; Pape, L.; Patzer, L.; Billing, H.; Weber, L.; et al. Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol. Dial. Transpl. 2012, 27, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Ravani, P.; Magnasco, A.; Edefonti, A.; Murer, L.; Ghio, L.; Belingheri, M.; Benetti, E.; Murtas, C.; Messina, G.; Massella, L.; et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: A randomized controlled trial. Clin. J. Am. Soc. Nephrol. 2011, 6, 1308–1315. [Google Scholar] [CrossRef]
- Cravedi, P.; Ruggenenti, P.; Sghirlanzoni, M.C.; Remuzzi, G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2007, 2, 932–937. [Google Scholar] [CrossRef]
- Ramachandran, R.; Bharati, J.; Rao, I.; Kashif, A.W.; Nada, R.; Minz, R.; Gupta, K.L.; Kohli, H.S. Persistent CD-19 depletion by rituximab is cost-effective in maintaining remission in calcineurin-inhibitor dependent podocytopathy. Nephrology 2019, 24, 1241–1247. [Google Scholar] [CrossRef]
- Kamei, K.; Ito, S.; Nozu, K.; Iijima, K. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatric Nephrol. 2009, 24, 1321–1328. [Google Scholar] [CrossRef]
- Fujinaga, S.; Hirano, D.; Nishizaki, N.; Kamei, K.; Ito, S.; Ohtomo, Y.; Shimizu, T.; Kaneko, K. Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatric Nephrol. 2010, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Sellier-Leclerc, A.L.; Baudouin, V.; Kwon, T.; Macher, M.A.; Guérin, V.; Lapillonne, H.; Deschênes, G.; Ulinski, T. Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood--follow-up after CD19 recovery. Nephrol. Dial. Transpl. 2012, 27, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Nayak, S.; Kumar, V.; Sethi, J.; Minz, R.; Kumar, V.; Rathi, M.; Kohli, H.S. Rituximab in primary membranous nephropathy: A comparative study of three dosing regimens. Nephrol. Dial. Transpl. 2021. ahead of print. [Google Scholar] [CrossRef]
- Ramachandran, R.; Bharati, J.; Nada, R.; Minz, R.; Kohli, H.S. Rituximab in maintaining remission in adults with podocytopathy. Nephrology 2020, 25, 616–624. [Google Scholar] [CrossRef]
- Fernandez-Fresnedo, G.; Segarra, A.; Alexandru, S.; Delgado, R.; Ramos, N.; Egido, J.; Praga, M.; Trabajo de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN). Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1317–1323. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Chiurchiu, C.; Abbate, M.; Perna, A.; Cravedi, P.; Bontempelli, M.; Remuzzi, G. Rituximab for idiopathic membranous nephropathy: Who can benefit? Clin. J. Am. Soc. Nephrol. 2006, 1, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Debiec, H.; Ruggiero, B.; Chianca, A.; Pellé, T.; Gaspari, F.; Suardi, F.; Gagliardini, E.; Orisio, S.; Benigni, A.; et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J. Am. Soc. Nephrol. 2015, 26, 2545–2558. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Bhatia, D.; Gulati, A.; Rawat, M.; Dinda, A.K.; Hari, P.; Bagga, A. Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol. Dial. Transpl. 2015, 30, 96–106. [Google Scholar] [CrossRef]
- Colucci, M.; Carsetti, R.; Cascioli, S.; Casiraghi, F.; Perna, A.; Ravà, L.; Ruggiero, B.; Emma, F.; Vivarelli, M. B Cell Reconstitution after Rituximab Treatment in Idiopathic Nephrotic Syndrome. J. Am. Soc. Nephrol. 2016, 27, 1811–1822. [Google Scholar] [CrossRef]
- Sato, M.; Kamei, K.; Ogura, M.; Ishikura, K.; Ito, S. Relapse of nephrotic syndrome during post-rituximab peripheral blood B-lymphocyte depletion. Clin. Exp. Nephrol. 2018, 22, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Okamoto, T.; Sato, Y.; Yamazaki, T.; Hayashi, A.; Aoyagi, H.; Ueno, M.; Kobayashi, N.; Uetake, K.; Nakanishi, M.; et al. Periodically repeated rituximab administrations in children with refractory nephrotic syndrome: 2-year multicenter observational study. Pediatric Nephrol. 2019, 34, 87–96. [Google Scholar] [CrossRef]
- Fenoglio, R.; Sciascia, S.; Beltrame, G.; Mesiano, P.; Ferro, M.; Quattrocchio, G.; Menegatti, E.; Roccatello, D. Rituximab as a front-line therapy for adult-onset minimal change disease with nephrotic syndrome. Oncotarget 2018, 9, 28799–28804. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, T.; Masuda, T.; Saito, S.; Kato, N.; Ishimoto, T.; Kato, S.; Kosugi, T.; Tsuboi, N.; Kitamura, H.; Tsuzuki, T.; et al. Therapeutic efficacy of rituximab for the management of adult-onset steroid-dependent nephrotic syndrome: A retrospective study. Clin. Exp. Nephrol. 2019, 23, 207–214. [Google Scholar] [CrossRef]
- Hiepe, F.; Dörner, T.; Hauser, A.E.; Hoyer, B.F.; Mei, H.; Radbruch, A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 2011, 7, 170–178. [Google Scholar] [CrossRef]
- Dass, S.; Rawstron, A.C.; Vital, E.M.; Henshaw, K.; McGonagle, D.; Emery, P. Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis Rheum. 2008, 58, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Gatti, A.; Buccisano, F.; Scupoli, M.T.; Brando, B. The ISCCA flow protocol for the monitoring of anti-CD20 therapies in autoimmune disorders. Cytom. B Clin. Cytom. 2021, 100, 194–205. [Google Scholar] [CrossRef]
- Sentís, A.; Diekmann, F.; Llobell, A.; de Moner, N.; Espinosa, G.; Yagüe, J.; Campistol, J.M.; Mirapeix, E.; Juan, M. Kinetic analysis of changes in T- and B-lymphocytes after anti-CD20treatment in renal pathology. Immunobiology 2017, 222, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Rosenzwajg, M.; Languille, E.; Debiec, H.; Hygino, J.; Dahan, K.; Simon, T.; Klatzmann, D.; Ronco, P. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. 2017, 92, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Sako, M.; Nozu, K. Rituximab for nephrotic syndrome in children. Clin. Exp. Nephrol. 2017, 21, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Leibler, C.; Moktefi, A.; Matignon, M.; Debiais-Delpech, C.; Oniszczuk, J.; Sahali, D.; Cohen, J.L.; Grimbert, P.; Audard, V. Rituximab and fibrillary glomerulonephritis: Interest of B cell reconstitution monitoring. J. Clin. Med. 2018, 7, 430. [Google Scholar] [CrossRef]
- Pozdzik, A.; Beukinga, I.; Gu-Trantien, C.; Willard-Gallo, K.; Nortier, J.; Pradier, O. Circulating (CD3(-)CD19(+)CD20(-)IgD(-)CD27(high)CD38(high)) plasmablasts: Promising cellular biomarker for immune activity for anti-PLA2R1 related membranous nephropathy? Mediat. Inflamm. 2016, 2016, 7651024. [Google Scholar] [CrossRef]
- Cambridge, G.; Leandro, M.J.; Lauren, J.; Fairhead, T.; Robinson, W.H.; Sokolove, J. B cell depletion with rituximab in patients with rheumatoid arthritis: Multiplex bead array reveals the kinetics of IgG and IgA antibodies to citrullinated antigens. J. Autoimmun. 2016, 70, 22–30. [Google Scholar] [CrossRef]
- Reddy, V.; Klein, C.; Isenberg, D.A.; Glennie, M.J.; Cambridge, G.; Cragg, M.S.; Leandro, M.J. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology 2017, 56, 1227–1237. [Google Scholar] [CrossRef]
- Cartron, G.; Watier, H. Obinutuzumab: What is there to learn from clinical trials? Blood 2017, 130, 581–589. [Google Scholar] [CrossRef]
- Basu, B. Ofatumumab for rituximab-resistant nephrotic syndrome. N. Engl. J. Med. 2014, 370, 1268–1270. [Google Scholar] [CrossRef]
- Podestà, M.A.; Gennarini, A.; Portalupi, V.; Rota, S.; Alessio, M.G.; Remuzzi, G.; Ruggenenti, P. Accelerating the depletion of circulating anti-phospholipase A2 receptor antibodies in patients with severe membranous nephropathy: Preliminary findings with double filtration plasmapheresis and ofatumumab. Nephron 2020, 144, 30–35. [Google Scholar] [CrossRef]
- Ravani, P.; Colucci, M.; Bruschi, M.; Vivarelli, M.; Cioni, M.; DiDonato, A.; Cravedi, P.; Lugani, F.; Antonini, F.; Prunotto, M.; et al. Human or Chimeric Monoclonal Anti-CD20 Antibodies for Children with Nephrotic Syndrome: A Superiority Randomized Trial. J. Am. Soc. Nephrol. 2021, 32, 2652–2663. [Google Scholar] [CrossRef]
- Jain, P.; Kanagal-Shamanna, R.; Wierda, W.; Ferrajoli, A.; Keating, M.; Jain, N. Membranoproliferative glomerulonephritis and acute renal failure in a patient with chronic lymphocytic leukemia: Response to obinutuzumab. Hematol. Oncol. Stem. Cell Ther. 2017, 10, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Klomjit, N.; Fervenza, F.C.; Zand, L. Successful treatment of patients with refractory PLA2R-associated membranous nephropathy with obinutuzumab: A report of 3 cases. Am. J. Kidney Dis. 2020, 76, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Mulero, P.; Midaglia, L.; Montalban, X. Ocrelizumab: A new milestone in multiple sclerosis therapy. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418773025. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Schulze, M.; Harendza, S.; Hoxha, E. Successful treatment of PLA 2 R1-antibody positive membranous nephropathy with ocrelizumab. J. Nephrol. 2021, 34, 603–606. [Google Scholar] [CrossRef]
- Crickx, E.; Chappert, P.; Sokal, A.; Weller, S.; Azzaoui, I.; Vandenberghe, A.; Bonnard, G.; Rossi, G.; Fadeev, T.; Storck, S.; et al. Rituximab-resistant splenic memory B cells and newly engaged naive B cells fuel relapses in patients with immune thrombocytopenia. Sci. Transl. Med. 2021, 13, eabc3961. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Ng, J.C.; Wallis, G.; Tsioligka, V.; Fraternali, F.; Dunn-Walters, D.K. Single-cell transcriptomic analyses define distinct peripheral b cell subsets and discrete development pathways. Front. Immunol. 2021, 12, 602539. [Google Scholar] [CrossRef] [PubMed]
- Nissimov, N.; Hajiyeva, Z.; Torke, S.; Grondey, K.; Brück, W.; Häusser-Kinzel, S.; Weber, M.S. B cells reappear less mature and more activated after their anti-CD20-mediated depletion in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 25690–25699. [Google Scholar] [CrossRef] [PubMed]

| CLINICAL TRIAL | GEMRITUX [23] | MENTOR [24] | STARMEN [27] | RI-CYCLO [28] |
|---|---|---|---|---|
| Country | France | The United States and Canada | Spain, France and the Netherlands | Italy and Switzerland |
| Publication Year | 2017 | 2019 | 2020 | 2021 |
| Randomized patients (n) | 75 | 130 | 86 | 74 |
| Inclusion criteria |
|
|
|
|
| Rituximab group | 375 mg/m2 on days 1 and 8 in association with NIAT |
|
|
|
| Control group | NIAT |
|
|
|
| Baseline characteristics: | ||||
| 56 | Around 52 | 55.7 | 55 |
| 76 | Around 90 | 78.5 | 76 |
| 7363.2 (4702.9–9735.0) | NA | NA | NA |
| NA | 8.9 (median) | 7.4 (median) | 6 |
| 68.6 | NA | 79.8 | 84 |
| NA | Around 86 | NA | NA |
| 73.3 | 73.8 | 77% | 66% |
| Primary endpoint | Complete or partial remission of proteinuria at 6 months | Complete or partial remission of proteinuria at 24 months | Complete or partial remission of proteinuria at 24 months | Complete or partial remission of proteinuria at 12 months |
| Primary outcome | n = 13 (35.1%; 95% [95% CI, 19.7 to 50.5) in the rituximab group and n = 8 (21.1%; 95% CI, 8.1 to 34.0) in the NIAT group; p = 0.21 | n = 39 (60%) in the rituximab group and n = 13 (20%) in the cyclosporine group (risk difference, 40 percentage points; 95% CI, 25 to 55; p < 0.001 for both non-inferiority and superiority). | n = 36 (83.7%) in the corticosteroid-cyclophosphamide group and n = 25 (58.1%) in the tacrolimus-rituximab group (RR 1.44; 95% CI 1.08 to 1.92) | n = 23 (62%) in the rituximab group and n = 27 (73%) receiving the cyclic regimen (OR, 0.61; 95% CI, 0.23 to 1.63). |
| Anti–PLA2R-Ab trend | At 6 months deletion in13 of 26 (50%) in the rituximab group and 3 out of 25 (12%) in NIAT group (p = 0.004) | In the subgroup achieving partial or complete remission higher reduction of anti PLAR2 ab titre for rituximab group in comparison to cyclosporine at all-time points | Significant decrease in both treatment groups. A higher proportion of anti-PLA2R-positive patients achieved immunological response at 3 and 6 months in the corticosteroid-cyclophosphamide group (77% and 92%, respectively), as compared to the tacrolimus-rituximab group (45% and 70%, respectively) | Anti-PLA2R levels decreased in both arms during follow-up, more rapidly in the rituximab arm. |
| Safety | Eight (21%) serious adverse events in each group | Serious adverse events in 11 patients (17%) in the rituximab group and in 20 (31%) in the cyclosporine group (p = 0.06) | More adverse events and more adverse events per patient in the corticosteroids-cyclophosphamide group than in the tacrolimus-rituximab group (p = 0.04). | Serious adverse events occurred in 19% of patients receiving rituximab and in 14% receiving SOFIJA the cyclic regimen. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Vecchio, L.; Allinovi, M.; Rocco, P.; Brando, B. Rituximab Therapy for Adults with Nephrotic Syndromes: Standard Schedules or B Cell-Targeted Therapy? J. Clin. Med. 2021, 10, 5847. https://doi.org/10.3390/jcm10245847
Del Vecchio L, Allinovi M, Rocco P, Brando B. Rituximab Therapy for Adults with Nephrotic Syndromes: Standard Schedules or B Cell-Targeted Therapy? Journal of Clinical Medicine. 2021; 10(24):5847. https://doi.org/10.3390/jcm10245847
Chicago/Turabian StyleDel Vecchio, Lucia, Marco Allinovi, Paolo Rocco, and Bruno Brando. 2021. "Rituximab Therapy for Adults with Nephrotic Syndromes: Standard Schedules or B Cell-Targeted Therapy?" Journal of Clinical Medicine 10, no. 24: 5847. https://doi.org/10.3390/jcm10245847
APA StyleDel Vecchio, L., Allinovi, M., Rocco, P., & Brando, B. (2021). Rituximab Therapy for Adults with Nephrotic Syndromes: Standard Schedules or B Cell-Targeted Therapy? Journal of Clinical Medicine, 10(24), 5847. https://doi.org/10.3390/jcm10245847








