NTBC Treatment Monitoring in Chilean Patients with Tyrosinemia Type 1 and Its Association with Biochemical Parameters and Liver Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Clinical Samples
2.3. Sample Preparation for NTBC, Succinylacetone, and Amino Acids
2.4. Instrumentation and Analysis by LC-MS/MS
2.5. Alpha-Fetoprotein and Liver Biomarker Determination
2.6. Statistical Analysis
3. Results
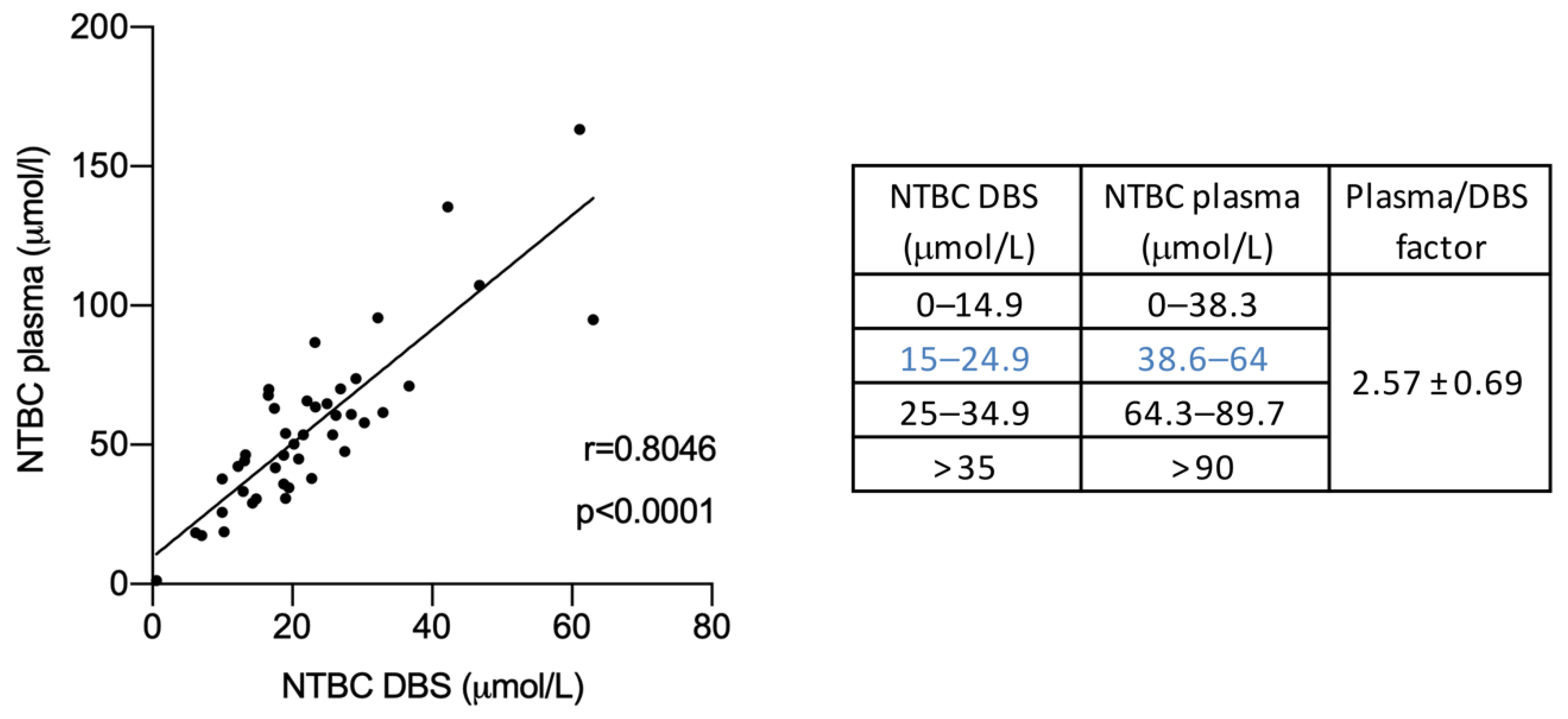
3.1. Comparison of NTBC Concentration in Plasma and DBS
3.2. Association of Succinylacetone Excretion in Urine with NTBC Concentration
3.3. Determination of Urinary Levels of Succinylacetone and Alpha-Fetoprotein According to NTBC Concentration Range
3.4. Association of Plasma Amino Acids with NTBC Concentration
3.5. Association of Liver Biomarkers with NTBC Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cycle Pharmaceuticals Ltd. Clinical Review Report: Nitisinone (Nitisinone Tablets). In Indication: For the Treatment of Patients with Hereditary Tyrosinemia Type 1 in Combination with Dietary Restriction of Tyrosine and Phenylalanine; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2018. Available online: http://www.ncbi.nlm.nih.gov/books/NBK539976/ (accessed on 10 September 2021).
- Angileri, F.; Bergeron, A.; Morrow, G.; Lettre, F.; Gray, G.; Hutchin, T.; Ball, S.; Tanguay, R.M. Geographical and Ethnic Distribution of Mutations of the Fumarylacetoacetate Hydrolase Gene in Hereditary Tyrosinemia Type 1. In JIMD Reports Volume 19; Zschocke, J., Baumgartner, M., Morava, E., Patterson, M., Rahman, S., Peters, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 43–58. [Google Scholar] [CrossRef] [Green Version]
- Morrow, G.; Tanguay, R.M. Biochemical and Clinical Aspects of Hereditary Tyrosinemia Type 1. In Hereditary Tyrosinemia; Tanguay, R.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 9–21. [Google Scholar] [CrossRef]
- Couce, M.L.; Dalmau, J.; del Toro, M.; Pintos-Morell, G.; Aldámiz-Echevarría, L.; Spanish Working Group on Tyrosinemia Type 1. Tyrosinemia type 1 in Spain: Mutational analysis, treatment and long-term outcome: Tyrosinemia type 1 in Spain. Pediatr. Int. 2011, 53, 985–989. [Google Scholar] [CrossRef]
- Holme, E.; Lindstedt, S. Tyrosinaemia type I and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione). J. Inherit. Metab. Dis. 1998, 21, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Larochelle, J.; Alvarez, F.; Bussières, J.F.; Chevalier, I.; Dallaire, L.; Dubois, J.; Faucher, F.; Fenyves, D.; Goodyer, P.; Grenier, A.; et al. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol. Genet. Metab. 2012, 107, 49–54. [Google Scholar] [CrossRef] [PubMed]
- van Ginkel, W.G.; Rodenburg, I.L.; Harding, C.O.; Hollak, C.E.M.; Heiner-Fokkema, M.R.; van Spronsen, F.J. Long-Term Outcomes and Practical Considerations in the Pharmacological Management of Tyrosinemia Type 1. Pediatr. Drugs 2019, 21, 413–426. [Google Scholar] [CrossRef] [Green Version]
- van Ginkel, W.G.; Gouw, A.S.H.; van der Jagt, E.J.; de Jong, K.P.; Verkade, H.J.; van Spronsen, F.J. Hepatocellular carcinoma in tyrosinemia type 1 without clear increase of AFP. Pediatrics 2015, 135, e749–e752. [Google Scholar] [CrossRef] [Green Version]
- van Spronsen, F.J.; van Rijn, M.; Meyer, U.; Das, A.M. Dietary Considerations in Tyrosinemia Type, I. Adv. Exp. Med. Biol. 2017, 959, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kienstra, N.S.; van Reemst, H.E.; van Ginkel, W.G.; Daly, A.; van Dam, E.; MacDonald, A.; Burgerhof, J.G.; de Blaauw, P.; McKiernan, P.J.; Heiner-Fokkema, M.R.; et al. Daily variation of NTBC and its relation to succinylacetone in tyrosinemia type 1 patients comparing a single dose to two doses a day. J. Inherit. Metab. Dis. 2018, 41, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Schultz, M.J.; Netzel, B.C.; Singh, R.H.; Pino, G.B.; Gavrilov, D.K.; Oglesbee, D.; Raymond, K.M.; Rinaldo, P.; Tortorelli, S.; Smith, W.E.; et al. Laboratory monitoring of patients with hereditary tyrosinemia type I. Mol. Genet. Metab. 2020, 130, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Mayorandan, S.; Meyer, U.; Gokcay, G.; Segarra, N.G.; De Baulny, H.O.; Van Spronsen, F.; Zeman, J.; De Laet, C.; Spiekerkoetter, U.; Thimm, E.; et al. Cross-sectional study of 168 patients with hepatorenal tyrosinaemia and implications for clinical practice. Orphanet J. Rare Dis. 2014, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Jack, R.M.; Scott, C.R. Validation of a therapeutic range for nitisinone in patients treated for tyrosinemia type 1 based on reduction of succinylacetone excretion. JIMD Rep. 2019, 46, 75–78. [Google Scholar] [CrossRef]
- Yeo, M.; Turner, C.; Dalton, N.R.; Rahman, Y.; Vara, R. Clinical utilization of dried blood spot nitisinone (NTBC) and succinylacetone (SA) concentrations in hereditary tyrosinaemia type 1—A UK centre experience. Ann. Clin. Biochem. Int. J. Lab. Med. 2020, 57, 412–419. [Google Scholar] [CrossRef]
- Public Health Division, Ministery of Health, Chile. Clinical Management of Tyrosinemia. Available online: https://www.minsal.cl/wp-content/uploads/2015/08/Orientaciones-Tirosinemia-rev-15122015.pdf (accessed on 10 September 2021).
- Chinsky, J.M.; Singh, R.; Ficicioglu, C.; Van Karnebeek, C.D.; Grompe, M.; Mitchell, G.; Waisbren, S.E.; Gucsavas-Calikoglu, M.; Wasserstein, M.P.; Coakley, K.; et al. Diagnosis and treatment of tyrosinemia type I: A US and Canadian consensus group review and recommendations. Genet. Med. 2017, 19, 1380. [Google Scholar] [CrossRef] [Green Version]
- Laeremans, H.; Turner, C.; Andersson, T.; de Juan, J.A.C.; Gerrard, A.; Heiner-Fokkema, M.R.; Herebian, D.; Janzen, N.; La Marca, G.; Rudebeck, M. Inter-laboratory analytical improvement of succinylacetone and nitisinone quantification from dried blood spot samples. JIMD Rep. 2020, 53, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Prieto, J.A.; Andrade, F.; Lage, S.; Aldámiz-Echevarría, L. Comparison of plasma and dry blood spots as samples for the determination of nitisinone (NTBC) by high-performance liquid chromatography–tandem mass spectrometry. Study of the stability of the samples at different temperatures. J. Chromatogr. B 2011, 879, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Wiley, V.; Webster, D.; Loeber, G. Screening Pathways through China, the Asia Pacific Region, the World. Int. J. Neonatal Screen. 2019, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Sander, J.; Janzen, N.; Terhardt, M.; Sander, S.; Gökcay, G.; Demirkol, M.; Ozer, I.; Peter, M.; Das, A.M. Monitoring tyrosinaemia type I: Blood spot test for nitisinone (NTBC). Clin. Chim. Acta 2011, 412, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Schlune, A.; Thimm, E.; Herebian, D.; Spiekerkoetter, U. Single dose NTBC-treatment of hereditary tyrosinemia type I. J. Inherit. Metab. Dis. 2012, 35, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Das, A. Clinical utility of nitisinone for the treatment of hereditary tyrosinemia type-1 (HT-1). Appl. Clin. Genet. 2017, 10, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Herebian, D.; Spiekerkötter, U.; Lamshöft, M.; Thimm, E.; Laryea, M.; Mayatepek, E. Liquid chromatography tandem mass spectrometry method for the quantitation of NTBC (2-(nitro-4-trifluoromethylbenzoyl)1,3-cyclohexanedione) in plasma of tyrosinemia type 1 patients. J. Chromatogr. B 2009, 877, 1453–1459. [Google Scholar] [CrossRef]
- La Marca, G.; Malvagia, S.; Materazzi, S.; Della Bona, M.L.; Boenzi, S.; Martinelli, D.; Dionisi-Vici, C. LC-MS/MS Method for Simultaneous Determination on a Dried Blood Spot of Multiple Analytes Relevant for Treatment Monitoring in Patients with Tyrosinemia Type, I. Anal. Chem. 2012, 84, 1184–1188. [Google Scholar] [CrossRef]
- De Laet, C.; Dionisi-Vici, C.; Leonard, J.V.; McKiernan, P.; Mitchell, G.; Monti, L.; De Baulny, H.O.; Pintos-Morell, G.; Spiekerkötter, U. Recommendations for the management of tyrosinaemia type 1. Orphanet J. Rare Dis. 2013, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Karaksy, H.; Rashed, M.; El-Sayed, R.; El-Raziky, M.; El-Koofy, N.; El-Hawary, M.; Al-Dirbashi, O. Clinical practice: NTBC therapy for tyrosinemia type 1: How much is enough? Eur. J. Pediatr. 2010, 169, 689–693. [Google Scholar] [CrossRef]
- Koelink, C.J.L.; van Hasselt, P.; van der Ploeg, A.; Van den Heuvel-Eibrink, M.M.; Wijburg, F.A.; Bijleveld, C.M.A.; Van Spronsen, F.J. Tyrosinemia type I treated by NTBC: How does AFP predict liver cancer? Mol. Genet. Metab. 2006, 89, 310–315. [Google Scholar] [CrossRef]
- Zeybek, C.A.; Zubarioglu, T. Nitisinone: A review. Orphan Drugs Res. Rev. 2017, 7, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Introne, W.J.; Perry, M.B.; Troendle, J.; Tsilou, E.; Kayser, M.A.; Suwannarat, P.; O’Brien, K.E.; Bryant, J.; Sachdev, V.; Reynolds, J.C.; et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol. Genet. Metab. 2011, 103, 307–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Lamuño, D.; Sánchez-Pintos, P.; Andrade, F.; Couce, M.L.; Aldámiz-Echevarría, L. Treatment adherence in tyrosinemia type 1 patients. Orphanet J. Rare Dis. 2021, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, F.; Liang, B.; Su, Y.; Sun, S.; Xia, S.; Shao, J.; Zhang, Z.; Hong, M.; Zhang, F.; et al. Methionine metabolism in chronic liver diseases: An update on molecular mechanism and therapeutic implication. Signal Transduct. Target. Ther. 2020, 5, 280. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Analysis | Value | Samples (n) | Recommended Reference Value * |
|---|---|---|---|---|
| NTBC doses (mg/kg/day) | Median | 0.97 | 57 | 1.0 mg/kg/day |
| Mean ± SD | 0.95 ± 0.17 | |||
| Min–max | 0.66–1.31 | |||
| NTBC concentration (μmol/L) DBS | Median | 21.3 | 57 | 20–40 μmol/L |
| Mean ± SD | 23.6 ± 12.6 | |||
| Min–max | 3.46–60.14 | |||
| NTBC concentration (μmol/L) Plasma | Median | 50.6 | 43 | 40–60 μmol/L |
| Mean ± SD | 50.4 ± 21.8 | |||
| Min–max | 9.8–101.6 | |||
| Tyrosine plasma (μmol/L) | Median | 460.7 | 58 | 400–600 μmol/L |
| Mean ± SD | 584.2 ± 253.4 | |||
| Min–max | 319.4–1181.6 | |||
| Phenylalanine plasma (μmol/L) | Median | 47.4 | 58 | 20–80 μmol/L |
| Mean ± SD | 47.8 ± 19.5 | |||
| Min–max | 18.05–76.7 | |||
| Methionine plasma (μmol/L) | Median | 23.83 | 58 | 14–43 μmol/L |
| Mean ± SD | 25.18 ± 6.4 | |||
| Min–max | 16.5–40.5 | |||
| Succinylacetone (mmol/mol creatinine) | <0.5 | 89% | 53 | <0.5 mmol/mol creatinine |
| >0.5 | 11% |
| NTBC DBS | NTBC Plasma | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| % of Total Samples | Mean | Number of Samples | Median | p-Value | Mean | Number of Samples | Median | p-Value | |
| Samples with SA < 0.25 mmol/mol creatinine | 79.5 | 22.9 | 31 | 22.1 | * 0.027 | 57.9 | 31 | 60.5 | * 0.025 |
| Samples with SA > 0.25 mmol/mol creatinine | 20.5 | 14.4 | 8 | 14.8 | 35.8 | 8 | 42.1 | ||
| Samples with SA < 0.5 mmol/mol creatinine | 89.7 | 23 | 35 | 21.2 | 0.323 | 55.1 | 35 | 53.5 | 0.357 |
| Samples with SA > 0.5 mmol/mol creatinine | 10.3 | 15.5 | 4 | 15.6 | 38.8 | 4 | 48.2 | ||
| Range of NTBC in DBS (µmol/L) | Number of Paired Samples | SA in Urine (mmol/mol Creatinine) | Number of Paired Samples | αFP (µg/L) |
|---|---|---|---|---|
| 0–14.9 | 14 | Mean ± SD: 3.9 ± 13.1; CI 95%: 0–11 | 13 | Mean ± SD: 23 ± 37.8; CI 95%: 0.1–44.5 |
| 15–24.9 | 19 | Mean ± SD: 0.2 ± 0.5; CI 95%: 0–0.41 | 19 | Mean ± SD: 8.3 ± 7.2; CI 95%: 3.5–10.6 |
| 25–34.9 | 14 | Mean ± SD: 0.1 ± 0.2; CI 95%: 0–0.22 | 15 | Mean ± SD: 8.7 ± 5.5; CI 95%: 4.8–10.8 |
| <35 | 5 | Mean ± SD: 0.2 ± 0.3; CI 95%: 0–0.46 | 6 | Mean ± SD: 5.5 ± 2.1; CI 95%: 2.3–6.8 |
| Values | Samples Analyzed (n) | Recommended Reference Value | ||
|---|---|---|---|---|
| Prothrombin time (sec) | Median | 13.7 | 48 | 11–13.5 sec |
| Mean ± SD | 14.6 ± 4.1 | |||
| Min–max | 7.45–31 | |||
| INR | Median | 1.06 | 48 | 0.8–1.1 |
| Mean ± SD | 1.09 ± 0.12 | |||
| Min–max | 1.0–1.8 | |||
| Aspartate aminotransferase AST (UI/L) | Median | 35.5 | 52 | 15–40 UI/L |
| Mean ± SD | 37.9 ± 18.5 | |||
| Min–max | 13–103 | |||
| Alanine aminotransferase ALT (UI/L) | Median | 25 | 52 | 10–34 UI/L |
| Mean ± SD | 30.2 ± 23 | |||
| Min–max | 10–154 | |||
| ALT/AST | Median | 0.72 | 52 | 1 |
| Mean ± SD | 0.79 ± 0.28 | |||
| Min–max | 0.37–1.56 | |||
| Gamma-glutamyl transferase (GGT) | Median | 27.5 | 52 | 11–21 UI/L |
| Mean ± SD | 38 ± 34 | |||
| Min–max | 10–202 | |||
| Bilirubin direct (mg/dL) | Median | 0.13 | 48 | 0.3 (mg/dL) |
| Mean ± SD | 0.19 ± 0.24 | |||
| Min–max | 0.02–1.72 | |||
| Bilirubin total (mg/dL) | Median | 0.39 | 48 | 0.2–1.2 (mg/dL) |
| Mean ± SD | 0.48 ± 0.31 | |||
| Min–max | 0.13–1.78 | |||
| Alkaline Phosphatase (UI/L) | Median | 204.5 | 45 | 44–147 (UI/L) * |
| Mean ± SD | 262 ± 149 | |||
| Min–max | 62–976 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuenzalida, K.; Leal-Witt, M.J.; Guerrero, P.; Hamilton, V.; Salazar, M.F.; Peñaloza, F.; Arias, C.; Cornejo, V. NTBC Treatment Monitoring in Chilean Patients with Tyrosinemia Type 1 and Its Association with Biochemical Parameters and Liver Biomarkers. J. Clin. Med. 2021, 10, 5832. https://doi.org/10.3390/jcm10245832
Fuenzalida K, Leal-Witt MJ, Guerrero P, Hamilton V, Salazar MF, Peñaloza F, Arias C, Cornejo V. NTBC Treatment Monitoring in Chilean Patients with Tyrosinemia Type 1 and Its Association with Biochemical Parameters and Liver Biomarkers. Journal of Clinical Medicine. 2021; 10(24):5832. https://doi.org/10.3390/jcm10245832
Chicago/Turabian StyleFuenzalida, Karen, María Jesús Leal-Witt, Patricio Guerrero, Valerie Hamilton, María Florencia Salazar, Felipe Peñaloza, Carolina Arias, and Verónica Cornejo. 2021. "NTBC Treatment Monitoring in Chilean Patients with Tyrosinemia Type 1 and Its Association with Biochemical Parameters and Liver Biomarkers" Journal of Clinical Medicine 10, no. 24: 5832. https://doi.org/10.3390/jcm10245832
APA StyleFuenzalida, K., Leal-Witt, M. J., Guerrero, P., Hamilton, V., Salazar, M. F., Peñaloza, F., Arias, C., & Cornejo, V. (2021). NTBC Treatment Monitoring in Chilean Patients with Tyrosinemia Type 1 and Its Association with Biochemical Parameters and Liver Biomarkers. Journal of Clinical Medicine, 10(24), 5832. https://doi.org/10.3390/jcm10245832






