SFRP4 Expression Is Linked to Immune-Driven Fibrotic Conditions, Correlates with Skin and Lung Fibrosis in SSc and a Potential EMT Biomarker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histology Studies
2.3. Immunofluorescence
2.4. Tissue Culture and Functional In Vitro Analysis
2.5. ELISA for SFRP4
2.6. Statistical Analysis
3. Results
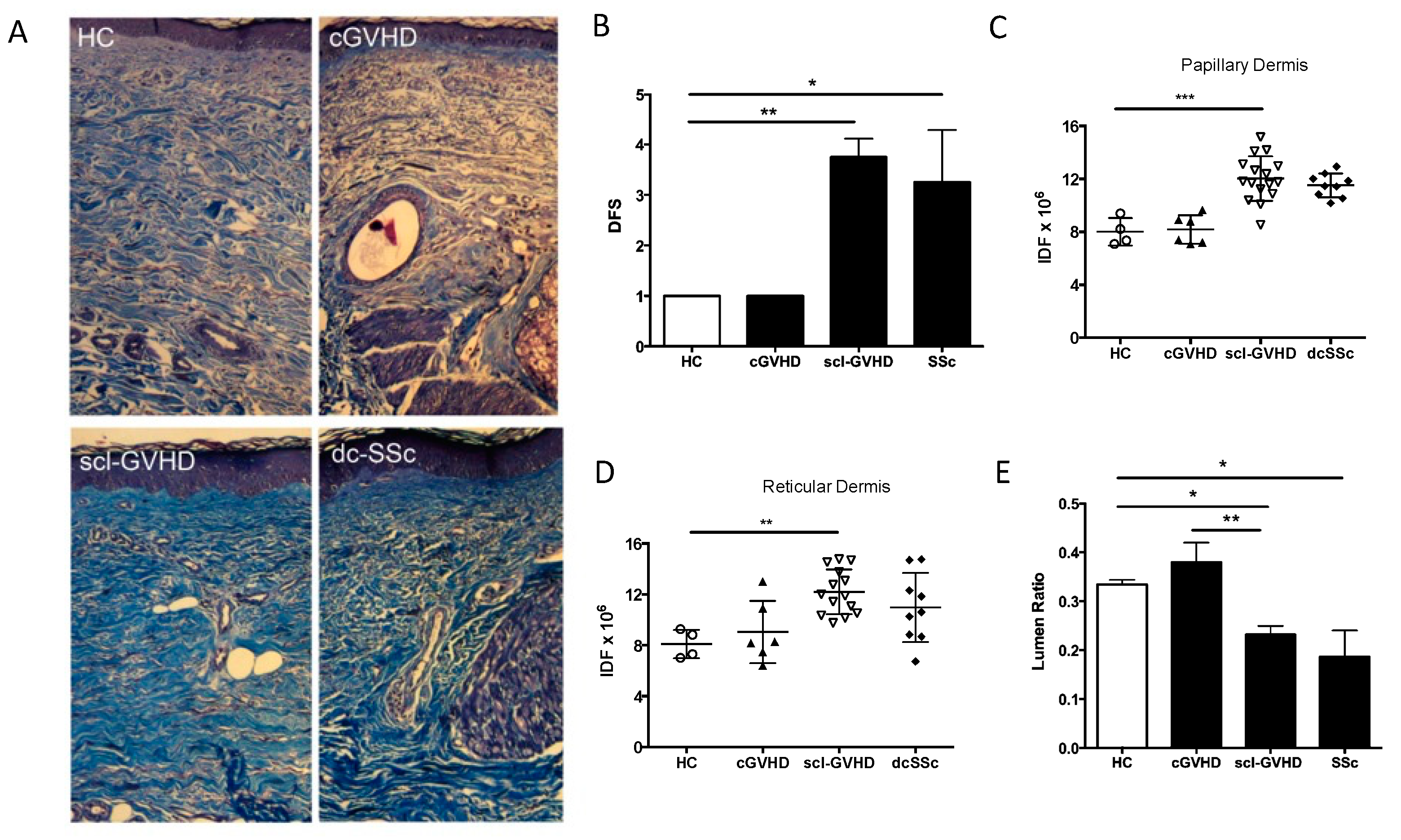
3.1. SSc and sclGVHD Share Similar Histologic Features
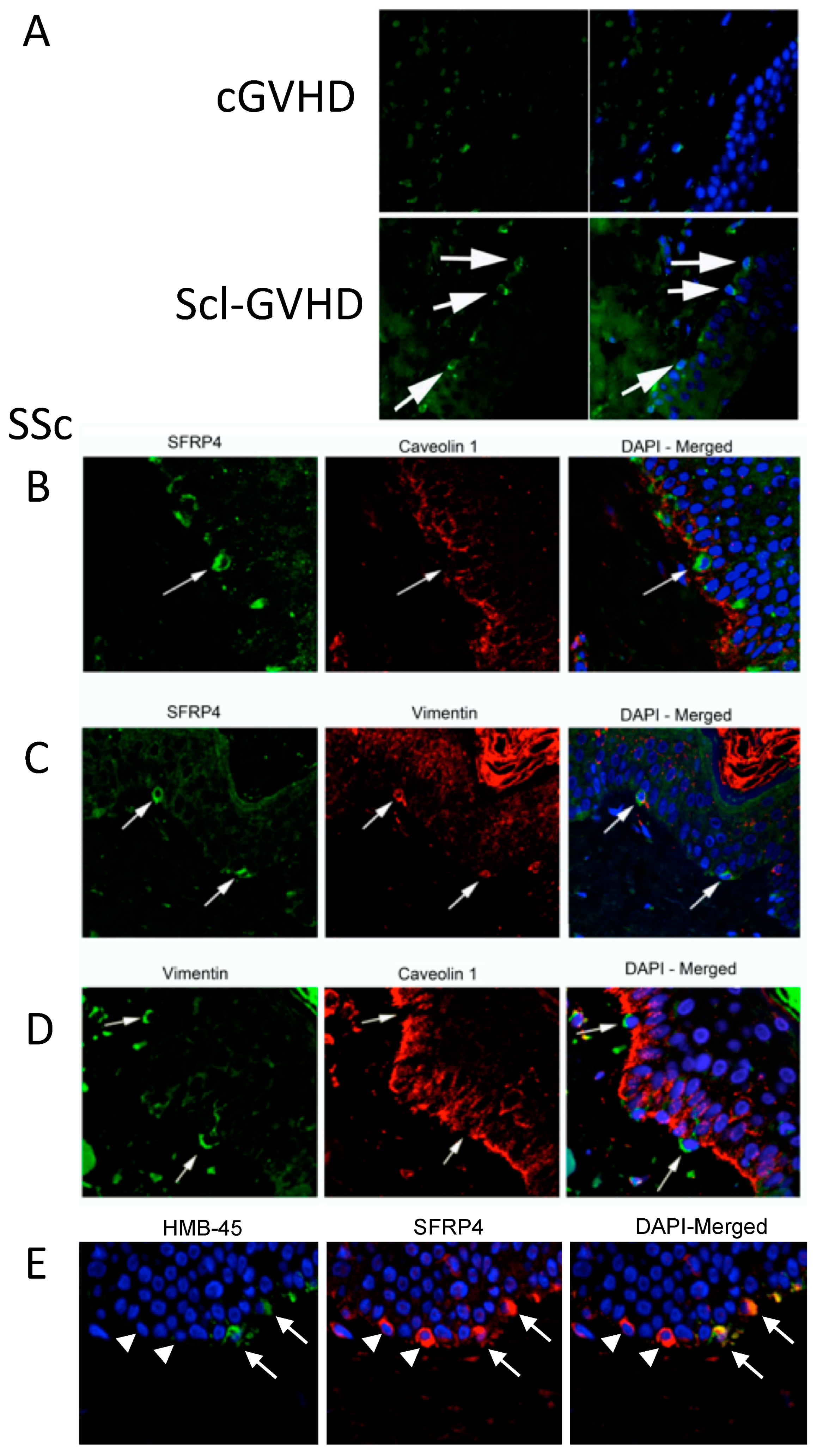
3.2. SFRP4 Expression Is Increased in sclGVHD Epidermis and the Germinal Layer
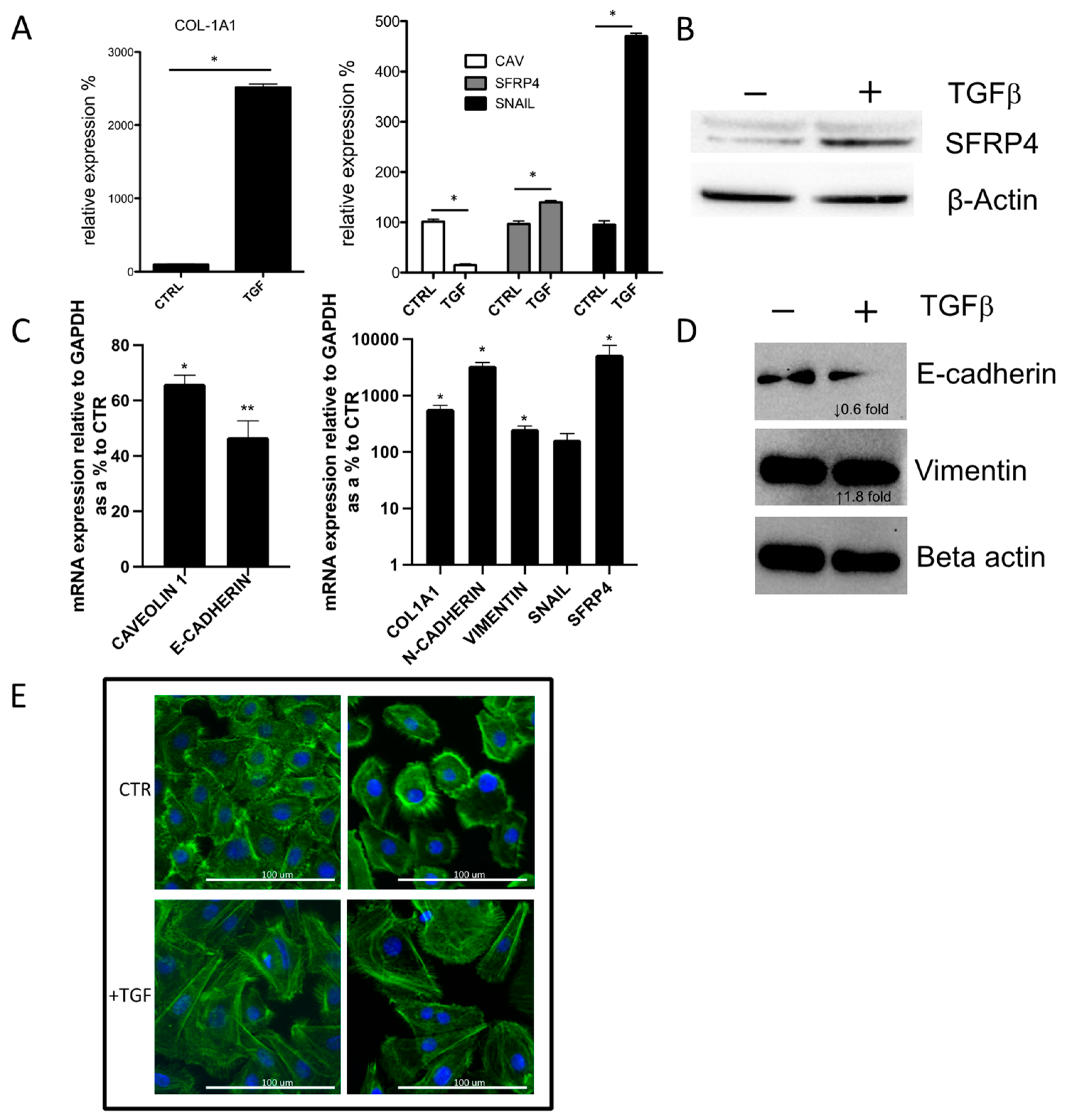
3.3. SFRP4 Is Induced during In Vitro TGFβ Driven Epithelial-to-Mesenchymal Transition (EMT)
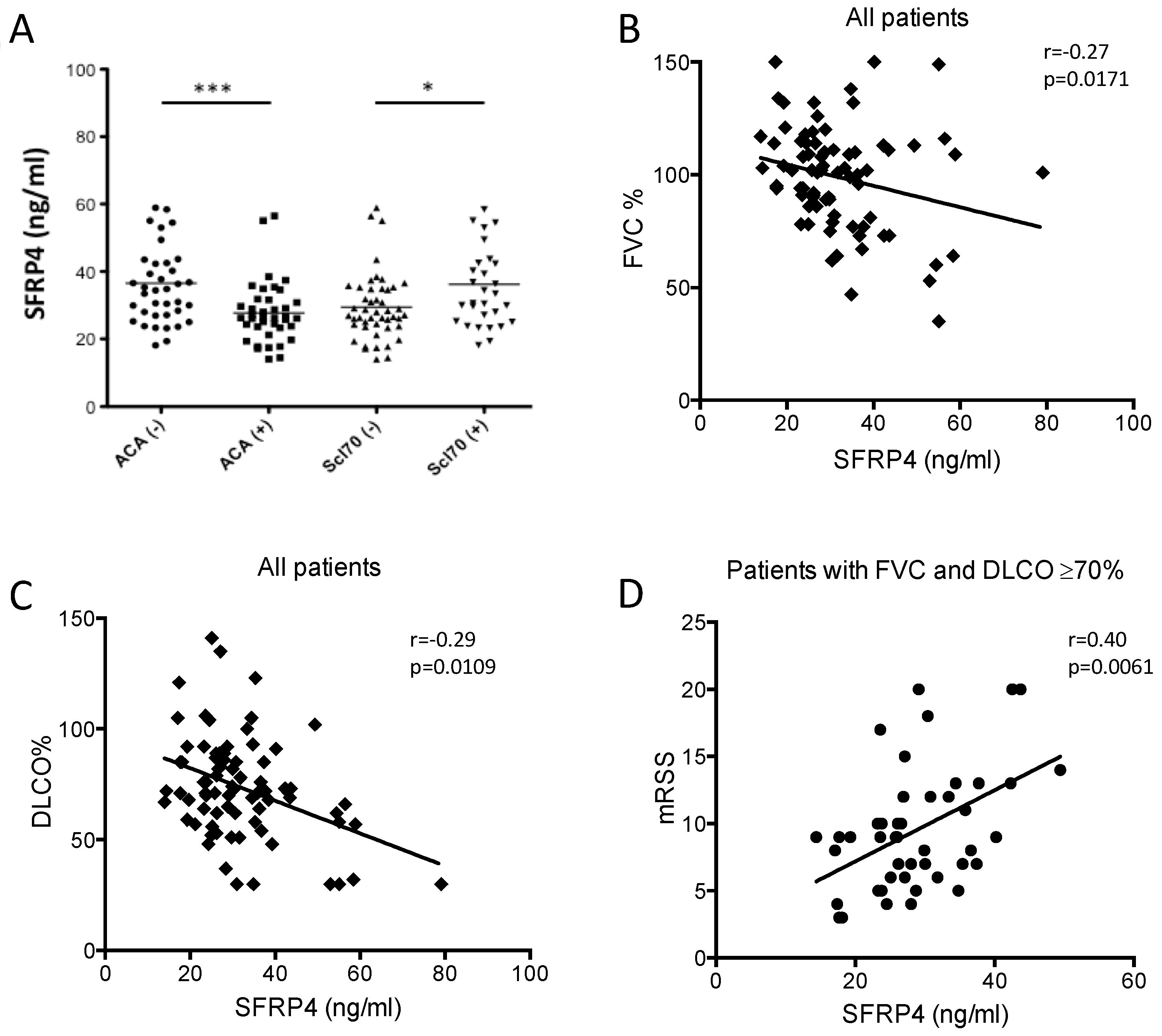
3.4. SFRP4 Serum Concentration as Putative Biomarker of Lung or Skin Fibrosis in SSc
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denton, C.P.; Black, C.M.; Abraham, D.J. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat. Clin. Pract. Rheumatol. 2006, 2, 134–144. [Google Scholar] [CrossRef]
- Sakkas, L.I. New developments in the pathogenesis of systemic sclerosis. Autoimmunity 2005, 38, 113–116. [Google Scholar] [CrossRef]
- Varga, J.; Abraham, D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef]
- Milano, A.; Pendergrass, S.A.; Sargent, J.L.; George, L.K.; McCalmont, T.H.; Connolly, M.K.; Whitfield, M.L. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE 2008, 3, e2696. [Google Scholar] [CrossRef]
- Wuyts, W.A.; Agostini, C.; Antoniou, K.M.; Bouros, D.; Chambers, R.; Cottin, V.; Egan, J.J.; Lambrecht, B.N.; Lories, R.; Parfrey, H.; et al. The pathogenesis of pulmonary fibrosis: A moving target. Eur. Respir. J. 2012, 41, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Postlethwaite, A.; Shigemitsu, H.; Kanangat, S. Cellular origins of fibroblasts: Possible implications for organ fibrosis in systemic sclerosis. Curr. Opin. Rheumatol. 2004, 16, 733–738. [Google Scholar] [CrossRef]
- Bergmann, C.; Distler, J.H.W. Canonical Wnt signaling in systemic sclerosis. Lab. Investig. 2016, 96, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Mii, Y.; Taira, M. Secreted Wnt “inhibitors” are not just inhibitors: Regulation of extracellular Wnt by secreted Frizzled-related proteins. Dev. Growth Differ. 2011, 53, 911–923. [Google Scholar] [CrossRef]
- Mii, Y.; Taira, M. Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development 2009, 136, 4083–4088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frost, J.; Estivill, X.; Ramsay, M.; Tikly, M. Dysregulation of the Wnt signaling pathway in South African patients with diffuse systemic sclerosis. Clin. Rheumatol. 2018, 38, 933–938. [Google Scholar] [CrossRef]
- Yang, I.V.; Burch, L.H.; Steele, M.P.; Savov, J.D.; Hollingsworth, J.W.; McElvania-Tekippe, E.; Berman, K.G.; Speer, M.C.; Sporn, T.A.; Brown, K.K.; et al. Gene expression profiling of familial and sporadic interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2007, 175, 45–54. [Google Scholar] [CrossRef]
- Zhou, X.; Tan, F.K.; Xiong, M.; Milewicz, D.M.; Feghali, C.A.; Fritzler, M.J.; Reveille, J.D.; Arnett, F.C. Systemic sclerosis (scleroderma): Specific autoantigen genes are selectively overexpressed in scleroderma fibroblasts. J. Immunol. 2001, 167, 7126–7133. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, M.L.; Finlay, D.R.; Murray, J.I.; Troyanskaya, O.G.; Chi, J.T.; Pergamenschikov, A.; McCalmont, T.H.; Brown, P.O.; Botstein, D.; Connolly, M.K. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc. Natl. Acad. Sci. USA 2003, 100, 12319–12324. [Google Scholar] [CrossRef] [Green Version]
- Gardner, H.; Shearstone, J.R.; Bandaru, R.; Crowell, T.; Lynes, M.; Trojanowska, M.; Pannu, J.; Smith, E.; Jablonska, S.; Blaszczyk, M.; et al. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum. 2006, 54, 1961–1973. [Google Scholar] [CrossRef]
- Bayle, J.; Fitch, J.; Jacobsen, K.; Kumar, R.; Lafyatis, R.; Lemaire, R. Increased Expression of Wnt2 and SFRP4 in Tsk Mouse Skin: Role of Wnt Signaling in Altered Dermal Fibrillin Deposition and Systemic Sclerosis. J. Investig. Dermatol. 2008, 128, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Valenzi, E.; Bulik, M.; Tabib, T.; Morse, C.; Sembrat, J.; Bittar, H.T.; Rojas, M.; Lafyatis, R. Single-cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis-associated interstitial lung disease. Ann. Rheum. Dis. 2019, 78, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.E.N.; Gak, N.; Campochiaro, C.; Ong, V.H.; Denton, C.P.; Derrett-Smith, E. Developing a molecular classifier for scleroderma spectrum disorders to augment clinical categorisation. Rheumatology 2019, 58, 3. [Google Scholar] [CrossRef] [Green Version]
- Skert, C.; Patriarca, F.; Sperotto, A.; Cerno, M.; Filì, C.; Zaja, F.; Stocchi, R.; Geromin, A.; Damiani, D.; Fanin, R. Sclerodermatous chronic graft-versus-host disease after allogenic hematopoietic stem cells transplantation: Incidence, predictors and outcome. Hematologica 2006, 91, 258–261. [Google Scholar]
- Barausse, G.; Caramaschi, P.; Scambi, C.; Benedetti, F.; Sorio, M.; Tinelli, M.; Tinazzi, I.; Benini, L.; Bambara, L.; Biasi, D. Clinical, Serologic and Instrumental Data of Ten Patients Affected by Sclerodermatous Chronic Graft versus Host Disease: Similarities and Differences in Respect to Systemic Sclerosis. Int. J. Immunopathol. Pharmacol. 2010, 23, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; McCormick, L.L.; Desai, S.R.; Wu, C.; Gilliam, A.C. Murine sclerodermatous graft-versus-host disease, a model for human scleroderma: Cutaneous cytokines, chemokines, and immune cell activation. J. Immunol. 2002, 168, 3088–3098. [Google Scholar] [CrossRef] [Green Version]
- Zerr, P.; Distler, A.; Palumbo-Zerr, K.; Tomcik, M.; Vollath, S.; Dees, C.; Egberts, F.; Tinazzi, I.; del Galdo, F.; Distler, O.; et al. Combined Inhibition of c-Abl and PDGF Receptors for Prevention and Treatment of Murine Sclerodermatous Chronic Graft-versus-Host Disease. Am. J. Pathol. 2012, 181, 1672–1680. [Google Scholar] [CrossRef]
- Zerr, P.; Palumbo-Zerr, K.; Distler, A.; Tomcik, M.; Vollath, S.; Munoz, L.E.; Beyer, C.; Dees, C.; Egberts, F.; Tinazzi, I.; et al. Inhibition of hedgehog signaling for the treatment of murine sclerodermatous chronic graft-versus-host disease. Blood 2012, 120, 2909–2917. [Google Scholar] [CrossRef] [Green Version]
- Le Roy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Akesson, A.; Fiori, G.; Krieg, T.; van den Hoogen, F.H.; Seibold, J.R. Assessment of skin, joint, tendon and muscle involvement. Clin. Exp. Rheumatol. 2003, 21, S5–S8. [Google Scholar]
- Masi, A.T. Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheumatol. 1980, 23, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.A.; McSweeney, P.A.; Nelson, J.L.; Wener, M.; Georges, G.E.; Langston, A.A.; Shulman, H.; Sullivan, K.M.; Lee, J.; Henstorf, G.; et al. Allogeneic marrow transplantation in patients with severe systemic sclerosis: Resolution of dermal fibrosis. Arthritis Rheumatol. 2006, 54, 1982–1986. [Google Scholar] [CrossRef] [Green Version]
- Busquets, J.; Del Galdo, F.; Kissin, E.Y.; Jimenez, S.A. Assessment of tissue fibrosis in skin biopsies from patients with systemic sclerosis employing confocal laser scanning microscopy: An objective outcome measure for clinical trials? Rheumatology 2010, 49, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, J.; Ross, R.L.; Corinaldesi, C.; Esteves, F.; Derrett-Smith, E.; McDermott, M.F.; Doody, G.M.; Denton, C.P.; Emery, P.; Del Galdo, F. Transforming Growth Factor β Activation Primes Canonical Wnt Signaling through Down-Regulation of Axin-2. Arthritis Rheumatol. 2018, 70, 932–942. [Google Scholar] [CrossRef]
- Kasai, H.; Allen, J.T.; Mason, R.M.; Kamimura, T.; Zhang, Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir. Res. 2005, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Del Galdo, F.; Shaw, M.A.; Jimenez, S.A. Proteomic analysis identification of a pattern of shared alterations in the secretome of dermal fibroblasts from systemic sclerosis and nephrogenic systemic fibrosis. Am. J. Pathol. 2010, 177, 1638–1646. [Google Scholar] [CrossRef]
- Fleming, J.N.; Shulman, H.M.; Nash, R.A.; Johnson, P.Y.; Wight, T.N.; Gown, A.; Schwartz, S.M. Cutaneous Chronic Graft-Versus-Host Disease Does Not Have the Abnormal Endothelial Phenotype or Vascular Rarefaction Characteristic of Systemic Sclerosis. PLoS ONE 2009, 4, e6203. [Google Scholar] [CrossRef] [PubMed]
- Tabib, T.; Huang, M.; Morse, N.; Papazoglou, A.; Behera, R.; Jia, M.; Bulik, M.; Monier, D.E.; Benos, P.V.; Chen, W.; et al. Myofibroblast transcriptome indicates SFRP2hi fibroblast progenitors in systemic sclerosis skin. Nat. Commun. 2021, 12, 4384. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, R.; Farina, G.; Bayle, J.; Dimarzio, M.; Pendergrass, S.; Milano, A.; Perbal, B.; Whitfield, M.; Lafyatis, R. Antagonistic effect of the matricellular signaling protein CCN3 on TGF-beta- and Wnt-mediated fibrillinogenesis in systemic sclerosis and Marfan syndrome. J. Investig. Dermatol. 2010, 130, 1514–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.Y.; Kang, W.H. Leukomelanoderma following acute cutaneous graft-versus-host disease. Eur. J. Dermatol. EJD 2004, 14, 146–149. [Google Scholar] [PubMed]
| HC | Scl-GVHD | cGVHD | SSc | |
|---|---|---|---|---|
| N | 3 | 8 | 5 | 8 |
| Sex | ||||
| F (%) | 1 (33) | 4 (50) | 3 (60) | 6 (75) |
| M (%) | 2 (66) | 4 (50) | 2 (40) | 2 (25) |
| Age (M ± SD) | 46.3 ± 12.8 | 51.3 ± 13.2 | 48.2 ± 18.1 | 42.7 ± 9.5 |
| Age of HSTC (M ± SD) | - | 48.2 ± 12.5 | 46.4 ± 17.1 | - |
| HSTC-cGVHD onset (M ± SD) | - | 8.7 ± 2.1 | 7.6 ± 1.6 | - |
| Site biopsy | ||||
| Thigh (%) | 2 (66) | 6 (75) | 2 (40) | 6 (75) |
| Forearm (%) | 1 (33) | 2 (25) | 3 (60) | 2 (25) |
| mRSS site biopsy | 0 | 2 | 0 | 2 |
| DFS score | ||||
| grade 1 | 3 | 0 | 5 | 0 |
| grade 2 | 0 | 1 | 0 | 2 |
| grade 3 | 0 | 2 | 0 | 3 |
| grade 4 | 0 | 3 | 0 | 2 |
| grade 5 | 0 | 2 | 0 | 1 |
| Therapy | ||||
| CsA (%) | 2 (25) | 1 (20) | 0 | |
| MMP (%) | - | 7 (87) | 4 (80) | 1 (12) |
| Sex, F/M | 62/15 |
| Age, mean (SD), years | 57.5 (14.3) |
| Disease duration, mean (SD), years | 15.2 (13.6) |
| Disease subset, D/L | 38/39 |
| ANA + | 77 (100 %) |
| ACA + | 39 (50.6%) |
| Anti-topoisomerase I + | 30 (39%) |
| mRSS, mean (S.D.)/range | 9.6 (4.5)/3–20 |
| FVC %, mean (S.D.)/range | 98.9 (23.2)/35–150 |
| DLCO %, mean (S.D.)/range | 73.4 (23.1)/30–141 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinazzi, I.; Mulipa, P.; Colato, C.; Abignano, G.; Ballarin, A.; Biasi, D.; Emery, P.; Ross, R.L.; Del Galdo, F. SFRP4 Expression Is Linked to Immune-Driven Fibrotic Conditions, Correlates with Skin and Lung Fibrosis in SSc and a Potential EMT Biomarker. J. Clin. Med. 2021, 10, 5820. https://doi.org/10.3390/jcm10245820
Tinazzi I, Mulipa P, Colato C, Abignano G, Ballarin A, Biasi D, Emery P, Ross RL, Del Galdo F. SFRP4 Expression Is Linked to Immune-Driven Fibrotic Conditions, Correlates with Skin and Lung Fibrosis in SSc and a Potential EMT Biomarker. Journal of Clinical Medicine. 2021; 10(24):5820. https://doi.org/10.3390/jcm10245820
Chicago/Turabian StyleTinazzi, Ilaria, Panji Mulipa, Chiara Colato, Giuseppina Abignano, Andrea Ballarin, Domenico Biasi, Paul Emery, Rebecca L. Ross, and Francesco Del Galdo. 2021. "SFRP4 Expression Is Linked to Immune-Driven Fibrotic Conditions, Correlates with Skin and Lung Fibrosis in SSc and a Potential EMT Biomarker" Journal of Clinical Medicine 10, no. 24: 5820. https://doi.org/10.3390/jcm10245820
APA StyleTinazzi, I., Mulipa, P., Colato, C., Abignano, G., Ballarin, A., Biasi, D., Emery, P., Ross, R. L., & Del Galdo, F. (2021). SFRP4 Expression Is Linked to Immune-Driven Fibrotic Conditions, Correlates with Skin and Lung Fibrosis in SSc and a Potential EMT Biomarker. Journal of Clinical Medicine, 10(24), 5820. https://doi.org/10.3390/jcm10245820







