Intra-Cochlear Current Spread Correlates with Speech Perception in Experienced Adult Cochlear Implant Users
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Procedure
2.2.1. Speech Recognition Tests
2.2.2. Impedance and Field Telemetry (IFT) Recording and Exponential Spread Coefficient (ESC) Computation
2.3. Statistical Analyses
3. Results
3.1. Feasibility
3.2. Correlations between ESC and Speech Recognition
3.2.1. Among All Subjects with Measurable SRT50
3.2.2. Among Subjects with Measurable SRT50 and All Electrodes Activated
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hood, L.J. Auditory Neuropathy/Dys-Synchrony Disorder: Diagnosis and Management. Otolaryngol. Clin. N. Am. 2015, 48, 1027–1040. [Google Scholar] [CrossRef]
- Petit, C.; El-Amraoui, A.; Avan, P. Audition: Hearing and Deafness. In Neuroscience in the 21st Century: From Basic to Clinical, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Niparko, J.K.; Tobey, E.A.; Thal, D.J.; Eisenberg, L.S.; Wang, N.-Y.; Quittner, A.L.; Fink, N.E.; CDaCI Investigative Team. Spoken Language Development in Children Following Cochlear Implantation. JAMA 2010, 303, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Dorman, M.F.; Spahr, A.J. A Sensitive Period for the Development of the Central Auditory System in Children with Cochlear Implants: Implications for Age of Implantation. Ear Hear. 2002, 23, 532. [Google Scholar] [CrossRef]
- Blamey, P.J.; Maat, B.; Başkent, D.; Mawman, D.; Burke, E.; Dillier, N.; Beynon, A.; Kleine-Punte, A.; Govaerts, P.J.; Skarzynski, P.H.; et al. A Retrospective Multicenter Study Comparing Speech Perception Outcomes for Bilateral Implantation and Bimodal Rehabilitation. Ear Hear. 2015, 36, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Lazard, D.S.; Vincent, C.; Venail, F.; Van de Heyning, P.; Truy, E.; Sterkers, O.; Skarzynski, P.H.; Skarzynski, H.; Schauwers, K.; O’Leary, S.; et al. Pre-, per- and Postoperative Factors Affecting Performance of Postlinguistically Deaf Adults Using Cochlear Implants: A New Conceptual Model over Time. PLoS ONE 2012, 7, e48739. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, M.; Shannon, R.V. Forward Masked Excitation Patterns in Multielectrode Electrical Stimulation. J. Acoust. Soc. Am. 1998, 103, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Bingabr, M.; Espinoza-Varas, B.; Loizou, P.C. Simulating the Effect of Spread of Excitation in Cochlear Implants. Hear. Res. 2008, 241, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, L.T. Practical Model Description of Peripheral Neural Excitation in Cochlear Implant Recipients: 2. Spread of the Effective Stimulation Field (ESF), from ECAP and FEA. Hear. Res. 2009, 247, 100–111. [Google Scholar] [CrossRef]
- Tang, Q.; Benítez, R.; Zeng, F.-G. Spatial Channel Interactions in Cochlear Implants. J. Neural Eng. 2011, 8, 046029. [Google Scholar] [CrossRef] [PubMed]
- Karg, S.A.; Lackner, C.; Hemmert, W. Temporal Interaction in Electrical Hearing Elucidates Auditory Nerve Dynamics in Humans. Hear. Res. 2013, 299, 10–18. [Google Scholar] [CrossRef]
- Boulet, J.; White, M.; Bruce, I.C. Temporal Considerations for Stimulating Spiral Ganglion Neurons with Cochlear Implants. J. Assoc. Res. Otolaryngol. 2016, 17, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, B.C.J. Coding of Sounds in the Auditory System and Its Relevance to Signal Processing and Coding in Cochlear Implants. Otol. Neurotol. 2003, 24, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Bierer, J.A.; Middlebrooks, J.C. Cortical Responses to Cochlear Implant Stimulation: Channel Interactions. J. Assoc. Res. Otolaryngol. 2004, 5, 32–48. [Google Scholar] [CrossRef]
- Cucis, P.-A.; Berger-Vachon, C.; Thaï-Van, H.; Hermann, R.; Gallego, S.; Truy, E. Word Recognition and Frequency Selectivity in Cochlear Implant Simulation: Effect of Channel Interaction. J. Clin. Med. 2021, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Goehring, T.; Archer-Boyd, A.W.; Arenberg, J.G.; Carlyon, R.P. The Effect of Increased Channel Interaction on Speech Perception with Cochlear Implants. Sci. Rep. 2021, 11, 10383. [Google Scholar] [CrossRef]
- Cohen, L.T.; Richardson, L.M.; Saunders, E.; Cowan, R.S.C. Spatial Spread of Neural Excitation in Cochlear Implant Recipients: Comparison of Improved ECAP Method and Psychophysical Forward Masking. Hear. Res. 2003, 179, 72–87. [Google Scholar] [CrossRef]
- Cohen, L.T.; Saunders, E.; Richardson, L.M. Spatial Spread of Neural Excitation: Comparison of Compound Action Potential and Forward-Masking Data in Cochlear Implant Recipients. Int. J. Audiol. 2004, 43, 346–355. [Google Scholar] [CrossRef]
- Guevara, N.; Hoen, M.; Truy, E.; Gallego, S. A Cochlear Implant Performance Prognostic Test Based on Electrical Field Interactions Evaluated by EABR (Electrical Auditory Brainstem Responses). PLoS ONE 2016, 11, e0155008. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.L.; Stille, L.J. Psychophysical and Physiological Measures of Electrical-Field Interaction in Cochlear Implants. J. Acoust. Soc. Am. 2009, 125, 247–260. [Google Scholar] [CrossRef]
- Dawson, P.W.; McKay, C.M.; Busby, P.A.; Grayden, D.B.; Clark, G.M. Electrode Discrimination and Speech Perception in Young Children Using Cochlear Implants. Ear Hear. 2000, 21, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Henry, B.A.; McKay, C.M.; McDermott, H.J.; Clark, G.M. The Relationship between Speech Perception and Electrode Discrimination in Cochlear Implantees. J. Acoust. Soc. Am. 2000, 108, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.L.; Abbas, P.J. The Relation between Electrophysiologic Channel Interaction and Electrode Pitch Ranking in Cochlear Implant Recipients. J. Acoust. Soc. Am. 2006, 119, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Throckmorton, C.S.; Collins, L.M. Investigation of the Effects of Temporal and Spatial Interactions on Speech-Recognition Skills in Cochlear-Implant Subjects. J. Acoust. Soc. Am. 1999, 105, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Zwolan, T.A.; Collins, L.M.; Wakefield, G.H. Electrode Discrimination and Speech Recognition in Postlingually Deafened Adult Cochlear Implant Subjects. J. Acoust. Soc. Am. 1997, 102, 3673–3685. [Google Scholar] [CrossRef]
- Schafer, F.; Enke, J.; Bohnke, F.; Hemmert, W.; Bai, S. Influence of the Cochlear Implant Electrode Array Placement on the Current Spread in the Cochlea. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 6145–6148. [Google Scholar] [CrossRef]
- Jürgens, T.; Hohmann, V.; Büchner, A.; Nogueira, W. The Effects of Electrical Field Spatial Spread and Some Cognitive Factors on Speech-in-Noise Performance of Individual Cochlear Implant Users—A Computer Model Study. PLoS ONE 2018, 13, e0193842. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Singhal, S.; Landry, T.; Roberts, I.; De Rijk, S.; Brochier, T.; Goehring, T.; Tan, Y.C.; Carlyon, R.P.; Bance, M.; et al. An Instrumented Cochlea Model for the Evaluation of Cochlear Implant Electrical Stimulus Spread. IEEE Trans. Biomed. Eng. 2021, 68, 2281–2288. [Google Scholar] [CrossRef]
- Söderqvist, S.; Lamminmäki, S.; Aarnisalo, A.; Hirvonen, T.; Sinkkonen, S.T.; Sivonen, V. Intraoperative Transimpedance and Spread of Excitation Profile Correlations with a Lateral-Wall Cochlear Implant Electrode Array. Hear. Res. 2021, 405, 108235. [Google Scholar] [CrossRef]
- Vanpoucke, F.J.; Zarowski, A.J.; Peeters, S.A. Identification of the Impedance Model of an Implanted Cochlear Prosthesis from Intracochlear Potential Measurements. IEEE Trans. Biomed. Eng. 2004, 51, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.Y.; Clark, G.M.; Cowan, R.S.C. A Study of Intra-Cochlear Electrodes and Tissue Interface by Electrochemical Impedance Methods in Vivo. Biomaterials 2004, 25, 3813–3828. [Google Scholar] [CrossRef]
- Micco, A.G.; Richter, C.-P. Tissue Resistivities Determine the Current Flow in the Cochlea. Curr. Opin. Otolaryngol. Head Neck Surg. 2006, 14, 352–355. [Google Scholar] [CrossRef]
- Fournier, J.E. Audiométrie Vocale: Les Épreuves D’intelligibilité et Leurs Applications au Diagnostic, à L’expertise et à la Correction Prothétique des Surdités; Maloine: Paris, France, 1951. [Google Scholar]
- Chan, Y.H. Biostatistics 104: Correlational Analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Miller, C.A.; Brown, C.J.; Abbas, P.J.; Chi, S.-L. The Clinical Application of Potentials Evoked from the Peripheral Auditory System. Hear. Res. 2008, 242, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.L. Fundamentals of Clinical ECAP Measures in Cochlear Implants: Part 1: Use of the ECAP in Speech Processor Programming (2nd ed.). Available online: http://www.audiologyonline.com/articles/fundamentals-clinical-ecap-measures-in-846 (accessed on 18 September 2015).
- Ji, F.; Liu, K.; Yang, S. Clinical Application of Electrically Evoked Compound Action Potentials. J. Otol. 2014, 9, 117–121. [Google Scholar] [CrossRef] [Green Version]
- De Vos, J.J.; Biesheuvel, J.D.; Briaire, J.J.; Boot, P.S.; van Gendt, M.J.; Dekkers, O.M.; Fiocco, M.; Frijns, J.H.M. Use of Electrically Evoked Compound Action Potentials for Cochlear Implant Fitting: A Systematic Review. Ear Hear. 2017, 39, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Joly, C.-A.; Péan, V.; Hermann, R.; Seldran, F.; Thai-Van, H.; Truy, E. Using Electrically-Evoked Compound Action Potentials to Estimate Perceptive Levels in Experienced Adult Cochlear Implant Users. Otol. Neurotol. 2017, 38, 1278–1289. [Google Scholar] [CrossRef]
- Hughes, M.L.; Stille, L.J. Effect of Stimulus and Recording Parameters on Spatial Spread of Excitation and Masking Patterns Obtained with the Electrically Evoked Compound Action Potential in Cochlear Implants. Ear Hear. 2010, 31, 679–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, P.J.; Hughes, M.L.; Brown, C.J.; Miller, C.A.; South, H. Channel Interaction in Cochlear Implant Users Evaluated Using the Electrically Evoked Compound Action Potential. Audiol. Neurotol. 2004, 9, 203–213. [Google Scholar] [CrossRef]
- Van der Beek, F.B.; Briaire, J.J.; Frijns, J.H.M. Effects of Parameter Manipulations on Spread of Excitation Measured with Electrically-Evoked Compound Action Potentials. Int. J. Audiol. 2012, 51, 465–474. [Google Scholar] [CrossRef]
- Won, J.H.; Humphrey, E.L.; Yeager, K.R.; Martinez, A.A.; Robinson, C.H.; Mills, K.E.; Johnstone, P.M.; Moon, I.J.; Woo, J. Relationship among the Physiologic Channel Interactions, Spectral-Ripple Discrimination, and Vowel Identification in Cochlear Implant Users. J. Acoust. Soc. Am. 2014, 136, 2714–2725. [Google Scholar] [CrossRef]
- Scheperle, R.A.; Abbas, P.J. Relationships Among Peripheral and Central Electrophysiological Measures of Spatial and Spectral Selectivity and Speech Perception in Cochlear Implant Users. Ear Hear. 2015, 36, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Fredelake, S.; Hohmann, V. Factors Affecting Predicted Speech Intelligibility with Cochlear Implants in an Auditory Model for Electrical Stimulation. Hear. Res. 2012, 287, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Holden, L.K.; Finley, C.C.; Firszt, J.B.; Holden, T.A.; Brenner, C.; Potts, L.G.; Gotter, B.D.; Vanderhoof, S.S.; Mispagel, K.; Heydebrand, G.; et al. Factors Affecting Open-Set Word Recognition in Adults with Cochlear Implants. Ear Hear. 2013, 34, 342–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
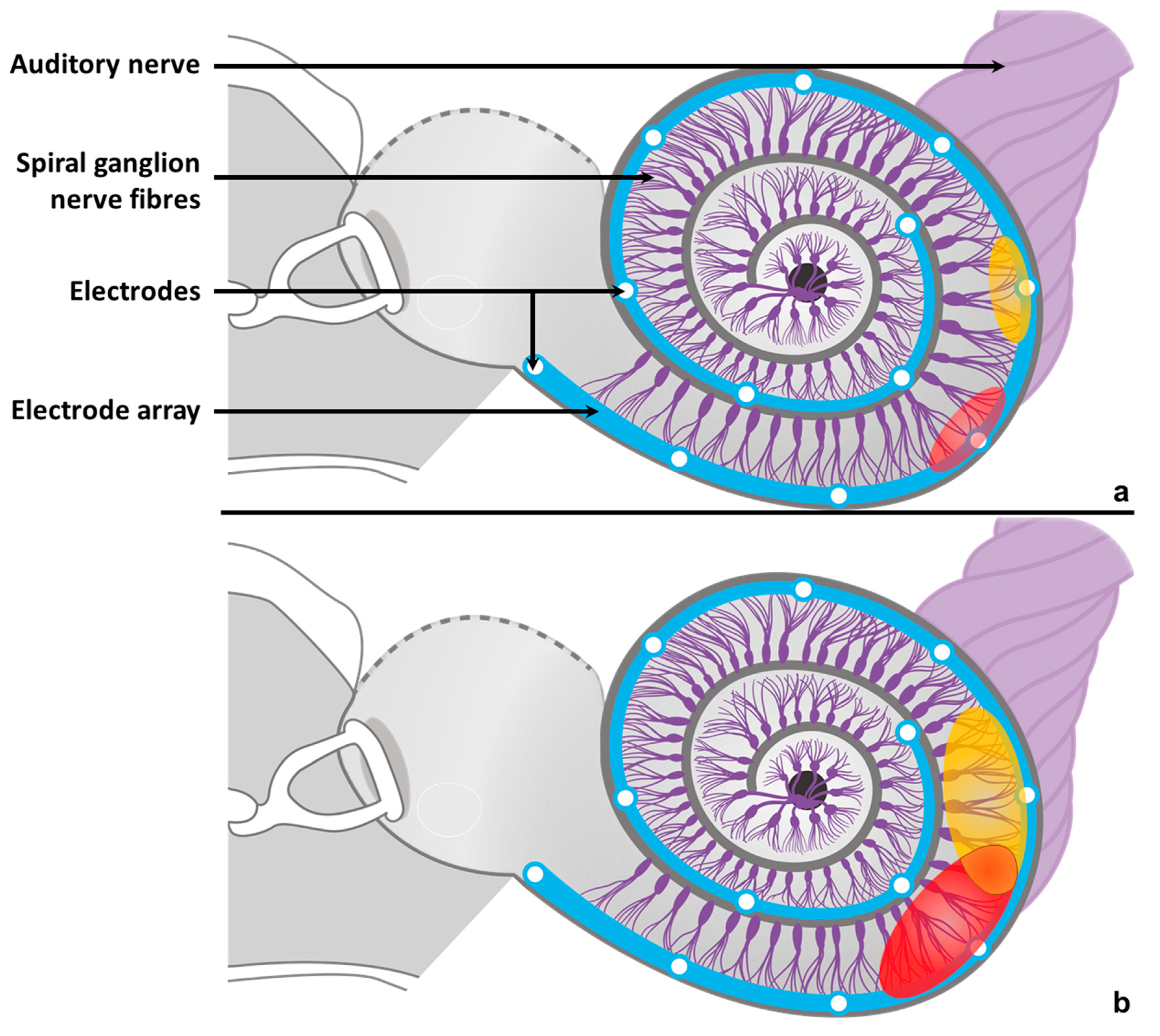
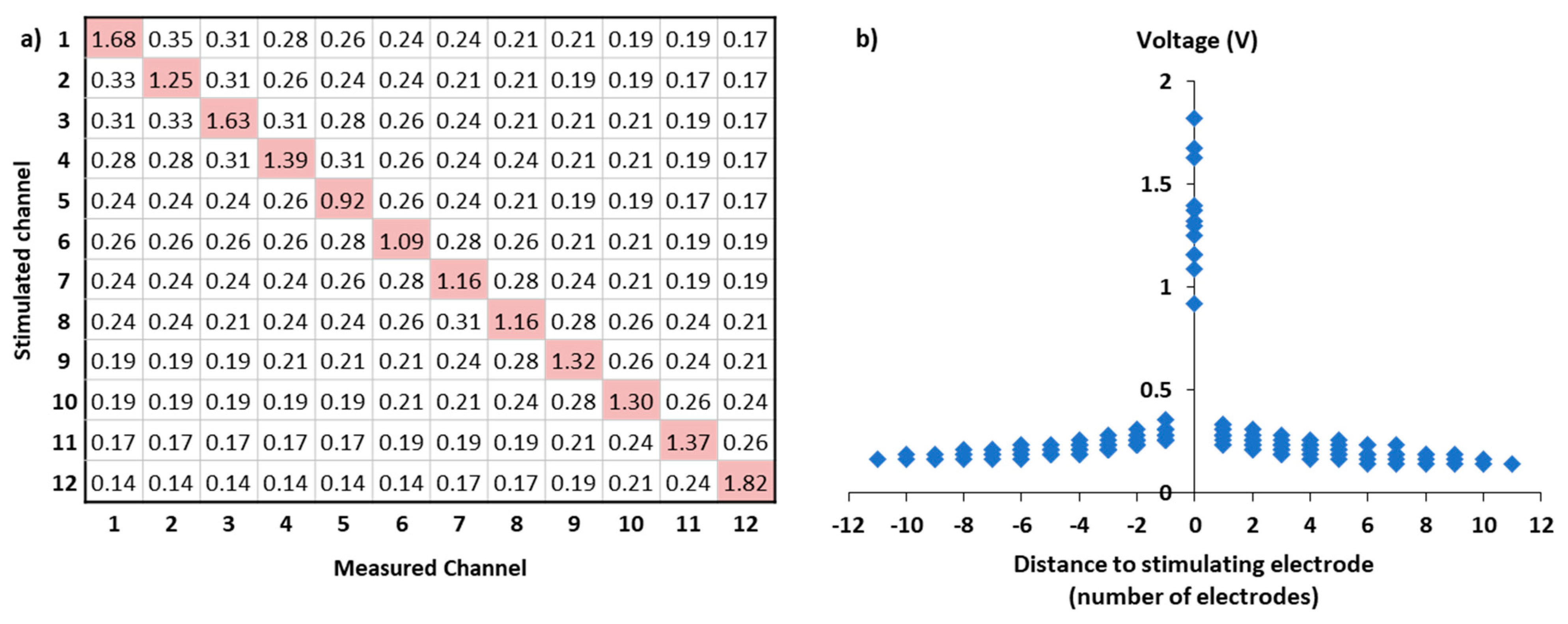



| Subject | Sex | Aetiology | Implanted Side | DU (Months) | NIE | Reason of Inactivation | ESC | I40 (%) | SRT50 (dB) |
|---|---|---|---|---|---|---|---|---|---|
| S-01 | male | Pre lingual, Progressive | Right | 24 | 0 | −0.0686 | 70 | 33.33 | |
| S-02 | female | Pre lingual, Progressive, Meningitis | Right | 15 | 0 | −0.0677 | 60 | 38.33 | |
| S-03 | female | Post lingual, Progressive, Genetic | Right | 27 | 0 | −0.1032 | 90 | 26.25 | |
| S-04 | female | Pre lingual, Congenital, Genetic | Left | 17 | 0 | −0.0985 | 80 | 24.00 | |
| S-05 | male | Post lingual, Meniere syndrome | Right | 12 | 0 | −0.1030 | 70 | 35.00 | |
| S-06 | male | Pre lingual, Usher | Right | 73 | 0 | −0.0804 | 10 | NA | |
| S-07 | female | Post lingual, Progressive | Right | 25 | 3 | Extra-cochlear | −0.1193 | 20 | 44.29 |
| S-08 | female | Post lingual, Progressive | Right | 20 | 1 | Extra-cochlear | −0.1069 | 80 | 27.78 |
| S-09 | female | Post lingual, Usher | Right | 81 | 2 | Extra-cochlear | −0.0739 | 70 | 37.14 |
| S-10 | female | Post lingual, Progressive, Genetic | Left | 25 | 4 | Non auditory side effect (3 electrodes) or Extra-cochlear | −0.0931 | 0 | 58.33 |
| S-11 | female | Pre lingual, Progressive | Right | 25 | 0 | −0.0746 | 60 | 38.33 | |
| S-12 | female | Post lingual, Ototoxicity | Right | 18 | 0 | −0.0650 | 20 | 53.33 | |
| S-13 | female | Post lingual, Ototoxicity | Right | 47 | 0 | −0.0618 | 10 | 45.00 | |
| S-14 | male | Post lingual, Progressive | Left | 129 | 2 | No auditory percept | −0.0923 | 50 | 40.00 |
| S-15 | female | Post lingual, Progressive | Right | 23 | 0 | −0.0722 | 30 | 45.00 | |
| S-16 | female | Post lingual, Progressive | Right | 56 | 0 | −0.0657 | 40 | 42.50 | |
| S-17 | female | Post lingual, Genetic | Left | 34 | 0 | −0.0830 | 70 | 37.14 | |
| S-18 | male | Pre lingual, Congenital, Progressive | Right | 22 | 0 | −0.0929 | 40 | 42.00 | |
| S-19 | male | Pre lingual, Congenital | Left | 23 | 0 | −0.0795 | 30 | 46.67 | |
| S-20 | female | Post lingual, Genetic | Right | 12 | 2 | Extra-cochlear | −0.0928 | 40 | 42.50 |
| S-21 | female | Post lingual | Right | 11 | 0 | −0.0933 | 70 | 37.14 | |
| S-22 | female | Post lingual, Progressive | Left | 72 | 1 | No auditory percept | −0.0904 | 40 | 42.00 |
| S-23 | male | Pre lingual | Left | 123 | 1 | Non auditory side effect | −0.0629 | 60 | 38.33 |
| S-24 | female | Post lingual, Otosclerosis | Left | 72 | 0 | −0.0874 | 100 | 25.00 | |
| S-25 | male | Post lingual, Ototoxicity | Right | 23 | 0 | −0.0942 | 100 | 24.00 | |
| S-26 | male | Post lingual, Meniere syndrome | Right | 18 | 2 | No auditory percept | −0.1094 | 20 | 47.50 |
| S-27 | female | Post lingual, Autoimmune | Right | 28 | 0 | −0.0674 | 80 | 35.00 | |
| S-28 | male | Post lingual, Progressive | Right | 47 | 0 | −0.0733 | 50 | 40.00 | |
| S-29 | female | Pre lingual, Congenital | Right | 11 | 0 | −0.0743 | 0 | NA | |
| S-30 | female | Pre lingual, Progressive | Left | 23 | 0 | −0.1057 | 70 | 37.14 | |
| S-31 | male | Post lingual, MELAS Syndrome | Right | 12 | 1 | Extra-cochlear | −0.0844 | 70 | 38.00 |
| S-32 | male | Post lingual, Progressive, Genetic | Left | 11 | 0 | −0.0349 | 80 | NA | |
| S-33 | female | Post lingual, Progressive | Right | 25 | 0 | −0.0834 | 30 | 43.33 | |
| S-34 | male | Post lingual, Progressive | Right | 11 | 2 | Extra-cochlear | −0.1099 | 80 | 34.00 |
| S-35 | male | Pre lingual, Congenital, Connexin 26 | Right | 23 | −0.0808 | 70 | 36.00 | ||
| S-36 | female | Post lingual, Sudden hearing loss | Left | 23 | 3 | Poor sound quality or No auditory percept (2 electrodes) | −0.1287 | 30 | 46.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joly, C.-A.; Reynard, P.; Hermann, R.; Seldran, F.; Gallego, S.; Idriss, S.; Thai-Van, H. Intra-Cochlear Current Spread Correlates with Speech Perception in Experienced Adult Cochlear Implant Users. J. Clin. Med. 2021, 10, 5819. https://doi.org/10.3390/jcm10245819
Joly C-A, Reynard P, Hermann R, Seldran F, Gallego S, Idriss S, Thai-Van H. Intra-Cochlear Current Spread Correlates with Speech Perception in Experienced Adult Cochlear Implant Users. Journal of Clinical Medicine. 2021; 10(24):5819. https://doi.org/10.3390/jcm10245819
Chicago/Turabian StyleJoly, Charles-Alexandre, Pierre Reynard, Ruben Hermann, Fabien Seldran, Stéphane Gallego, Samar Idriss, and Hung Thai-Van. 2021. "Intra-Cochlear Current Spread Correlates with Speech Perception in Experienced Adult Cochlear Implant Users" Journal of Clinical Medicine 10, no. 24: 5819. https://doi.org/10.3390/jcm10245819
APA StyleJoly, C.-A., Reynard, P., Hermann, R., Seldran, F., Gallego, S., Idriss, S., & Thai-Van, H. (2021). Intra-Cochlear Current Spread Correlates with Speech Perception in Experienced Adult Cochlear Implant Users. Journal of Clinical Medicine, 10(24), 5819. https://doi.org/10.3390/jcm10245819






