Hypogonadism in Women with Prader-Willi Syndrome—Clinical Recommendations Based on a Dutch Cohort Study, Review of the Literature and an International Expert Panel Discussion
Abstract
1. Introduction
2. Materials and Methods
2.1. Terminology
2.2. Definition of Hypogonadism
2.3. Laboratory Measurements
2.4. Expert Panel Discussion on Diagnosis and Treatment of Hypogonadism
2.5. Literature Search
2.6. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Hypogonadism
3.3. Types of Hypogonadism
3.4. Hormone Treatment
3.5. Expert Panel Discussion on Diagnosis and Treatment of Hypogonadism
3.5.1. Start Treatment for Hypogonadism
3.5.2. Stop Treatment for Hypogonadism
3.6. Literature Review
4. Discussion
4.1. Type of Hypogonadism
4.2. Ovarian Histology
4.3. Factors Influencing Hypogonadism in PWS
4.4. Importance of Treatment of Hypogonadism
4.4.1. Bone Health
4.4.2. Muscle and Fat
4.4.3. Psychological Effects
4.4.4. Cardiovascular Risk
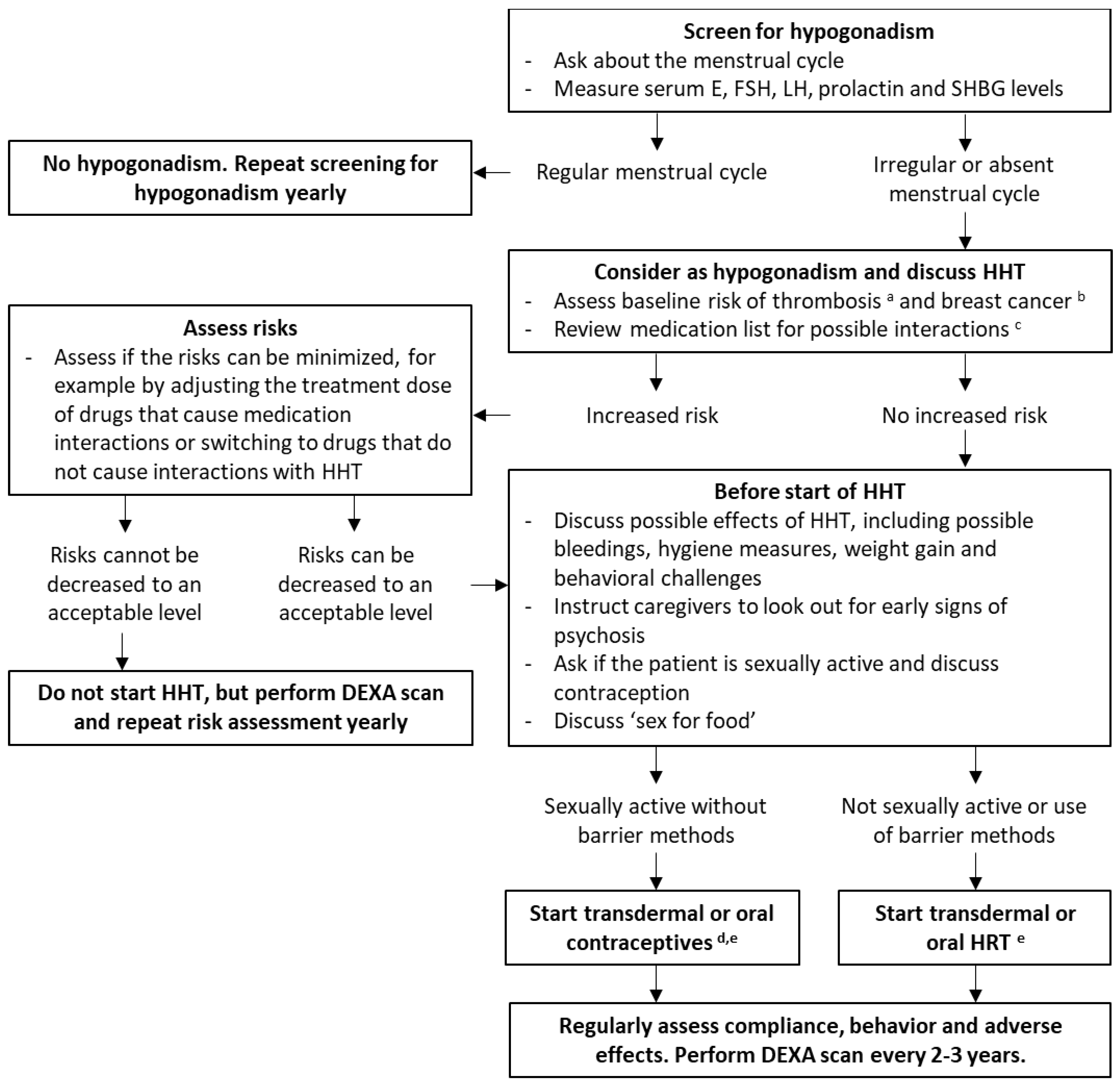
4.5. Recommendations
4.5.1. Assessment of Gonadal Function
4.5.2. Treatment Regimen
4.5.3. Drug Interactions
Psychotropic Medication
Recombinant Human Growth Hormone Treatment
4.5.4. Intellectual Disability and Menstrual Hygiene
4.5.5. Challenging Behavior and Psychotic Symptoms
4.5.6. Thrombosis
4.5.7. Weight Gain
4.5.8. Breast Cancer
4.5.9. Sex for Food
4.5.10. Non-Compliance
4.5.11. Discontinuation of HHT
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef]
- Cheon, C.K. Genetics of Prader-Willi syndrome and Prader-Will-Like syndrome. Ann. Pediatr. Endocrinol. Metab. 2016, 21, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Hartin, S.N.; Hossain, W.A.; Manzardo, A.M.; Kimonis, V.; Dykens, E.; Gold, J.A.; Kim, S.J.; Weisensel, N.; Tamura, R.; et al. Molecular genetic classification in Prader-Willi syndrome: A multisite cohort study. J. Med. Genet. 2019, 56, 149–153. [Google Scholar] [CrossRef]
- Angulo, M.A.; Butler, M.G.; Cataletto, M.E. Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. J. Endocrinol. Investig. 2015, 38, 1249–1263. [Google Scholar] [CrossRef]
- Goldstone, A.P.; Holland, A.J.; Hauffa, B.P.; Hokken-Koelega, A.C.; Tauber, M.; Speakers Contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 4183–4197. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.B. Prader-Willi syndrome. J. Med. Genet. 1997, 34, 917–923. [Google Scholar] [CrossRef]
- Holm, V.A.; Cassidy, S.B.; Butler, M.G.; Hanchett, J.M.; Greenswag, L.R.; Whitman, B.Y.; Greenberg, F. Prader-Willi syndrome: Consensus diagnostic criteria. Pediatrics 1993, 91, 398–402. [Google Scholar]
- Dykens, E.; Shah, B. Psychiatric disorders in Prader-Willi syndrome: Epidemiology and management. CNS Drugs 2003, 17, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Feighan, S.M.; Hughes, M.; Maunder, K.; Roche, E.; Gallagher, L. A profile of mental health and behaviour in Prader-Willi syndrome. J. Intellect. Disabil. Res. 2020, 64, 158–169. [Google Scholar] [CrossRef]
- Rice, L.J.; Einfeld, S.L. Cognitive and behavioural aspects of Prader-Willi syndrome. Curr. Opin. Psychiatry 2015, 28, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Sinnema, M.; Boer, H.; Collin, P.; Maaskant, M.A.; van Roozendaal, K.E.; Schrander-Stumpel, C.T.; Curfs, L.M. Psychiatric illness in a cohort of adults with Prader-Willi syndrome. Res. Dev. Disabil. 2011, 32, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Pellikaan, K.; Ben Brahim, Y.; Rosenberg, A.G.W.; Davidse, K.; Poitou, C.; Coupaye, M.; Goldstone, A.P.; Høybye, C.; Markovic, T.P.; Grugni, G.; et al. Hypogonadism in adult males with Prader-Willi syndrome—Clinical recommendations based on a Dutch cohort study, review of the literature and an international expert panel discussion. J. Clin. Med. 2021, 10, 4361. [Google Scholar] [CrossRef] [PubMed]
- Partsch, C.J.; Lammer, C.; Gillessen-Kaesbach, G.; Pankau, R. Adult patients with Prader-Willi syndrome: Clinical characteristics, life circumstances and growth hormone secretion. Growth Horm. IGF Res. 2000, 10, S81–S85. [Google Scholar] [CrossRef]
- Whittington, J.; Holland, A.; Webb, T.; Butler, J.; Clarke, D.; Boer, H. Relationship between clinical and genetic diagnosis of Prader-Willi syndrome. J. Med. Genet. 2002, 39, 926–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grugni, G.; Morabito, F.; Crino, A. Gonadal function and its disorders in simple obesity and in Prader-Willi syndrome. In Prader-Willi Syndrome as a Model for Obesity; Eiholzer, U., l’Allemad, D., Zipf, W.B., Eds.; Karger: Basel, Switzerland, 2003; pp. 140–155. [Google Scholar] [CrossRef]
- Hoybye, C.; Thoren, M.; Bohm, B. Cognitive, emotional, physical and social effects of growth hormone treatment in adults with Prader-Willi syndrome. J. Intellect. Disabil. Res. 2005, 49, 245–252. [Google Scholar] [CrossRef]
- Miller, J.L.; Goldstone, A.P.; Couch, J.A. Pituitary abnormalities in Prader–Willi syndrome and early onset morbid obesity. Am. J. Med. Genet. A 2008, 146, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Brandau, D.T.; Theodoro, M.; Garg, U.; Butler, M.G. Follicle stimulating and leutinizing hormones, estradiol and testosterone in Prader-Willi syndrome. Am. J. Med. Genet. A 2008, 146, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Sode-Carlsen, R.; Farholt, S.; Rabben, K.F.; Bollerslev, J.; Schreiner, T.; Jurik, A.G.; Christiansen, J.S.; Hoybye, C. Body composition, endocrine and metabolic profiles in adults with Prader-Willi syndrome. Growth Horm. IGF Res. 2010, 20, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Van Nieuwpoort, I.C.; Sinnema, M.; Castelijns, J.A.; Twisk, J.W.; Curfs, L.M.; Drent, M.L. The GH/IGF-I axis and pituitary function and size in adults with Prader-Willi syndrome. Horm. Res. Paediatr. 2011, 75, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.J.; Eldar-Geva, T.; Bennaroch, F.; Pollak, Y.; Gross-Tsur, V. Sexual dichotomy of gonadal function in Prader-Willi syndrome from early infancy through the fourth decade. Hum. Reprod. 2015, 30, 2587–2596. [Google Scholar] [CrossRef]
- Coupaye, M.; Tauber, M.; Cuisset, L.; Laurier, V.; Bieth, E.; Lacorte, J.M.; Oppert, J.M.; Clement, K.; Poitou, C. Effect of genotype and previous GH treatment on adiposity in adults with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 4895–4903. [Google Scholar] [CrossRef] [PubMed]
- Noordam, C.; Hoybye, C.; Eiholzer, U. Prader-Willi syndrome and hypogonadism: A review article. Int. J. Mol. Sci. 2021, 22, 2705. [Google Scholar] [CrossRef]
- Eldar-Geva, T.; Hirsch, H.J.; Rabinowitz, R.; Benarroch, F.; Rubinstein, O.; Gross-Tsur, V. Primary ovarian dysfunction contributes to the hypogonadism in women with Prader-Willi syndrome. Horm. Res. 2009, 72, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Crino, A.; Schiaffini, R.; Ciampalini, P.; Spera, S.; Beccaria, L.; Benzi, F.; Bosio, L.; Corrias, A.; Gargantini, L.; Salvatoni, A.; et al. Hypogonadism and pubertal development in Prader-Willi syndrome. Eur. J. Pediatr. 2003, 162, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Siemensma, E.P.; van Alfen-van der Velden, A.A.; Otten, B.J.; Laven, J.S.; Hokken-Koelega, A.C. Ovarian function and reproductive hormone levels in girls with Prader-Willi syndrome: A longitudinal study. J. Clin. Endocrinol. Metab. 2012, 97, E1766–E1773. [Google Scholar] [CrossRef] [PubMed]
- Gross-Tsur, V.; Hirsch, H.J.; Benarroch, F.; Eldar-Geva, T. The FSH-inhibin axis in prader-willi syndrome: Heterogeneity of gonadal dysfunction. Reprod. Biol. Endocrinol. 2012, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Akefeldt, A.; Tornhage, C.J.; Gillberg, C. A woman with Prader-Willi syndrome gives birth to a healthy baby girl. Dev. Med. Child Neurol. 1999, 41, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Mogensen, H.; Hamborg-Petersen, B.; Graem, N.; Ostergaard, J.R.; Brondum-Nielsen, K. Fertility in Prader-Willi syndrome: A case report with Angelman syndrome in the offspring. Acta Paediatr. 2001, 90, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Formoso, G.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Colao, A. Prader- Willi syndrome: An uptodate on endocrine and metabolic complications. Rev. Endocr. Metab. Disord. 2019, 20, 239–250. [Google Scholar] [CrossRef]
- Hockey, A.; Byrne, G.; Cohen, A. Precocious puberty in the male offspring of a mother and daughter with the Prader-Willi syndrome. Am. J. Med. Genet. 1987, 26, 749. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Vetri, L.; Ragusa, L.; Vinci, M.; Gloria, A.; Occhipinti, P.; Costanzo, A.A.; Quatrosi, G.; Roccella, M.; Buono, S.; et al. Prader-Willi syndrome with Angelman syndrome in the offspring. Medicina 2021, 57, 460. [Google Scholar] [CrossRef] [PubMed]
- Dzemaili, S.; Tiemensma, J.; Quinton, R.; Pitteloud, N.; Morin, D.; Dwyer, A.A. Beyond hormone replacement: Quality of life in women with congenital hypogonadotropic hypogonadism. Endocr. Connect. 2017, 6, 404–412. [Google Scholar] [CrossRef]
- Richard-Eaglin, A. Male and female hypogonadism. Nurs. Clin. N. Am. 2018, 53, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Spangenburg, E.E.; Geiger, P.C.; Leinwand, L.A.; Lowe, D.A. Regulation of physiological and metabolic function of muscle by female sex steroids. Med. Sci. Sports Exerc. 2012, 44, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Tsiligiannis, S.; Panay, N.; Stevenson, J.C. Premature ovarian insufficiency and long-term health consequences. Curr. Vasc. Pharmacol. 2019, 17, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.C.; Kim, S.C.; Yin, K.J.; Lee, J.P.; Hamblin, M.H. Sex differences in vascular physiology and pathophysiology: Estrogen and androgen signaling in health and disease. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H524–H545. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Albrand, G.; Munoz, F.; Sornay-Rendu, E.; DuBoeuf, F.; Delmas, P.D. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: The OFELY study. Bone 2003, 32, 78–85. [Google Scholar] [CrossRef]
- Cummings, S.R.; Nevitt, M.C.; Browner, W.S.; Stone, K.; Fox, K.M.; Ensrud, K.E.; Cauley, J.; Black, D.; Vogt, T.M. Risk factors for hip fracture in white women. Study of osteoporotic fractures research group. N. Engl. J. Med. 1995, 332, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Segev, D.; Hellerstein, D.; Dunsky, A. Physical activity—Does it really increase bone density in postmenopausal women? A review of articles published between 2001–2016. Curr. Aging Sci. 2018, 11, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Bellicha, A.; Coupaye, M.; Mosbah, H.; Tauber, M.; Oppert, J.-M.; Poitou, C. Physical activity in patients with Prader-Willi syndrome—A systematic review of observational and interventional studies. J. Clin. Med. 2021, 10, 2528. [Google Scholar] [CrossRef] [PubMed]
- Doga, M.; Bonadonna, S.; Gola, M.; Mazziotti, G.; Giustina, A. Growth hormone deficiency in the adult. Pituitary 2006, 9, 305–311. [Google Scholar] [CrossRef]
- Butler, M.G.; Haber, L.; Mernaugh, R.; Carlson, M.G.; Price, R.; Feurer, I.D. Decreased bone mineral density in Prader-Willi syndrome: Comparison with obese subjects. Am. J. Med. Genet. 2001, 103, 216–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakamura, Y.; Murakami, N.; Iida, T.; Asano, S.; Ozeki, S.; Nagai, T. Growth hormone treatment for osteoporosis in patients with scoliosis of Prader-Willi syndrome. J. Orthop. Sci. 2014, 19, 877–882. [Google Scholar] [CrossRef]
- Napolitano, L.; Barone, B.; Morra, S.; Celentano, G.; La Rocca, R.; Capece, M.; Morgera, V.; Turco, C.; Caputo, V.F.; Spena, G.; et al. Hypogonadism in patients with Prader Willi syndrome: A narrative review. Int. J. Mol. Sci. 2021, 22, 1993. [Google Scholar] [CrossRef] [PubMed]
- Pellikaan, K.; Rosenberg, A.G.W.; Kattentidt-Mouravieva, A.A.; Kersseboom, R.; Bos-Roubos, A.G.; Veen-Roelofs, J.M.C.; van Wieringen, N.; Hoekstra, F.M.E.; van den Berg, S.A.A.; van der Lely, A.J.; et al. Missed diagnoses and health problems in adults with Prader-Willi syndrome: Recommendations for screening and treatment. J. Clin. Endocrinol. Metab. 2020, 105, e4671–e4687. [Google Scholar] [CrossRef]
- Bulun, S.E.; Chen, D.; Moy, I.; Brooks, D.C.; Zhao, H. Aromatase, breast cancer and obesity: A complex interaction. Trends Endocrinol. Metab. 2012, 23, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Kailasam, C.; Cahill, D. Review of the safety, efficacy and patient acceptability of the levonorgestrel-releasing intrauterine system. Patient Prefer. Adherence 2008, 2, 293–302. [Google Scholar] [CrossRef]
- Lijfering, W.M.; Rosendaal, F.R.; Cannegieter, S.C. Risk factors for venous thrombosis—Current understanding from an epidemiological point of view. Br. J. Haematol. 2010, 149, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, A.F.; Di Giorgio, G.; Grugni, G.; Cuttini, M.; Losacco, V.; Anzuini, A.; Spera, S.; Marzano, C.; Lenzi, A.; Cappa, M.; et al. Multiple forms of hypogonadism of central, peripheral or combined origin in males with Prader-Willi syndrome. Clin. Endocrinol. 2012, 76, 72–77. [Google Scholar] [CrossRef]
- Hirsch, H.J.; Eldar-Geva, T.; Benarroch, F.; Rubinstein, O.; Gross-Tsur, V. Primary testicular dysfunction is a major contributor to abnormal pubertal development in males with Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Dahms, W.T.; Swerdloff, R.S.; Fiser, R.H.; Atkinson, R.L.; Carrel, R.E. The Prader-Willi syndrome: A study of 40 patients and a review of the literature. Medicine 1983, 62, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Grugni, G.; Tringali, G.; Agosti, F.; Sartorio, A. Assessment of fat-free mass from bioelectrical impedance analysis in obese women with Prader-Willi syndrome. Ann. Hum. Biol. 2015, 42, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Cartwright, B.; Robinson, J.; Seed, P.T.; Fogelman, I.; Rymer, J. Hormone replacement therapy versus the combined oral contraceptive pill in premature ovarian failure: A randomized controlled trial of the effects on bone mineral density. J. Clin. Endocrinol. Metab. 2016, 101, 3497–3505. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Claustrat, B.; Delmas, P.D. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J. Bone. Miner. Res. 2000, 15, 1526–1536. [Google Scholar] [CrossRef]
- Lee, J.S.; LaCroix, A.Z.; Wu, L.; Cauley, J.A.; Jackson, R.D.; Kooperberg, C.; Leboff, M.S.; Robbins, J.; Lewis, C.E.; Bauer, D.C.; et al. Associations of serum sex hormone-binding globulin and sex hormone concentrations with hip fracture risk in postmenopausal women. J. Clin. Endocrinol. Metab. 2008, 93, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Hart, D.M.; Aitken, J.M.; MacDonald, E.B.; Anderson, J.B.; Clarke, A.C. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet 1976, 1, 1038–1041. [Google Scholar] [CrossRef]
- Christiansen, C.; Christensen, M.S.; Transbøl, I. Bone mass in postmenopausal women after withdrawal of oestrogen/gestagen replacement therapy. Lancet 1981, 1, 459–461. [Google Scholar] [CrossRef]
- Li, L.; Qiu, X.; Lash, G.E.; Yuan, L.; Liang, Z.; Liu, L. Effect of hormone replacement therapy on bone mineral density and body composition in Chinese adolescent and young adult Turner syndrome patients. Front. Endocrinol. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, A.P.; Ueland, T.; Sode-Carlsen, R.; Schreiner, T.; Rabben, K.F.; Farholt, S.; Hoybye, C.; Christiansen, J.S.; Bollerslev, J. Two years of growth hormone treatment in adults with Prader-Willi syndrome do not improve the low BMD. J. Clin. Endocrinol. Metab. 2013, 98, E753–E760. [Google Scholar] [CrossRef] [PubMed]
- Longhi, S.; Grugni, G.; Gatti, D.; Spinozzi, E.; Sartorio, A.; Adami, S.; Fanolla, A.; Radetti, G. Adults with Prader-Willi syndrome have weaker bones: Effect of treatment with GH and sex steroids. Calcif. Tissue Int. 2015, 96, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Birzniece, V.; Meinhardt, U.J.; Gibney, J.; Johannsson, G.; Armstrong, N.; Baxter, R.C.; Ho, K.K. Differential effects of raloxifene and estrogen on body composition in growth hormone-replaced hypopituitary women. J. Clin. Endocrinol. Metab. 2012, 97, 1005–1012. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Sarrel, P.M.; Nelson, L.M. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil. Steril. 2016, 106, 1588–1599. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.N.; Almeida, O.P.; Joffe, H.; Cohen, L.S. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: A double-blind, randomized, placebo-controlled trial. Arch. Gen. Psychiatry 2001, 58, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, J.E.; O’Brien, W.H. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology 1997, 22, 189–212. [Google Scholar] [CrossRef]
- Webber, L.; Anderson, R.A.; Davies, M.; Janse, F.; Vermeulen, N. HRT for women with premature ovarian insufficiency: A comprehensive review. Hum. Reprod. Open 2017, 2017, hox007. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, L.L.; Morch, L.S.; Lokkegaard, E. Hormone replacement therapy and the risk of endometrial cancer: A systematic review. Maturitas 2016, 91, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Gross-Tsur, V.; Eldar-Geva, T.; Benarroch, F.; Rubinstein, O.; Hirsch, H.J. Body image and sexual interests in adolescents and young adults with Prader-Willi syndrome. J. Pediatr. Endocrinol. Metab. 2011, 24, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Kyvernitakis, I.; Kostev, K.; Nassour, T.; Thomasius, F.; Hadji, P. The impact of depot medroxyprogesterone acetate on fracture risk: A case-control study from the UK. Osteoporos. Int. 2017, 28, 291–297. [Google Scholar] [CrossRef]
- Hadji, P.; Colli, E.; Regidor, P.A. Bone health in estrogen-free contraception. Osteoporos. Int. 2019, 30, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Cromer, B.A.; Bonny, A.E.; Stager, M.; Lazebnik, R.; Rome, E.; Ziegler, J.; Camlin-Shingler, K.; Secic, M. Bone mineral density in adolescent females using injectable or oral contraceptives: A 24-month prospective study. Fertil. Steril. 2008, 90, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Pellikaan, K.; Snijders, F.; Rosenberg, A.G.W.; Davidse, K.; van den Berg, S.A.A.; Visser, W.E.; van der Lely, A.J.; de Graaff, L.C.G. Thyroid function in adults with Prader–Willi syndrome; A cohort study and literature review. J. Clin. Med. 2021, 10, 3804. [Google Scholar] [CrossRef] [PubMed]
- Berry-Bibee, E.N.; Kim, M.J.; Simmons, K.B.; Tepper, N.K.; Riley, H.E.; Pagano, H.P.; Curtis, K.M. Drug interactions between hormonal contraceptives and psychotropic drugs: A systematic review. Contraception 2016, 94, 650–667. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, L.R.; Wisner, K.L.; Cattan, M.K.; Betcher, H.K.; Stika, C.S.; Kiley, J.W. Contraception for women with psychiatric disorders. Am. J. Psychiatry 2021, 178, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Petrenaite, V.; Sabers, A.; Hansen-Schwartz, J. Individual changes in lamotrigine plasma concentrations during pregnancy. Epilepsy Res. 2005, 65, 185–188. [Google Scholar] [CrossRef]
- Reimers, A. Hormone replacement therapy with estrogens may reduce lamotrigine serum concentrations: A matched case-control study. Epilepsia 2017, 58, e6–e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, K.; Choi, S.; Fischer, J.H.; Jeong, H. Up-regulation of UDP-glucuronosyltransferase (UGT) 1A4 by 17beta-estradiol: A potential mechanism of increased lamotrigine elimination in pregnancy. Drug Metab. Dispos. 2009, 37, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.G.; Blum, A.S.; Farina, E.L.; Maestri, X.E.; Newman, J.; Garcia, E.; Krishnamurthy, K.B.; Hoch, D.B.; Replansky, S.; Fowler, K.M.; et al. Valproate and lamotrigine level variation with menstrual cycle phase and oral contraceptive use. Neurology 2009, 72, 911–914. [Google Scholar] [CrossRef]
- Erickson-Ridout, K.K.; Sun, D.; Lazarus, P. Glucuronidation of the second-generation antipsychotic clozapine and its active metabolite N-desmethylclozapine. Potential importance of the UGT1A1 A(TA)(7)TAA and UGT1A4 L48V polymorphisms. Pharmacogenet. Genom. 2012, 22, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Linnet, K. Glucuronidation of olanzapine by cDNA-expressed human UDP-glucuronosyltransferases and human liver microsomes. Hum. Psychopharmacol. 2002, 17, 233–238. [Google Scholar] [CrossRef]
- Erickson-Ridout, K.K.; Zhu, J.; Lazarus, P. Olanzapine metabolism and the significance of UGT1A448V and UGT2B1067Y variants. Pharmacogenet. Genom. 2011, 21, 539–551. [Google Scholar] [CrossRef]
- Breyer-Pfaff, U.; Mey, U.; Green, M.D.; Tephly, T.R. Comparative N-glucuronidation kinetics of ketotifen and amitriptyline by expressed human UDP-glucuronosyltransferases and liver microsomes. Drug Metab. Dispos. 2000, 28, 869–872. [Google Scholar]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Shimada, T. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch. Biochem. Biophys. 1997, 346, 161–169. [Google Scholar] [CrossRef]
- Dutton, C.; Foldvary-Schaefer, N. Contraception in women with epilepsy: Pharmacokinetic interactions, contraceptive options, and management. Int. Rev. Neurobiol. 2008, 83, 113–134. [Google Scholar] [CrossRef]
- Sjöström, A.; Pellikaan, K.; Sjöström, H.; Goldstone, A.P.; Grugni, G.; Crinò, A.; De Graaff, L.C.G.; Höybye, C. Hyperprolactinemia in adults with Prader-Willi syndrome. J. Clin. Med. 2021, 10, 3613. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.M. Growth hormone and estrogen: A clinician’s approach. J. Pediatr. Endocrinol. Metab. 2004, 17, 1273–1276. [Google Scholar]
- Southmayd, E.A.; De Souza, M.J. A summary of the influence of exogenous estrogen administration across the lifespan on the GH/IGF-1 axis and implications for bone health. Growth Horm. IGF Res. 2017, 32, 2–13. [Google Scholar] [CrossRef]
- Wilbur, J.; Torondel, B.; Hameed, S.; Mahon, T.; Kuper, H. Systematic review of menstrual hygiene management requirements, its barriers and strategies for disabled people. PLoS ONE 2019, 14, e0210974. [Google Scholar] [CrossRef]
- Wilkinson, J.E.; Cerreto, M.C. Primary care for women with intellectual disabilities. J. Am. Board Fam. Med. 2008, 21, 215–222. [Google Scholar] [CrossRef]
- Quint, E.H. Adolescents with special needs: Clinical challenges in reproductive health care. J. Pediatr. Adolesc. Gynecol. 2016, 29, 2–6. [Google Scholar] [CrossRef]
- Wandresen, G.; Sgarbi, F.; Nisihara, R. Management of contraceptives and menstrual complaints in patients with Down syndrome. Gynecol. Endocrinol. 2019, 35, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.R. Menstrual and contraceptive management in women with an intellectual disability. Med. J. Aust. 2002, 176, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Marjoribanks, J.; Farquhar, C.; Roberts, H.; Lethaby, A.; Lee, J. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst. Rev. 2017, 1, CD004143. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G.; Oyetunji, A.; Manzardo, A.M. Age distribution, comorbidities and risk factors for thrombosis in Prader-Willi syndrome. Genes 2020, 11, 67. [Google Scholar] [CrossRef]
- Roach, R.E.; Lijfering, W.M.; Helmerhorst, F.M.; Cannegieter, S.C.; Rosendaal, F.R.; van Hylckama Vlieg, A. The risk of venous thrombosis in women over 50 years old using oral contraception or postmenopausal hormone therapy. J. Thromb. Haemost. 2013, 11, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Giordano Imbroll, M.; Gruppetta, M. A current perspective into young female sex hormone replacement: A review. Expert Rev. Endocrinol. Metab. 2020, 15, 405–414. [Google Scholar] [CrossRef]
- Olie, V.; Canonico, M.; Scarabin, P.Y. Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women. Curr. Opin. Hematol. 2010, 17, 457–463. [Google Scholar] [CrossRef]
- Zieman, M.; Guillebaud, J.; Weisberg, E.; Shangold, G.A.; Fisher, A.C.; Creasy, G.W. Contraceptive efficacy and cycle control with the Ortho Evra/Evra transdermal system: The analysis of pooled data. Fertil. Steril. 2002, 77, S13–S18. [Google Scholar] [CrossRef]
- Westhoff, C.L.; Reinecke, I.; Bangerter, K.; Merz, M. Impact of body mass index on suppression of follicular development and ovulation using a transdermal patch containing 0.55-mg ethinyl estradiol/2.1-mg gestodene: A multicenter, open-label, uncontrolled study over three treatment cycles. Contraception 2014, 90, 272–279. [Google Scholar] [CrossRef]
- Stachenfeld, N.S.; Keefe, D.L. Estrogen effects on osmotic regulation of AVP and fluid balance. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E711–E721. [Google Scholar] [CrossRef] [PubMed]
- Kristiina, P.; Reijo, S.; Markus, K.; Eero, P. Cancer incidence among persons Prader-Willi syndrome in Finland. Int. J. Disabil. Hum. Dev. 2008, 7, 69–72. [Google Scholar] [CrossRef]
- Davies, H.D.; Leusink, G.L.; McConnell, A.; Deyell, M.; Cassidy, S.B.; Fick, G.H.; Coppes, M.J. Myeloid leukemia in Prader-Willi syndrome. J. Pediatr. 2003, 142, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Havrilesky, L.J.; Gierisch, J.M.; Moorman, P.G.; Coeytaux, R.R.; Urrutia, R.P.; Lowery, W.J.; Dinan, M.; McBroom, A.J.; Wing, L.; Musty, M.D.; et al. Oral contraceptive use for the primary prevention of ovarian cancer. Evid. Rep. Technol. Assess. 2013, 212, 1–514. [Google Scholar]
- Hodis, H.N.; Sarrel, P.M. Oral contraceptive use for the primary prevention of ovarian cancer. Climacteric 2018, 21, 521–528. [Google Scholar] [CrossRef]
- Miller, J.L.; Lynn, C.H.; Driscoll, D.C.; Goldstone, A.P.; Gold, J.A.; Kimonis, V.; Dykens, E.; Butler, M.G.; Shuster, J.J.; Driscoll, D.J. Nutritional phases in Prader-Willi syndrome. Am. J. Med. Genet. A 2011, 155, 1040–1049. [Google Scholar] [CrossRef]
- Benarroch, F.; Srebnik-Moshe, N.; Hirsch, H.J.; Genstil, L.; Derei, D.; Shay, A.; Gross-Tsur, V. Syndrome-related risk factors for sexual abuse: The example of Prader-Willi syndrome. Arch. Sex. Behav. 2021, 50, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.J.; Boer, H.; Chung, M.C.; Sturmey, P.; Webb, T. Maladaptive behaviour in Prader-Willi syndrome in adult life. J. Intellect. Disabil. Res. 1996, 40, 159–165. [Google Scholar] [CrossRef] [PubMed]
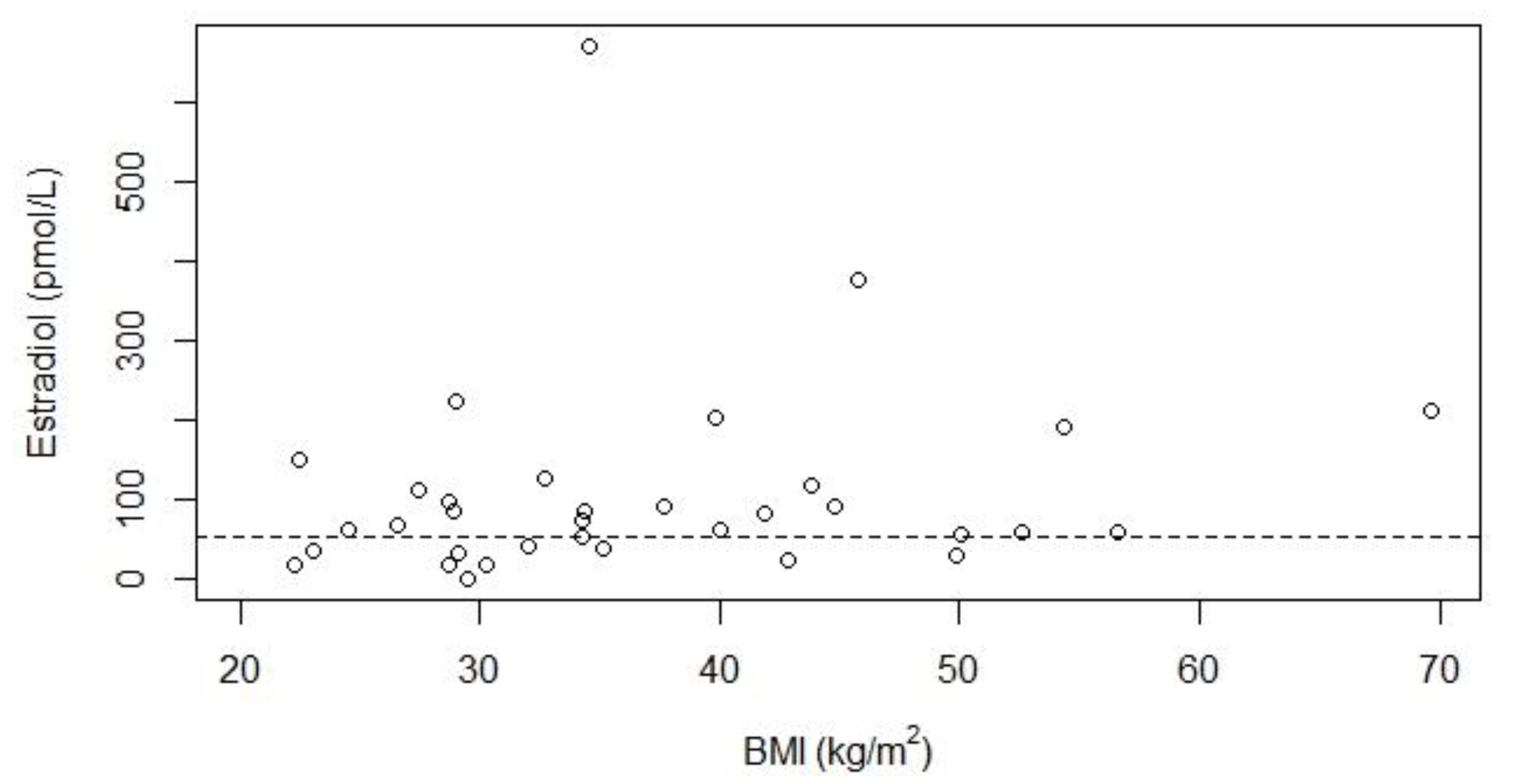
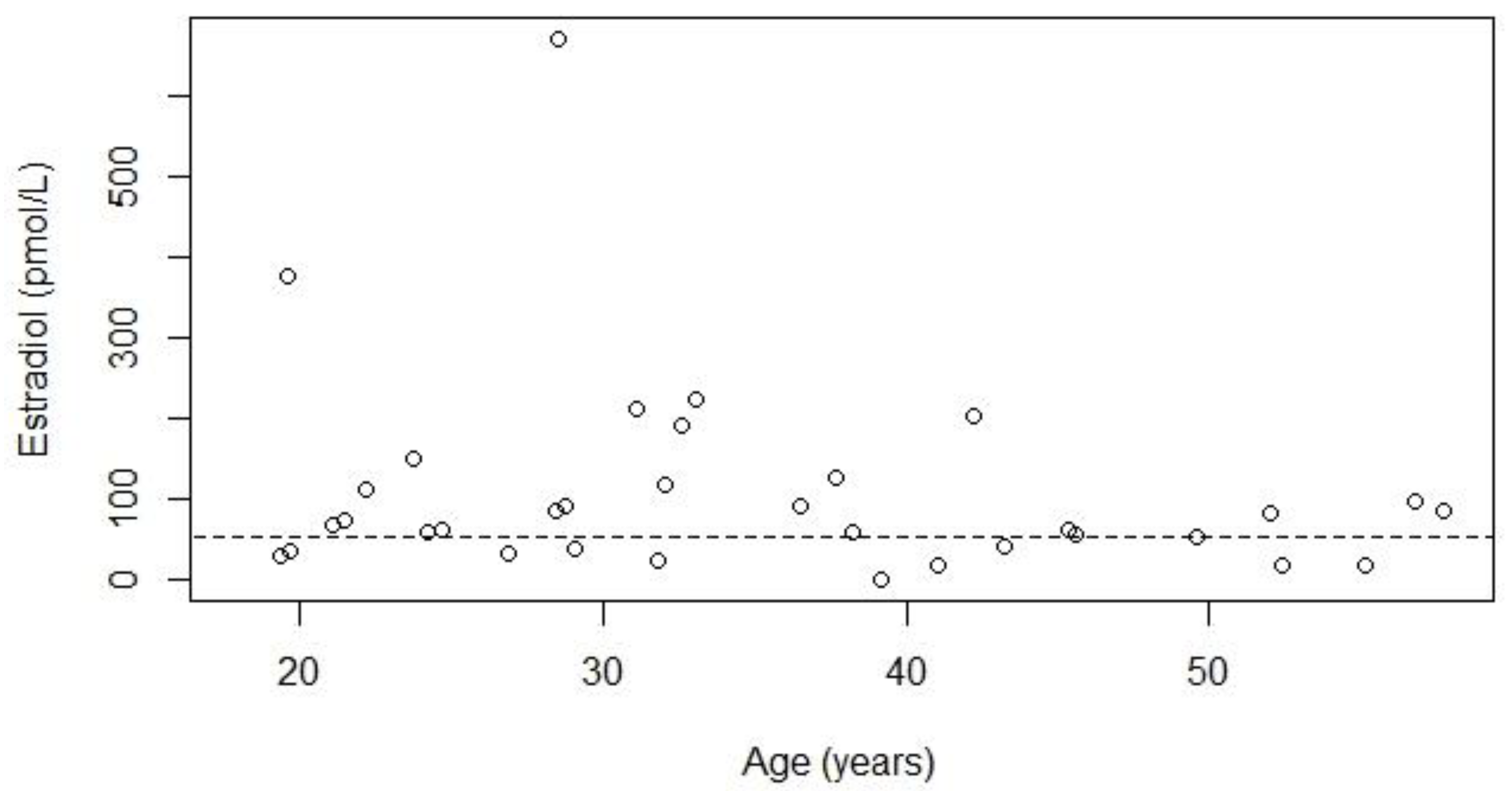
| Hypogonadism Known n = 50 | Hypogonadism Unknown due to Treatment a n = 10 | Hypogonadism Unknown due to Age b n = 4 | Total n = 64 | |
|---|---|---|---|---|
| Age in years, median (IQR) | 27 (21–34) | 25 (19–38) | 56 (51–58) | 28 (22–37) |
| Age range in years | 18–52 | 18–49 | 49–58 | 18–58 |
| BMI in kg/m2, median (IQR) | 32 (27–43) | 25 (22–34) | 31 (24–34) | 32 (27–40) |
| Genetic subtype | ||||
| Deletion | 30 (60%) | 6 (60%) | 1 (25%) | 37 (58%) |
| mUPD c | 16 (32%) | 3 (30%) | 3 (75%) | 22 (34%) |
| ICD | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Unknown | 3 (6%) | 1 (10%) | 0 (0%) | 4 (6%) |
| rhGH treatment d | ||||
| Only during childhood | 6 (12%) | 2 (20%) | 0 (0%) | 8 (13%) |
| Only during adulthood | 2 (4%) | 0 (0%) | 0 (0%) | 2 (3%) |
| Both | 18 (36%) | 6 (60%) | 0 (0%) | 24 (38%) |
| Never | 24 (48%) | 2 (20%) | 4 (100%) | 30 (47%) |
| Current rhGH treatment | 18 (36%) | 6 (60%) | 0 (0%) | 24 (38%) |
| Psychotropic medication | 16 (32%) | 5 (50%) | 3 (75%) | 24 (38%) |
| Living situation | ||||
| With family | 12 (24%) | 3 (30%) | 0 (0%) | 15 (23%) |
| In a specialized PWS group home | 11 (22%) | 3 (30%) | 1 (25%) | 15 (23%) |
| In a non-specialized facility | 27 (54%) | 4 (40%) | 3 (75%) | 34 (53%) |
| Scholar level | ||||
| Secondary vocational education | 2 (4%) | 2 (20%) | 0 (0%) | 4 (6%) |
| Pre-vocational secondary education | 1 (2%) | 0 (0%) | 0 (0%) | 1 (2%) |
| Special education | 35 (70%) | 6 (60%) | 2 (50%) | 43 (67%) |
| No education | 2 (4%) | 1 (10%) | 1 (10%) | 4 (6%) |
| Unknown | 10 (20%) | 1 (10%) | 1 (10%) | 12 (19%) |
| Relationship status | ||||
| In a relationship with sexual intercourse | 5 (10%) | 1 (10%) | 0 (0%) | 6 (9%) |
| In a relationship without sexual intercourse | 8 (16%) | 0 (0%) | 1 (25%) | 9 (14%) |
| Not in a relationship | 33 (66%) | 7 (70%) | 2 (50%) | 42 (66%) |
| Unknown | 4 (8%) | 2 (20%) | 1 (25%) | 7 (11%) |
| Women with PWS n = 64 | |
|---|---|
| Hypogonadism before screening | 30/50 (60%) |
| Of whom untreated number of women with | 7/30 (23%) |
| hypogonadism (%) | |
| Hypogonadism revealed by screening | 17/50 (34%) |
| Hypogonadism after screening | 47/50 (94%) |
| Of whom untreated number of women with | 13/47 (28%) |
| hypogonadism (%) | |
| Age at start hypogonadism hormone therapy, median (IQR) | 20 (16–28) |
| (n = 33) | |
| Hypogonadism after screening according to BMI group | |
| In females with BMI < 25 kg/m2 | 7/7 (100%) |
| In females with BMI 25–30 kg/m2 | 13/14 (93%) |
| In females with BMI > 30 kg/m2 | 27/29 (93%) |
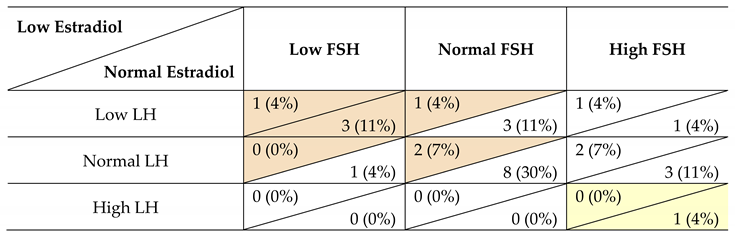 |
| Article | n | Country | Age Range (years) | Genotype (Deletion/mUPD/ICD/ Translocation) | Mean BMI (kg/m2) | Assays Used | Definition Hypogonadism |
|---|---|---|---|---|---|---|---|
| Partsch et al. (2000) [13] | 9 a | Germany | 18–34 b | NA/NA/0/0 | 46 c | Commercially available immunoassays | Low estradiol levels and absence of a regular MC |
| Whittington et al. (2002) [14] d | 24 | United Kingdom | 18–47 | NA e | NA | NA | absence of a regular MC |
| Grugni et al. (2003) [15] | 20 | Italy | 18–28 | 13/7/0/0 | 44 | LH/FSH: immunochemiluminescent assays Estradiol: chemiluminescent immunoassay | absence of a regular MC |
| Höybye et al. (2005) [16] d | 6 | Sweden | 19–37 | NA e | Median 36 | Commercially available immunoassays | low estradiol, absence of a spontaneous regular MC, or treatment with sex steroids |
| Miller et al. (2008) [17] d | 6 | Florida, USA | 18–29 | 4/2/0/0 | 32 | Commercially available radioimmunoassays | Hypogonadotropic hypogonadism: delayed onset of puberty (>13 year) in addition to low gonadotropin levels for age |
| Brandau et al. (2008) [18] d | 21 | Missouri, USA | 18–50 | 14/7/0/0 | 33 | FSH, LH: chemiluminescence assays Estradiol: radioimmunoassay | low estradiol levels |
| Sode-Carlsen et al. (2010) [19] d | 24 | Denmark, Norway, Sweden | 18–41 | 9/2/1/0 (12 NA) e | Median 28 | Commercially available immunoassays | low estradiol, absence of a spontaneous regular MC, or treatment with sex steroids |
| Van Nieuwpoort et al. (2011) [20] | 11 | The Netherlands | 19–41 | 14/1/0/0 c | 33 | Commercially available immunoassays | absence of a spontaneous regular MC |
| Hirsch et al. (2015) [21] d | 19 | Israel | 18–47 | 10/8/1/0 | 33 | LH, FSH, testosterone, estradiol: immunoassays Inhibin B, AMH: ELISA SHBG: immunochemiluminescence | absence of a regular MC |
| Coupaye et al. (2016) [22] d | 35 | France | 18–58 c | 42/24/0/0 c,f | 39 c | Routine techniques | absence of a spontaneous regular MC, treatment with sex steroid or estradiol level < 120 ng/L at any time |
| Article | Hypogonadism n (%) | Primary/ Central Hypogonadism | FSH | LH | Estradiol | SHBG | Inhibin B | AMH |
|---|---|---|---|---|---|---|---|---|
| Partsch et al. (2000) [13] | 9 (100%) | - a | - | - | - | - | - | - |
| Whittington et al. (2002) [14] | 20 (100%) (4 NA) | - | - | - | - | - | - | - |
| Grugni et al. (2003) [15] | 17 (85%) | - | 2.1 (0.1–5.1) IU/L | 1.3 (0.1–5.0) IU/L | 34 (15–72) pg/mL 123 (55–264) pmol/L | - | - | - |
| Höybye et al. (2005) [16] | 5 (83%) | 0/5 | 4.9 (1.0–7.8) IU/L | 2.1 (0.6–5.5) IU/L | 104 (72–203) pmol/L | - | - | - |
| Miller et al. (2008) [17] | 6 (100%) | 1/5 | - | - | - | - | - | - |
| Brandau et al. (2008) [18] | 14 (70%) (1 NA) | - | 3.8 (0.4–15.0) IU/L | 1.8 (0.1–5.3) IU/L | 23 (5–82) pg/mL 85 (18–301) pmol/L | - | - | - |
| Sode-Carlsen et al. (2010) [19] | 13 (54%) | 1/5 (7 NA) | 4.9 (<0.2–17.6) IU/L | 2.7 (<1.0–12.9) IU/L | 0.13 (0.08–0.54) nmol/L 130 (80–54) pmol/L | - | - | - |
| Van Nieuwpoort et al. (2011) [20] | 9 (81%) | 0/4 (5 NA) | Median (IQR) 4.65 (3.49) IU/L | Median (IQR) 2.75 (2.26) IU/L | Median (IQR) 92 (257) pmol/L | Median (IQR) 25.5 (20.2) nmol/L | - | - |
| Hirsch et al. (2015) [21] | 18 (95%) | 1/2 (15 combined b) | 6.1 (0.5–18.3) IU/L | 2.6 (0.1–6.8) IU/L | 144 (37–733) pmol/L | 47.1 (5.1–146.0) nmol/L | 26.9 (10.0–73.0) pg/mL (n = 18) | 1.04 (0.02–2.75) ng/mL (n = 17) |
| Coupaye et al. (2016) [22] | 33 (94%) | - | Mean ± SD 6.4 ± 9.6 IU/L | Mean ± SD 4.2 ± 4.3 IU/L | 50 (12–143) ng/L 183 (44–525) pmol/L | Mean ± SD 36.9 ± 26.4 nmol/L | Mean ± SD 5.6 ± 6.0 pg/mL (n = 5) | Mean ± SD 0.9 ± 0.6 ng/mL (n = 5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellikaan, K.; Ben Brahim, Y.; Rosenberg, A.G.W.; Davidse, K.; Poitou, C.; Coupaye, M.; Goldstone, A.P.; Høybye, C.; Markovic, T.P.; Grugni, G.; et al. Hypogonadism in Women with Prader-Willi Syndrome—Clinical Recommendations Based on a Dutch Cohort Study, Review of the Literature and an International Expert Panel Discussion. J. Clin. Med. 2021, 10, 5781. https://doi.org/10.3390/jcm10245781
Pellikaan K, Ben Brahim Y, Rosenberg AGW, Davidse K, Poitou C, Coupaye M, Goldstone AP, Høybye C, Markovic TP, Grugni G, et al. Hypogonadism in Women with Prader-Willi Syndrome—Clinical Recommendations Based on a Dutch Cohort Study, Review of the Literature and an International Expert Panel Discussion. Journal of Clinical Medicine. 2021; 10(24):5781. https://doi.org/10.3390/jcm10245781
Chicago/Turabian StylePellikaan, Karlijn, Yassine Ben Brahim, Anna G. W. Rosenberg, Kirsten Davidse, Christine Poitou, Muriel Coupaye, Anthony P. Goldstone, Charlotte Høybye, Tania P. Markovic, Graziano Grugni, and et al. 2021. "Hypogonadism in Women with Prader-Willi Syndrome—Clinical Recommendations Based on a Dutch Cohort Study, Review of the Literature and an International Expert Panel Discussion" Journal of Clinical Medicine 10, no. 24: 5781. https://doi.org/10.3390/jcm10245781
APA StylePellikaan, K., Ben Brahim, Y., Rosenberg, A. G. W., Davidse, K., Poitou, C., Coupaye, M., Goldstone, A. P., Høybye, C., Markovic, T. P., Grugni, G., Crinò, A., Caixàs, A., Eldar-Geva, T., Hirsch, H. J., Gross-Tsur, V., Butler, M. G., Miller, J. L., van der Kuy, P.-H. M., van den Berg, S. A. A., ... de Graaff, L. C. G. (2021). Hypogonadism in Women with Prader-Willi Syndrome—Clinical Recommendations Based on a Dutch Cohort Study, Review of the Literature and an International Expert Panel Discussion. Journal of Clinical Medicine, 10(24), 5781. https://doi.org/10.3390/jcm10245781








