Diagnostic Yield of Transbronchial Lung Cryobiopsy Compared to Transbronchial Forceps Biopsy in Patients with Sarcoidosis in a Prospective, Randomized, Multicentre Cross-Over Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Acquisition
2.5. Bronchoscopy
2.6. Tissue Sampling
2.7. Randomization
2.8. Pathological Evaluation
2.9. Evaluation of Adverse Effects
2.10. Primary and Secondary Objectives
2.11. Statistics
2.12. Approval and Registration
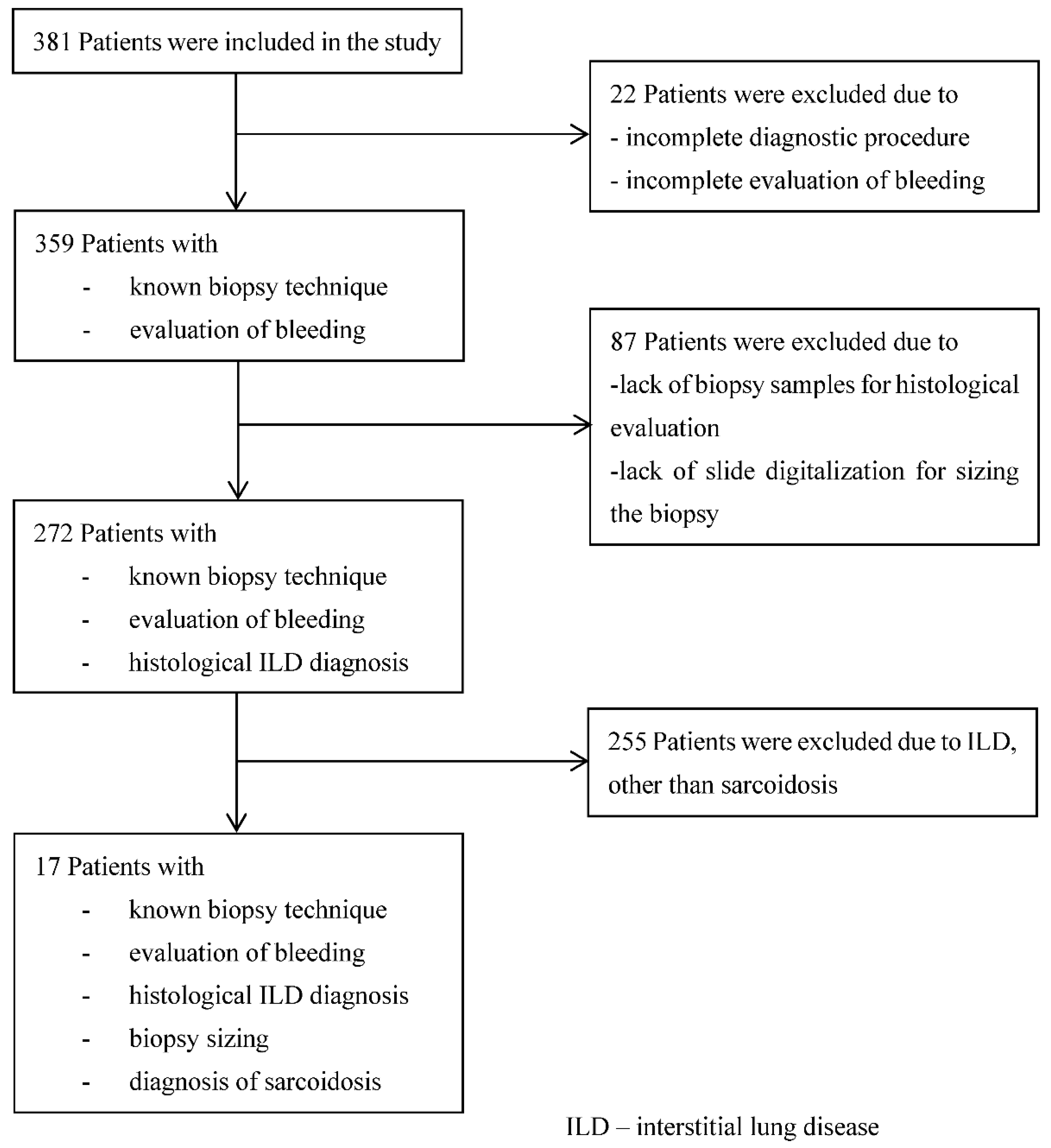
3. Results
Adverse Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.; Günther, A.; Bonella, F.; Dinkel, J.; Fink, L.; Geiser, T.; Geißler, K.; Gläser, S.; Handzhhiev, S.; Jonigk, D.; et al. German Guideline for Idiopathic Pulmonary Fibrosis. Pneumologie 2020, 74, 263–293. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Bradley, B.; Branley, H.M.; Egan, J.J.; Greaves, M.S.; Hansell, D.M.; Harrison, N.K.; Hirani, N.; Hubbard, R.; Lake, F.; Millar, A.B.; et al. Interstitial lung disease guideline: The British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008, 63 (Suppl. S5), v1–v58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govender, P.; Berman, J.S. The Diagnosis of Sarcoidosis. Clin. Chest Med. 2015, 36, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Gupta, D.; Agarwal, R.; Bal, A.; Nijhawan, R.; Aggarwal, A.N. Value of different bronchoscopic sampling techniques in diagnosis of sarcoidosis: A prospective study of 151 patients. J. Bronchol. Interv. Pulmonol. 2014, 21, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Benzaquen, S.; Aragaki-Nakahodo, A.A. Bronchoscopic modalities to diagnose sarcoidosis. Curr. Opin. Pulm. Med. 2017, 23, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Oki, M.; Saka, H.; Kitagawa, C.; Kogure, Y.; Murata, N.; Ichihara, S.; Moritani, S. Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J. Thorac. Cardiovasc. Surg. 2012, 143, 1324–1329. [Google Scholar] [CrossRef] [Green Version]
- Plit, M.; Pearson, R.; Havryk, A.; Da Costa, J.; Chang, C.; Glanville, A.R. Diagnostic utility of endobronchial ultrasound-guided transbronchial needle aspiration compared with transbronchial and endobronchial biopsy for suspected sarcoidosis. Intern. Med. J. 2012, 42, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Pedro, C.; Melo, N.; Novais, E.B.H.; Magalhães, A.; Fernandes, G.; Martins, N.; Morais, A.; Caetano Mota, P. Role of Bronchoscopic Techniques in the Diagnosis of Thoracic Sarcoidosis. J. Clin. Med. 2019, 8, 1327. [Google Scholar] [CrossRef] [Green Version]
- Jacob, M.; Bastos, H.N.; Mota, P.C.; Melo, N.; Cunha, R.; Pereira, J.M.; Guimarães, S.; Souto Moura, C.; Morais, A. Diagnostic yield and safety of transbronchial cryobiopsy in sarcoidosis. ERJ Open Res. 2019, 5, 00203–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragaki-Nakahodo, A.A.; Baughman, R.P.; Shipley, R.T.; Benzaquen, S. The complimentary role of transbronchial lung cryobiopsy and endobronchial ultrasound fine needle aspiration in the diagnosis of sarcoidosis. Respir. Med. 2017, 131, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetzel, J.; Eberhardt, R.; Petermann, C.; Gesierich, W.; Darwiche, K.; Hagmeyer, L.; Muche, R.; Kreuter, M.; Lewis, R.; Ehab, A.; et al. Bleeding risk of transbronchial cryobiopsy compared to transbronchial forceps biopsy in interstitial lung disease–a prospective, randomized, multicentre cross-over trial. Respir. Res. 2019, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Colella, S.; Haentschel, M.; Shah, P.; Poletti, V.; Hetzel, J. Transbronchial Lung Cryobiopsy in Interstitial Lung Diseases: Best Practice. Respir. Int. Rev. Thorac. Dis. 2018, 95, 383–391. [Google Scholar] [CrossRef]
- Troy, L.K.; Grainge, C.; Corte, T.J.; Williamson, J.P.; Vallely, M.P.; Cooper, W.A.; Mahar, A.; Myers, J.L.; Lai, S.; Mulyadi, E.; et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): A prospective, comparative study. Lancet Respir. Med. 2020, 8, 171–181. [Google Scholar] [CrossRef]
- Ravaglia, C.; Wells, A.U.; Tomassetti, S.; Gurioli, C.; Gurioli, C.; Dubini, A.; Cavazza, A.; Colby, T.V.; Piciucchi, S.; Puglisi, S.; et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: A large cohort of 699 patients. BMC Pulm. Med. 2019, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- Hetzel, J.; Wells, A.U.; Costabel, U.; Colby, T.V.; Walsh, S.L.F.; Verschakelen, J.; Cavazza, A.; Tomassetti, S.; Ravaglia, C.; Böckeler, M.; et al. Transbronchial cryobiopsy increases diagnostic confidence in interstitial lung disease: A prospective multicentre trial. Eur. Respir. J. 2020, 56, 1901520. [Google Scholar] [CrossRef]
- Descombes, E.; Gardiol, D.; Leuenberger, P. Transbronchial lung biopsy: An analysis of 530 cases with reference to the number of samples. Monaldi Arch. Chest Dis. 1997, 52, 324–329. [Google Scholar]
- Mitchell, D.M.; Mitchell, D.N.; Collins, J.V.; Emerson, C.J. Transbronchial lung biopsy through fibreoptic bronchoscope in diagnosis of sarcoidosis. Br. Med. J. 1980, 280, 679–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.M.; Emerson, C.J.; Collins, J.V.; Stableforth, D.E. Transbronchial lung biopsy with the fibreoptic bronchoscope: Analysis of results in 433 patients. Br. J. Dis. Chest 1981, 75, 258–262. [Google Scholar] [CrossRef]
- Puar, H.S.; Young, R.C., Jr.; Armstrong, E.M. Bronchial and transbronchial lung biopsy without fluoroscopy in sarcoidosis. Chest 1985, 87, 303–306. [Google Scholar] [CrossRef]
- Gupta, D.; Dadhwal, D.S.; Agarwal, R.; Gupta, N.; Bal, A.; Aggarwal, A.N. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest 2014, 146, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Bilaçeroğlu, S.; Perim, K.; Günel, O.; Cağirici, U.; Büyükşirin, M. Combining transbronchial aspiration with endobronchial and transbronchial biopsy in sarcoidosis. Monaldi Arch. Chest Dis. 1999, 54, 217–223. [Google Scholar] [PubMed]
- Trisolini, R.; Lazzari Agli, L.; Cancellieri, A.; Poletti, V.; Candoli, P.; Paioli, D.; Alifano, M.; Tinelli, C.; Patelli, M. Transbronchial needle aspiration improves the diagnostic yield of bronchoscopy in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2004, 21, 147–151. [Google Scholar]
- Christophi, G.P.; Caza, T.; Curtiss, C.; Gumber, D.; Massa, P.T.; Landas, S.K. Gene expression profiles in granuloma tissue reveal novel diagnostic markers in sarcoidosis. Exp. Mol. Pathol. 2014, 96, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Spagnolo, P.; Rossi, G.; Trisolini, R.; Sverzellati, N.; Baughman, R.P.; Wells, A.U. Pulmonary sarcoidosis. Lancet Respir. Med. 2018, 6, 389–402. [Google Scholar] [CrossRef]
- Spagnolo, P.; Rossi, G.; Trisolini, R.; Sverzellati, N.; Baughman, R.P.; Wells, A.U. Sarcoidosis: Is cryobiopsy not cool enough?–Authors’ reply. Lancet Respir. Med. 2018, 6, e45. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, F.; Danoff, S.K.; Wells, A.U.; Colby, T.V.; Ryu, J.H.; Liberman, M.; Wahidi, M.M.; Frazer, L.; Hetzel, J.; Rickman, O.B.; et al. Transbronchial Cryobiopsy for the Diagnosis of Interstitial Lung Diseases: CHEST Guideline and Expert Panel Report. Chest 2020, 157, 1030–1042. [Google Scholar] [CrossRef]
- Hetzel, J.; Maldonado, F.; Ravaglia, C.; Wells, A.U.; Colby, T.V.; Tomassetti, S.; Ryu, J.H.; Fruchter, O.; Piciucchi, S.; Dubini, A.; et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respir. Int. Rev. Thorac. Dis. 2018, 95, 188–200. [Google Scholar] [CrossRef] [PubMed]
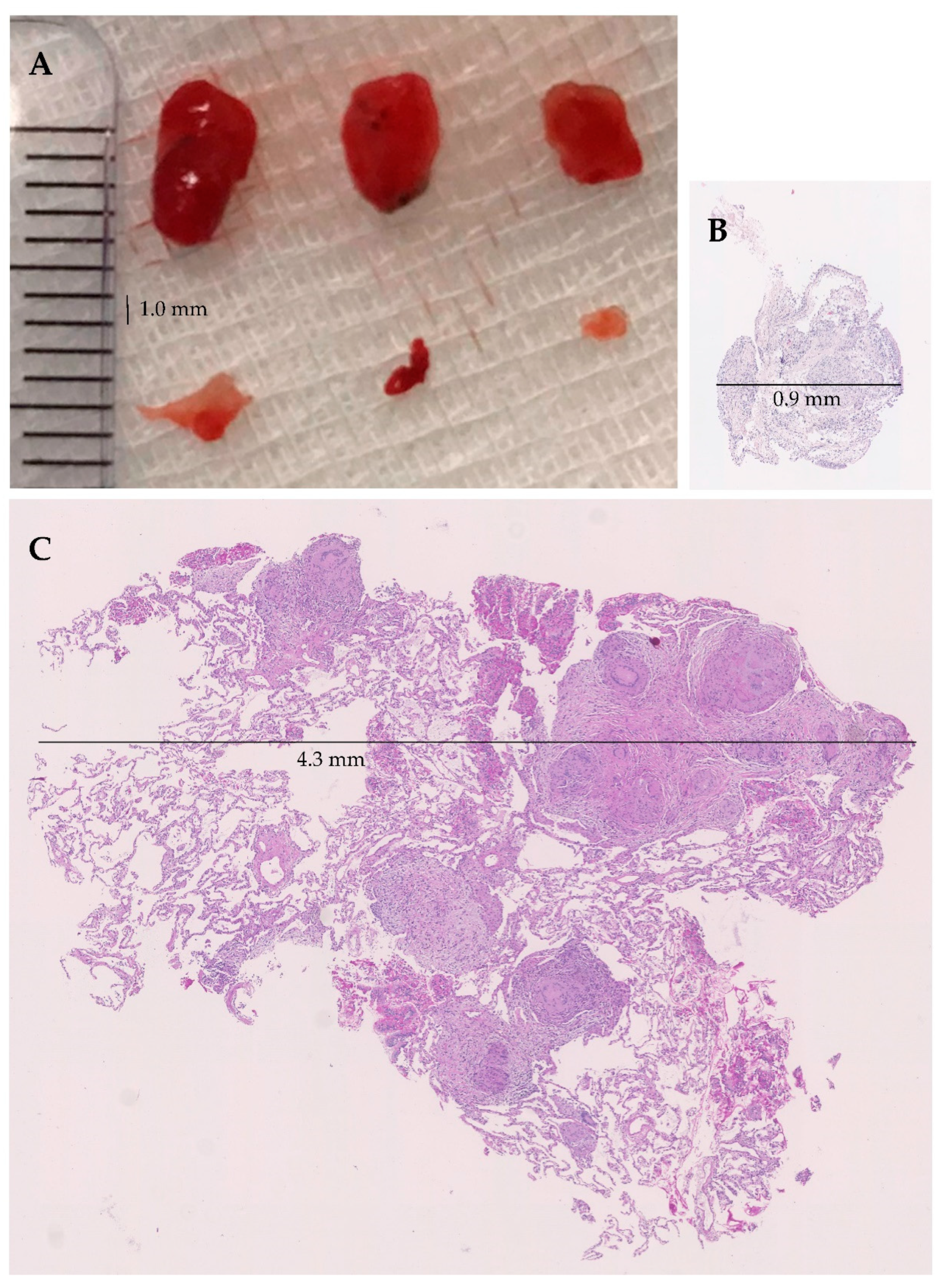

| Bronchoscopic Intervention | TBLC (N = 17) | TBLF (N = 17) | p-Value |
|---|---|---|---|
| Biopsy procedure | |||
| Number of biopsies—no. (%) Total number Number per patient—mean | 61 3.6 (±1.2) | 68 4.0 (±1.3) | n.s. |
| Size of biopsy probe †—no. (%) Small Large | 10 (62.5) 6 (37.5) | 8 (47.0) 9 (52.9) | n.s. |
| Biopsy location—no. (%) Upper lobe—right/left Middle lobe/Lingula Lower lobe—right/left | 19 (31.1)/5 (8.2) 2 (3.3)/6 (9.8) 25 (41.0)/4 (6.6) | 23 (33.8)/10 (14.7) 3 (4.4)/4 (5.9) 23 (33.8)/5 (7.4) | n.s. |
| Positioning of biopsy probe—no. (%) Easy Intermediate Difficult | 10 (58.8) 7 (41.2) 0 (0.0) | 14 (82.3) 3 (17.6) 0 (0.0) | n.s. |
| Duration of biopsy technique—mean (min) | 8.0 (±6.1) | 5.6 (±4.5) | |
| Biopsy sample characteristics | |||
| Area of biopsies Total area of all biopsies—median (mm2) Area per biopsy—median (mm2) | 26.07 8.11 | 8.19 1.72 | p < 0.05 |
| Artefacts (percentage of area) no. (%) No artefacts 0–10% 11–20% >20% | 13 (76.5) 3 (17.6) 1 (5.9) 0 (0.0) | 4 (23.5) 5 (29.4) 6 (35.3) 2 (11.8) | p < 0.05 |
| Diagnosis of Sarcoidosis in the Overall Cohort | TBLC 272 (100) | ||
|---|---|---|---|
| Yes 16 (5.9) | No 256 (94.1) | ||
| TBLF 272 (100) | Yes 7 (2.6) | 6 (2.2) | 1 (0.4) |
| No 265 (97.4) | 10 (3.7) | 255 (93.8) | |
| p < 0.0001 | |||
| Adverse Bleeding Events | TBLC (N = 17) | TBLF (N = 17) | p-Value | |
|---|---|---|---|---|
| Clinical relevance | Bleeding severity | |||
| Low | No | 7 (41.1) | 8 (50.0) | n.a. |
| Mild | 7 (41.1) | 8 (50.0) | ||
| High | Moderate | 2 (11.8) | 0 (0.0) | |
| Severe | 1 (5.9) | 0 (0.0) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häntschel, M.; Eberhardt, R.; Petermann, C.; Gesierich, W.; Darwiche, K.; Hagmeyer, L.; Colby, T.V.; Fend, F.; Theegarten, D.; Wintzer, H.-O.; et al. Diagnostic Yield of Transbronchial Lung Cryobiopsy Compared to Transbronchial Forceps Biopsy in Patients with Sarcoidosis in a Prospective, Randomized, Multicentre Cross-Over Trial. J. Clin. Med. 2021, 10, 5686. https://doi.org/10.3390/jcm10235686
Häntschel M, Eberhardt R, Petermann C, Gesierich W, Darwiche K, Hagmeyer L, Colby TV, Fend F, Theegarten D, Wintzer H-O, et al. Diagnostic Yield of Transbronchial Lung Cryobiopsy Compared to Transbronchial Forceps Biopsy in Patients with Sarcoidosis in a Prospective, Randomized, Multicentre Cross-Over Trial. Journal of Clinical Medicine. 2021; 10(23):5686. https://doi.org/10.3390/jcm10235686
Chicago/Turabian StyleHäntschel, Maik, Ralf Eberhardt, Christoph Petermann, Wolfgang Gesierich, Kaid Darwiche, Lars Hagmeyer, Thomas V. Colby, Falko Fend, Dirk Theegarten, Hanns-Olof Wintzer, and et al. 2021. "Diagnostic Yield of Transbronchial Lung Cryobiopsy Compared to Transbronchial Forceps Biopsy in Patients with Sarcoidosis in a Prospective, Randomized, Multicentre Cross-Over Trial" Journal of Clinical Medicine 10, no. 23: 5686. https://doi.org/10.3390/jcm10235686
APA StyleHäntschel, M., Eberhardt, R., Petermann, C., Gesierich, W., Darwiche, K., Hagmeyer, L., Colby, T. V., Fend, F., Theegarten, D., Wintzer, H.-O., Kreuter, M., Spengler, W., Behrens-Zemek, A. F., Lewis, R. A., Evrard, H. C., Ehab, A., Böckeler, M., & Hetzel, J. (2021). Diagnostic Yield of Transbronchial Lung Cryobiopsy Compared to Transbronchial Forceps Biopsy in Patients with Sarcoidosis in a Prospective, Randomized, Multicentre Cross-Over Trial. Journal of Clinical Medicine, 10(23), 5686. https://doi.org/10.3390/jcm10235686







