Mid-Diastolic Events (L Events): A Critical Review
Abstract
1. Introduction
2. Epidemiology
| Author/Year | Study | Population Studied | Age (Year) | N | % L Wave (+) | % Female L Wave (+) |
|---|---|---|---|---|---|---|
| Ha, 2006 [13] | Observational | Patients with L wave (83) from all the patients in SR (n = 9004) examined in the same period | 63 ± 10 | 9004 | 0.9 | 60 |
| Nakai, 2007 [15] | Observational | Patients with persistent nonvalvular AF. Patients in SR in the same period (for prevalence estimation of the L wave) | L wave (+): 81 ± 8 L wave (−): 73 ± 9 All in AF | 945 (SR) 99 (AF) | 2.4 (SR) 34 (AF) | 68 |
| Lam, 2008 [20] | Observational | Patients in SR with LVH and preserved LVEF, without significant VHD | 65 ± 11 | 177 | 20 | 63 |
| Ho, 2013 [17] | Observational | Patients with persistent nonvalvular AF | 70 ± 10 | 196 | - | - |
| Ari, 2013 [14] | Observational | Patients with persistent nonvalvular AF | L wave (+): 65.45 ± 6.45 L wave (−): 62.04 ± 7.79 | 70 | 31 | 73 |
| Kim, 2017 [12] | Observational | Patients in SR with L wave without significant VHD and normal LVEF from all (n = 20,854) the patients with diastolic dysfunction | 63 ± 12 | 144 | 1 | 61 |
| Masai, 2018 [16] | Observational | Patients with HF. No significant VHD, no FA or frequent EB or HR > 120 bpm | 70 ± 15 | 151 | 32 | ? |
| Sugiura, 2019 [10] | Observational retrospective | Patients with HCM with low SCD risk factor and functional class NYHA I–II | 58 ± 13 | 96 | 15 | 43 |
| Saito, 2020 [11] | Observational retrospective | Patients with the first clinical diagnosis of HCM | L wave (+): 49 ± 18 L wave (−): 52 ± 15 | 445 | 32 | 44 |
3. Pathophysiology
4. L Events in Sinus Rhythm
5. L Events in Atrial Fibrillation
6. L Events in Hypertrophic Cardiomyopathy
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Muscogiuri, G.; Chiesa, M.; Trotta, M.; Gatti, M.; Palmisano, V.; Dell’Aversana, S.; Baessato, F.; Cavaliere, A.; Cicala, G.; Loffreno, A.; et al. Performance of a deep learning algorithm for the evaluation of CAD-RADS classification with CCTA. Atherosclerosis 2020, 294, 25–32. [Google Scholar] [CrossRef]
- Pontone, G.; Guaricci, A.I.; Palmer, S.C.; Andreini, D.; Verdecchia, M.; Fusini, L.; Lorenzoni, V.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; et al. Diagnostic performance of non-invasive imaging for stable cor-onary artery disease: A meta-analysis. Int. J. Cardiol. 2020, 300, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Guglielmo, M.; Fusini, L.; Muscogiuri, G.; Baessato, F.; Loffreno, A.; Cavaliere, A.; Rizzon, G.; Baggiano, A.; Rabbat, M.G.; Muratori, M.; et al. T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur. Radiol. 2021, 31, 1100–1109. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Masci, P.G.; Muscogiuri, G.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Lorenzoni, V.; Martini, C.; Andreini, D.; Pavon, A.G.; et al. CarDiac magnEtic Resonance for prophylactic Implantable-cardioVerter defibrillAtor ThErapy in Non-Ischaemic dilated CardioMyopathy: An international Registry. Europace 2021, 23, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Martini, C.; Gatti, M.; Dell’Aversana, S.; Ricci, F.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Bracciani, A.; Scafuri, S.; et al. Feasibility of late gadolinium enhancement (LGE) in ischemic cardiomyopathy using 2D-multisegment LGE combined with artificial intelligence reconstruction deep learning noise reduction algorithm. Int. J. Cardiol. 2021, 343, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Fusini, L.; Ricci, F.; Sicuso, R.; Guglielmo, M.; Baggiano, A.; Gasperetti, A.; Casella, M.; Mushtaq, S.; Conte, E.; et al. Additional diagnostic value of cardiac magnetic resonance feature tracking in patients with biopsy-proven arrhythmogenic cardiomyopathy. Int. J. Cardiol. 2021, 339, 203–210. [Google Scholar] [CrossRef]
- Gaibazzi, N.; Porter, T.; Lorenzoni, V.; Pontone, G.; de Santis, D.; de Rosa, A.; Guaricci, A.I. Effect of Coronary Revascular-ization on the Prognostic Value of Stress Myocardial Contrast Wall Motion and Perfusion Imaging. J. Am. Heart. Assoc. 2017, 6, e006202. [Google Scholar] [CrossRef]
- Badano, L.P.; Caravita, S.; Rella, V.; Guida, V.; Parati, G.; Muraru, D. The Added Value of 3-Dimensional Echocardiography to Understand the Pathophysiology of Functional Tricuspid Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 683–689. [Google Scholar] [CrossRef]
- Edler, I. Part Three:Atrioventricular Valve Motility In The Living Human Heart Recorded By Ultrasound. Acta Medica Scand. 2009, 170, 83–113. [Google Scholar] [CrossRef]
- Sugiura, Y.; Morimoto, R.; Aoki, S.; Yamaguchi, S.; Haga, T.; Kuwayama, T.; Yokoi, T.; Hiraiwa, H.; Kondo, T.; Watanabe, N.; et al. Prognostic impact of mitral L-wave in patients with hypertrophic cardiomyopathy without risk factors for sudden cardiac death. Heart Vessel. 2019, 34, 2002–2010. [Google Scholar] [CrossRef]
- Saito, C.; Minami, Y.; Arai, K.; Haruki, S.; Shirotani, S.; Higuchi, S.; Ashihara, K.; Hagiwara, N. Prognostic Significance of the Mitral L-Wave in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2020, 130, 130–136. [Google Scholar] [CrossRef]
- Kim, S.-A.; Son, J.; Shim, C.-Y.; Choi, E.-Y.; Ha, J.-W. Long-term outcome of patients with triphasic mitral flow with a mid-diastolic L wave: Prognostic role of left atrial volume and N-terminal pro-brain natriuretic peptide. Int. J. Cardiovasc. Imaging 2017, 14, 88–1384. [Google Scholar] [CrossRef]
- Ha, J.W.; Ahn, J.A.; Moon, J.Y.; Suh, H.S.; Kang, S.M.; Rim, S.J.; Jang, Y.; Chung, N.; Shim, W.H.; Cho, S.Y. Triphasic mitral inflow velocity with mid-diastolic flow: The presence of mid-diastolic mitral annular velocity indicates advanced diastolic dys-function. Eur. J. Echocardiogr. 2006, 7, 16–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ari, H.; Ari, S.; Akkaya, M.; Sarigul, O.Y.; Emlek, N.; Aydin, C.; Çetinkaya, S.; Bozat, T.; Melek, M. A novel echocardio-graphic predictor of atrial fibrillation recurrence: L-wave. Echocardiography 2013, 30, 1180–1186. [Google Scholar] [CrossRef]
- Nakai, H.; Takeuchi, M.; Nishikage, T.; Nagakura, T.; Otani, S. The Mitral L Wave A Marker of Advanced Diastolic Dysfunction in Patients with Atrial Fibrillation. Circ. J. 2007, 71, 1244–1249. [Google Scholar] [CrossRef][Green Version]
- Masai, K.; Mano, T.; Goda, A.; Sugahara, M.; Daimon, A.; Asakura, M.; Ishihara, M.; Masuyama, T. Correlates and Prognostic Values of Appearance of L Wave in Heart Failure Patients with Preserved vs. Reduced Ejection Fraction. Circ. J. 2018, 82, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-M.; Lin, T.-H.; Hsu, P.-C.; Lee, W.-H.; Chu, C.-Y.; Lee, C.-S.; Lai, W.-T.; Sheu, S.-H.; Voon, W.-C. Incremental prognostic value of identifying mitral L wave in patients with atrial fibrillation. Int. J. Cardiol. 2013, 168, 4501–4503. [Google Scholar] [CrossRef]
- Lam, C.S.; Han, L.; Ha, J.-W.; Oh, J.K.; Ling, L.H. The mitral L wave: A marker of pseudonormal filling and predictor of heart failure in patients with left ventricular hypertrophy. J. Am. Soc. Echocardiogr. 2005, 18, 336–341. [Google Scholar] [CrossRef]
- Ha, J.-W.; Oh, J.K.; Redfield, M.M.; Ujino, K.; Seward, J.B.; Tajik, A. Triphasic mitral inflow velocity with middiastolic filling: Clinical implications and associated echocardiographic findings. J. Am. Soc. Echocardiogr. 2004, 17, 428–431. [Google Scholar] [CrossRef]
- Lam, C.S.; Han, L.; Oh, J.K.; Yang, H.; Ling, L.H. The Mitral Annular Middiastolic Velocity Curve: Functional Correlates and Clinical Significance in Patients with Left Ventricular Hypertrophy. J. Am. Soc. Echocardiogr. 2008, 21, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Podroužková, M.J.; Špinarová, H.; Hude, P.; Krejčí, J. flow and mid- diastolic mitral annular motion—Relation to pulmonary capillary wedge pressure in dilated cardiomyopathy patients. Kardiol. Rev. 2013, 15, 172–176. [Google Scholar]
- Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Andreini, D.; Mushtaq, S.; Conte, E.; Annoni, A.D.; Formenti, A.; Mancini, E.M.; et al. Multimodality imaging of left atrium in patients with atrial fibrillation. J. Cardiovasc. Comput. Tomogr. 2019, 13, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Sciaccaluga, C.; Loiacono, F.; Simova, I.; Miglioranza, M.H.; Nistor, D.O.; Bandera, F.; Emdin, M.; Giannoni, A.; Ciccone, M.M.; et al. The analysis of left atrial function predicts the severity of functional impairment in chronic heart failure: The FLASH multicenter study. Int. J. Cardiol. 2019, 286, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Pontone, G.; Andreini, D.; Bertella, E.; Petullà, M.; Russo, E.; Innocenti, E.; Mushtaq, S.; Gripari, P.; Loguercio, M.; Segurini, C.; et al. Comparison of cardiac computed tomography versus cardiac magnetic resonance for characterization of left atrium anatomy before radiofrequency catheter ablation of atrial fibrillation. Int. J. Cardiol. 2015, 179, 114–121. [Google Scholar] [CrossRef]
- Barbier, P.; Solomon, S.B.; Schiller, N.B.; Glantz, S.A. Left Atrial Relaxation and Left Ventricular Systolic Function Determine Left Atrial Reservoir Function. Circulation 1999, 100, 427–436. [Google Scholar] [CrossRef]
- Hoit, B.D.; Shao, Y.; Gabel, M.; Walsh, R.A. In vivo assessment of left atrial contractile performance in normal and patho-logical conditions using a time-varying elastance model. Circulation 1994, 89, 1829–1838. [Google Scholar] [CrossRef]
- Keren, G.; Meisner, J.S.; Sherez, J.; Yellin, E.L.; Laniado, S. Interrelationship of mid-diastolic mitral valve motion, pulmonary venous flow, and transmitral flow. Circulation 1986, 74, 36–44. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Hatle, L. Doppler echocardiographic evaluation of diastolic function in hypertensive cardiomyopathies. Eur. Heart J. 1993, 14, 88–94. [Google Scholar]
- Masai, M.T.; Mano, T.; Goda, A.; Soyama, Y.; Matsumoto, A.; Masuyama, T. Clinical Significance of Mid-Diastolic L Wave in Heart Failure Patients by Various Loading Test. J. Card. Fail. 2016, 22, S192. [Google Scholar] [CrossRef]
- Wang, J.; Khoury, D.S.; Thohan, V.; Torre-Amione, G.; Nagueh, S.F. Global Diastolic Strain Rate for the Assessment of Left Ventricular Relaxation and Filling Pressures. Circulation 2007, 115, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Magoon, R.; Malik, V.; Kapoor, P.M.; Ramakrishnan, S. Studying diastology with speckle tracking echo-cardiography: The essentials. Ann. Card. Anaesth. 2017, 20, S57–S60. [Google Scholar]
- Kasner, M.; Gaub, R.; Sinning, D.; Westermann, D.; Steendijk, P.; Hoffmann, W.; Schultheiss, H.-P.; Tschöpe, C. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure-volume loop analysis. Eur. J. Echocardiogr. 2010, 11, 743–751. [Google Scholar] [CrossRef][Green Version]
- Tanaka, M.; Sakamoto, T.; Sugawara, S.; Nakajima, H.; Kameyama, T.; Tabuchi, H.; Katahira, Y.; Ohtsuki, S.; Kanai, H. Physi-ological basis and clinical significance of left ventricular suction studied using echo-dynamography. J. Cardiol. 2011, 58, 232–244. [Google Scholar] [CrossRef]
- Ghosh, E.; Caruthers, S.D.; Kovács, S.J. E-wave generated intraventricular diastolic vortex to L-wave relation: Model-based prediction with in vivo validation. J. Appl. Physiol. 2014, 117, 316–324. [Google Scholar] [CrossRef]
- Hayabuchi, Y.; Ono, A.; Homma, Y.; Kagami, S. Tricuspid L and L’ waves. Int. J. Cardiol. 2016, 211, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Koitka, K.; Kelly, N.; Lau, K.; Lin, A.; Chan, J.; Scalia, G.; Hamilton-Craig, C. Does Mid-Diastolic Transmitral Flow (‘L-wave’) Correlate with Raised Left Ventricular End Diastolic Pressure. Heart Lung Circ. 2018, 27, S235. [Google Scholar] [CrossRef]
- Su, H.-M.; Lin, T.-H.; Lee, C.-S.; Lee, P.-C.; Yeh, S.-C.; Chen, W.-R.; Lai, W.-T.; Sheu, S.-H.; Voon, W.-C. Mid-Diastolic Mitral Annular Motion: A Useful Marker in the Evaluation of Left Ventricular Relaxation and End-Diastolic Pressure. Ultrasound Med. Biol. 2008, 34, 1909–1913. [Google Scholar] [CrossRef]
- Ecker, V.; Knoery, C.; Rushworth, G.; Rudd, I.; Ortner, A.; Begley, D.; Leslie, S.J. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin. Cardiol. 2018, 41, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Misumi, I.; Ishii, M. Triphasic mitral inflow pattern associated with hemodynamic deterioration in anemia or mitral regurgitation: A report of two cases. J. Med. Ultrason. 2017, 45, 501–507. [Google Scholar] [CrossRef]
- Misumi, I.; Ebihara, K.; Akahoshi, R.; Rokutanda, T.; Uramoto, H.; Esaki, T.; Matsumoto, M.; Sugiyama, S.; Ogawa, H. Triphasic mitral inflow in acute coronary syndrome: A case study. J. Cardiol. Cases 2011, 4, e156–e159. [Google Scholar] [CrossRef][Green Version]
- Morisawa, D.; Ohno, Y.; Ohta, Y.; Orihara, Y.; Masai, K.; Goda, A.; Asakura, M.; Ishihara, M. Serial changes of L wave according to heart rates in a heart failure patient with persistent atrial fibrillation. J. Cardiol. Cases 2019, 20, 213–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Misumi, I.; Motozato, K.; Usuku, H.; Sakamoto, K.; Kaikita, K.; Tsujita, K.; Fukui, T. A Mechanism for L-Wave Generation via Color M-Mode Imaging in a Patient with Mitral Regurgitation. CASE 2019, 4, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Link, M.S.; Lesser, J.R.; Chan, R.H.; Garberich, R.F.; Udelson, J.E.; Maron, M.S. Hypertrophic Cardiomyopathy in Adulthood Associated With Low Cardiovascular Mortality with Contemporary Management Strategies. J. Am. Coll. Cardiol. 2015, 65, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Debl, K.; Djavidani, B.; Buchner, S.; Poschenrieder, F.; Feuerbach, S.; Riegger, G.; Luchner, A. Triphasic Mitral Inflow Pattern and Regional Triphasic Mitral Annulus Velocity in Hypertrophic Cardiomyopathy. J. Am. Soc. Echocardiogr. 2008, 21, 407.e5–407.e6. [Google Scholar] [CrossRef]

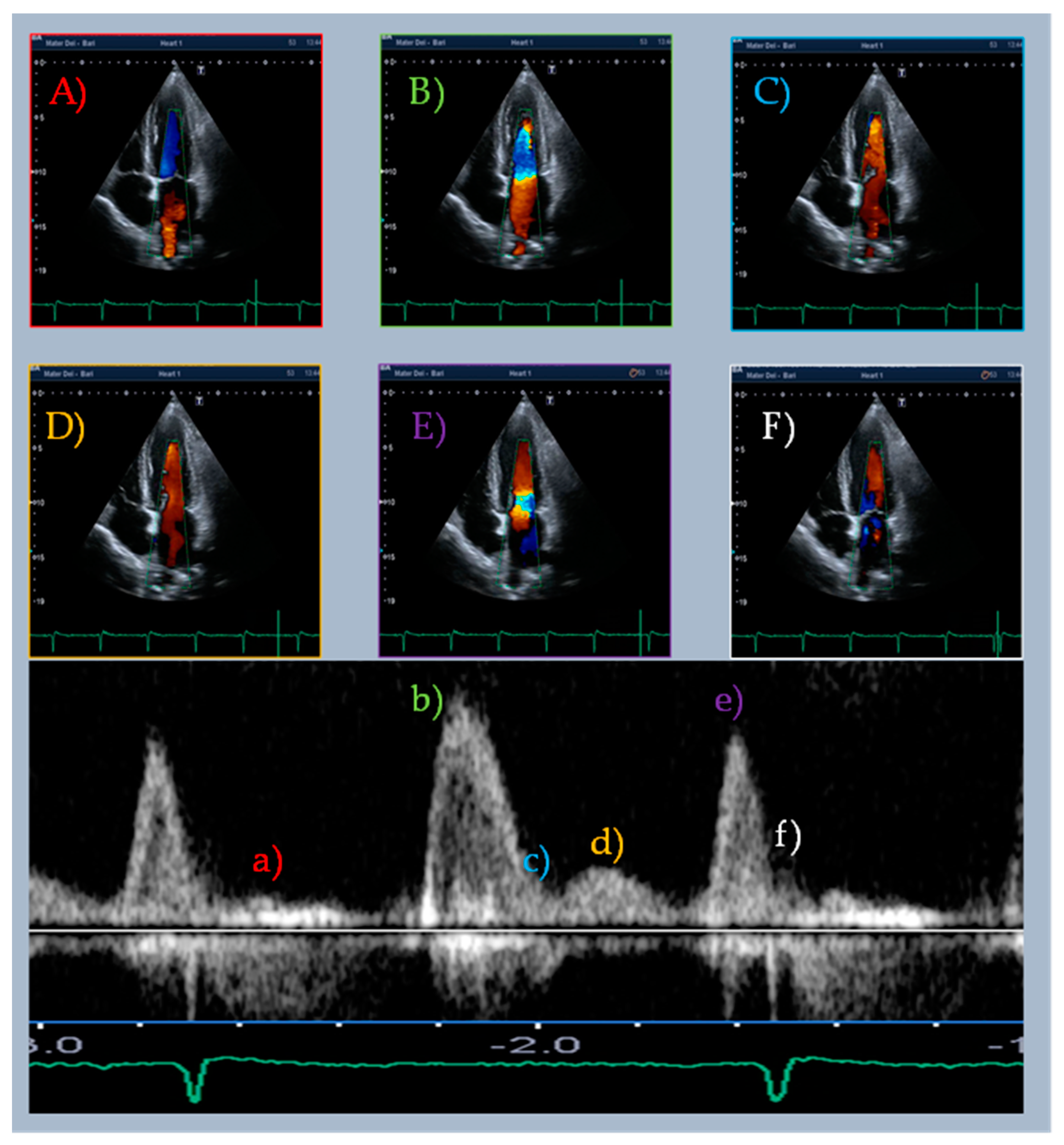

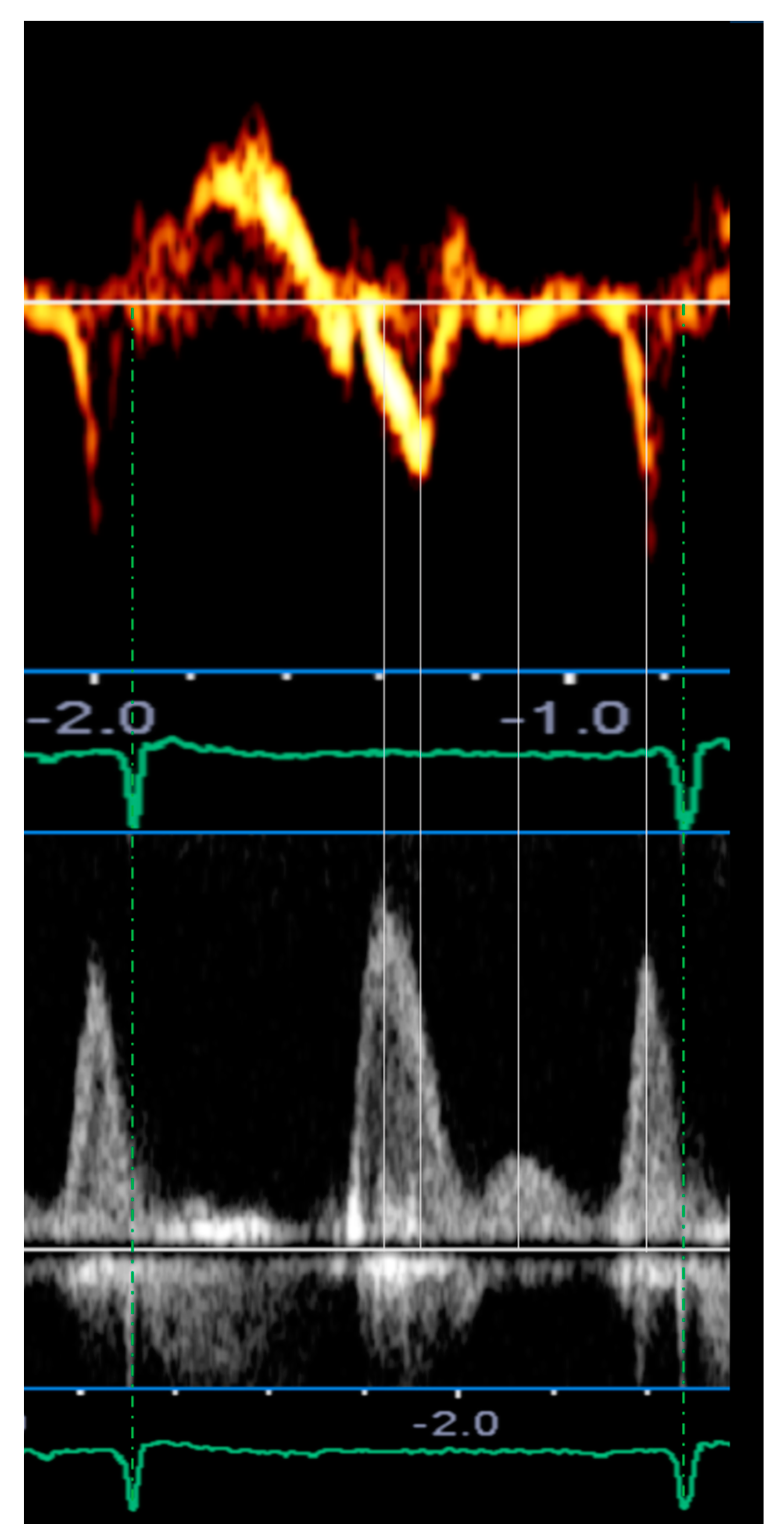
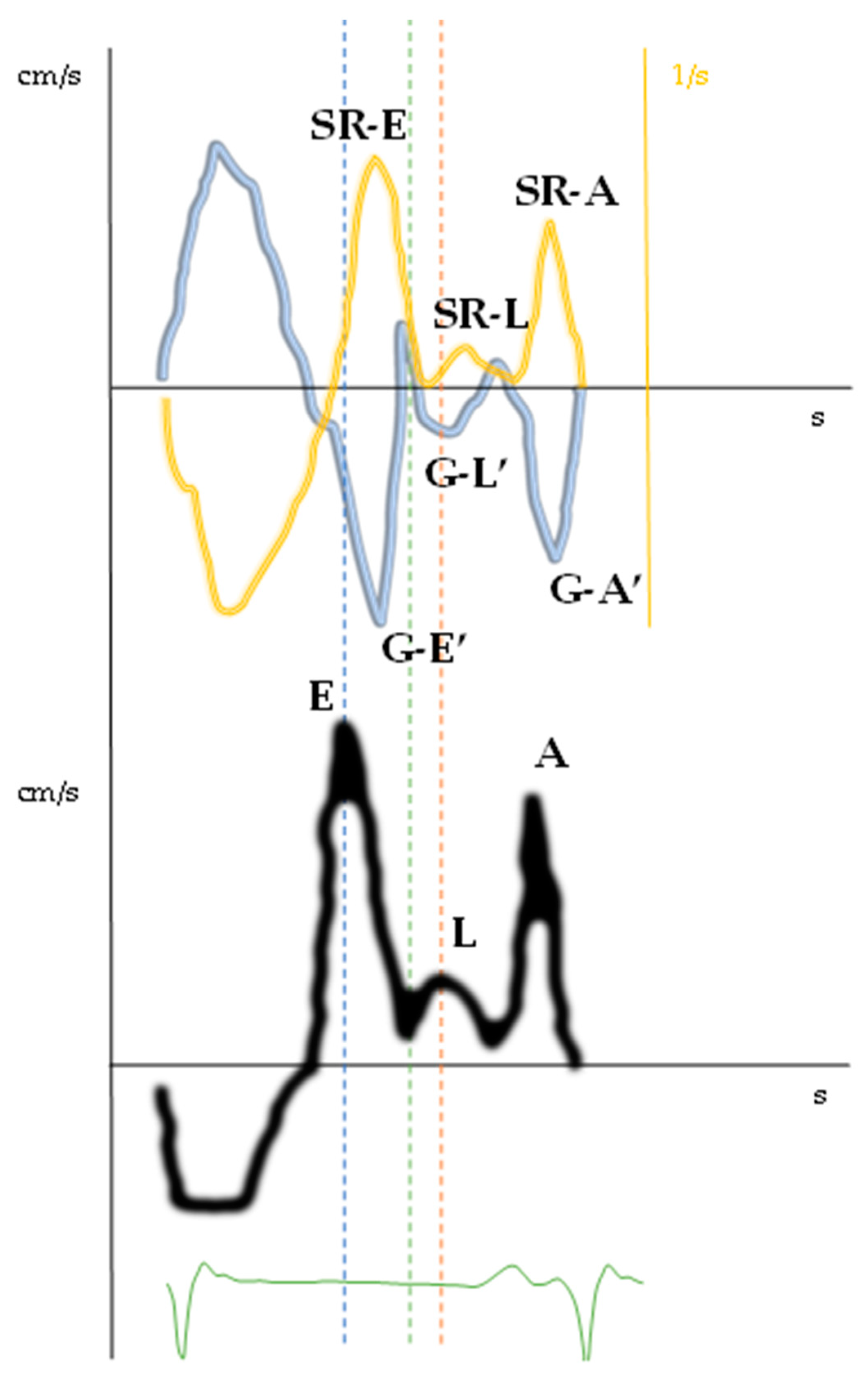
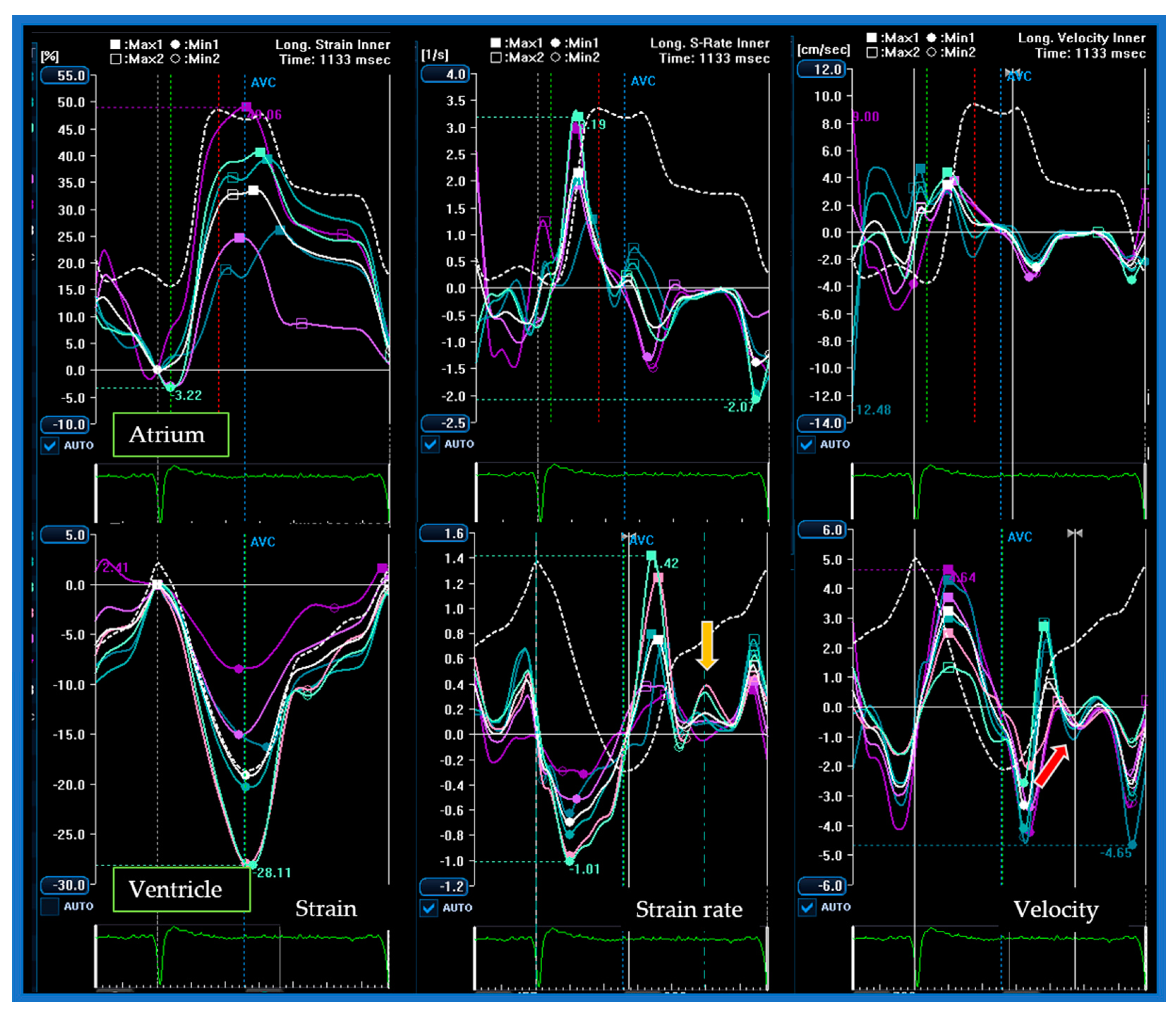
| Type | Definition | Sampling Site and Method |
|---|---|---|
| Mid-diastolic transmitral flow velocity (L wave) | Distinct forward flow velocity after E wave with peak velocity ≥ 20 cm/s | Apical four-chamber view, sample volume at the tips of mitral leaflets with PW Doppler |
| Mid-diastolic mitral valve motion (L motion) | Mid-diastolic opening and closing motion of the mitral valve | Parasternal long-axis view M-mode along the line-cutting mitral valve |
| Mid-diastolic mitral annular velocity (L’ wave) | Distinct basilar–apical tissue velocity after E’ wave present in all cardiac cycles * | Apical four-chamber view, sample volume at septal and lateral mitral annular corners (TDI and PW Doppler) |
| Doppler-Parameters | Values | Chambers | Values | Strain and Strain Rate Parameters of LV and LA | Values | Time to Peak (ms) | %RR |
|---|---|---|---|---|---|---|---|
| E wave (cm/s) | 119.8 | IVS (mm) | 11 | GLS (%) | −16.7 | E wave | 49 |
| L wave (cm/s) | 32.3 | EDD (mm) | 53 | SR-irt (1/s) | 0.06 | G–E’ | 53 |
| A wave (cm/s) | 100.6 | EDV (mL/m2) | 63.9 | SR-E (1/s) | 0.69 | SR-E | 56 |
| E/A ratio | 1.2 | ESV (mL/m2) | 23.3 | E/SR-ivrt ratio (cm) | 1997 | G–L’ | 73 |
| E’ lat wave (cm/s) | 8.2 | LVEF (%) | 63 | E/SR-E ratio (cm) | 1.7 | L wave | 73 |
| L’ lat wave (cm/s) | 3.0 | LAVi (mL/m2) | 42.2 | SR-L (1/s) | 0.13 | SR-L | 75 |
| A’ lat wave (cm/s) | 13.8 | G–L’ (cm/s) | 0.59 | ||||
| E’ ivs wave (cm/s) | 8.1 | LA strain reservoir (%) | 34.2 | ||||
| L’ ivs wave (cm/s) | 2.2 | LA strain conduit (%) | 13.6 | ||||
| A’ ivs wave (cm/s) | 5.8 | LA strain contraction (%) | 20.5 | ||||
| E/E’ | 14.7 | aSR-E (1/s) | −0.77 | ||||
| IVRT (PW) (ms) | 61 | aSR-A (1/s) | −1.39 | ||||
| DT (ms) | 178 | (E/E’)/LA strain reservoir | 0.44 | ||||
| S/D wave ratio | <1 | ||||||
| TRV (m/s) | 2.8 |
| Relevant Clinical Associations | Clinical Settings | |||
|---|---|---|---|---|
| Aymptomatic DD | HFrEF/HFpEF | HCM | NVAF | |
| Diagnostic Value | ||||
| BNP/NT-proBNP serum levels elevation | + | + | ||
| Grade II-III diastolic dysfunction | + | + | + | + |
| Prognostic Value | ||||
| Hospitalization for HF or new-onset HF | + | + | + | + |
| Cardiovascular mortality | + | + | + | |
| All-cause mortality | + | + | ||
| SCD, VAs | + | |||
| AF or Recurrent AF after ECV | + | + | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Virgilio, E.; Monitillo, F.; Santoro, D.; D’Alessandro, S.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Memeo, R.; Rabbat, M.G.; Favale, S.; et al. Mid-Diastolic Events (L Events): A Critical Review. J. Clin. Med. 2021, 10, 5654. https://doi.org/10.3390/jcm10235654
Di Virgilio E, Monitillo F, Santoro D, D’Alessandro S, Guglielmo M, Baggiano A, Fusini L, Memeo R, Rabbat MG, Favale S, et al. Mid-Diastolic Events (L Events): A Critical Review. Journal of Clinical Medicine. 2021; 10(23):5654. https://doi.org/10.3390/jcm10235654
Chicago/Turabian StyleDi Virgilio, Emanuele, Francesco Monitillo, Daniela Santoro, Silvia D’Alessandro, Marco Guglielmo, Andrea Baggiano, Laura Fusini, Riccardo Memeo, Mark G. Rabbat, Stefano Favale, and et al. 2021. "Mid-Diastolic Events (L Events): A Critical Review" Journal of Clinical Medicine 10, no. 23: 5654. https://doi.org/10.3390/jcm10235654
APA StyleDi Virgilio, E., Monitillo, F., Santoro, D., D’Alessandro, S., Guglielmo, M., Baggiano, A., Fusini, L., Memeo, R., Rabbat, M. G., Favale, S., Cameli, M., Guaricci, A. I., & Pontone, G. (2021). Mid-Diastolic Events (L Events): A Critical Review. Journal of Clinical Medicine, 10(23), 5654. https://doi.org/10.3390/jcm10235654













