Effects of Anesthetic Technique on the Occurrence of Acute Kidney Injury after Spine Surgery: A Retrospective Cohort Study
Abstract
:1. Introduction
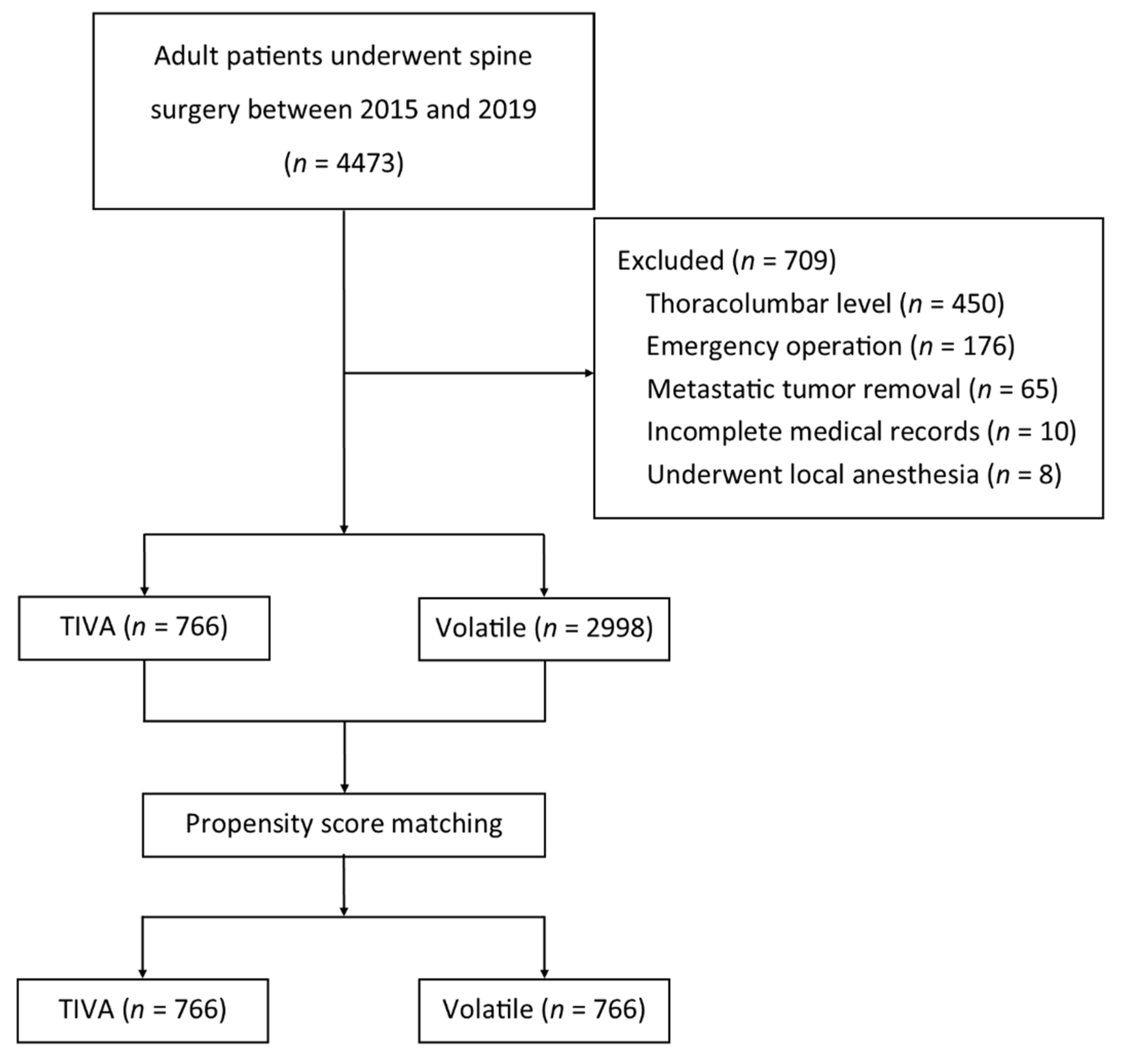
2. Materials and Methods
2.1. Study Design and Patient Data
2.2. Definition of AKI
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bagshaw, S.M. The long-term outcome after acute renal failure. Curr. Opin. Crit. Care 2006, 12, 561–566. [Google Scholar] [CrossRef]
- Motayagheni, N.; Phan, S.; Eshraghi, C.; Nozari, A.; Atala, A. A review of anesthetic effects on renal function: Potential organ protection. Am. J. Nephrol. 2017, 46, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, D.; Lei, L.; Jia, Y.; Zhou, H.; Yang, B. Propofol prevents renal ischemia-reperfusion injury via inhibiting the oxidative stress pathways. Cell Physiol. Biochem. 2015, 37, 14–26. [Google Scholar] [CrossRef]
- Sanchez, P.; Rodriguez, J.M.; Nicolas, J.L.; Lozano, F.S.; Garcia, F.J.; Cascajo, C.; Gonzalez, R.; Muriel, C. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth. Analg. 2008, 106, 371–378. [Google Scholar] [CrossRef]
- Yuzbasioglu, M.F.; Aykas, A.; Kurutas, E.B.; Sahinkanat, T. Protective effects of propofol against ischemia/reperfusion injury in rat kidneys. Ren. Fail. 2010, 32, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-J.; Park, Y.-S.; Kim, S.-H.; Kim, D.; Shim, W.-H.; Jang, D.-M.; Shaffrey, C.I.; Naik, B.I. Effect of prone positional apparatus on the occurrence of acute kidney injury after spine surgery. World Neurosurg. 2019, 128, e597–e602. [Google Scholar] [CrossRef]
- Park, J.T. Postoperative acute kidney injury. Korean J. Anesthesiol. 2017, 70, 258–266. [Google Scholar] [CrossRef] [Green Version]
- John, A.; Kellum, N.L.; Peter, A.; Rashad, S.B.; Emmanuel, A.B.; Stuart, L.G.; Charles, A.H.; Michael, J.; Andreas, K.; Andrew, S.L.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012, 2, 1–138. [Google Scholar] [CrossRef] [Green Version]
- Justus, J.; Randolph, K.F.; Austin, K.M.; Joseph, L.B. A step-by-step guide to propensity score matching in R. Prac. Assess. Res. Eval. 2014, 19, 1–6. [Google Scholar] [CrossRef]
- Lee, H.-J.; Bae, J.; Kwon, Y.; Jang, H.S.; Yoo, S.; Jeong, C.W.; Kim, J.-T.; Kim, W.H. General anesthetic agents and renal function after nephrectomy. J. Clin. Med. 2019, 8, 1530. [Google Scholar] [CrossRef] [Green Version]
- Bang, J.Y.; Lee, J.; Oh, J.; Song, J.G.; Hwang, G.S. The influence of propofol and sevoflurane on acute kidney injury after colorectal surgery: A retrospective cohort study. Anesth. Analg. 2016, 123, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.R.; Yoon, S.; Song, G.Y.; Lee, S.; Bahk, J.H.; Nam, K. The impact of total intravenous anesthesia versus inhalation anesthesia on acute kidney injury after major abdominal surgery: A propensity score analysis. J. Anesth. 2021, 35, 112–121. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Park, J.; Lee, S.-H.; Oh, A.-R.; Lee, J.-H.; Min, J.J. Effects of volatile versus total intravenous anesthesia on occurrence of myocardial injury after non-cardiac surgery. J. Clin. Med. 2019, 8, 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, A.S.; Mahmoud, K.M. Comparative effect of propofol versus sevoflurane on renal ischemia/reperfusion injury after elective open abdominal aortic aneurysm repair. Saudi J. Anaesth. 2016, 10, 301–307. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Shim, J.K.; Song, Y.; Yang, S.Y.; Kwak, Y.L. Anesthetics influence the incidence of acute kidney injury following valvular heart surgery. Kidney Int. 2014, 86, 414–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, T.K.; Kim, J.; Han, S.; Kim, K.; Jheon, S.; Ji, E. Effect of sevoflurane-based or propofol-based anaesthesia on the incidence of postoperative acute kidney injury: A retrospective propensity score-matched analysis. Eur. J. Anaesthesiol. 2019, 36, 649–655. [Google Scholar] [CrossRef]
- Schiffl, H. Gender differences in the susceptibility of hospital-acquired acute kidney injury: More questions than answers. Int. Urol Nephrol. 2020, 52, 1911–1914. [Google Scholar] [CrossRef]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [Green Version]
- Han, S.S.; Baek, S.H.; Ahn, S.Y.; Chin, H.J.; Na, K.Y.; Chae, D.W.; Kim, S. Anemia is a risk factor for acute kidney injury and long-term mortality in critically ill patients. Tohoku J. Exp. Med. 2015, 237, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Griffin, B.R.; Teixeira, J.P.; Ambruso, S.; Bronsert, M.; Pal, J.D.; Cleveland, J.C.; Reece, T.B.; Fullerton, D.A.; Faubel, S.; Aftab, M. Stage 1 acute kidney injury is independently associated with infection following cardiac surgery. J. Thorac. Cardiovasc. Surg. 2021, 161, 1346–1355. [Google Scholar] [CrossRef]
- Liotta, M.; Olsson, D.; Sartipy, U.; Holzmann, M.J. Minimal changes in postoperative creatinine values and early and late mortality and cardiovascular events after coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Variables | TIVA (n = 766) | Volatile (n = 2998) | ASD | TIVA (n = 766) | Volatile (n = 766) | ASD |
| Age (years) | 60.6 ± 13.5 | 64.1 ± 13.0 | 0.27 | 60.6 ± 13.5 | 61.5 ± 13.7 | 0.06 |
| Male sex | 470 (61.4%) | 1457 (48.6%) | 0.26 | 470 (61.4%) | 468 (61.1%) | 0.05 |
| BMI (kg m−2) | 27.9 ± 82.4 | 27.0 ± 25.2 | 0.01 | 27.9 ± 82.4 | 26.1 ± 14.4 | 0.02 |
| ASA | 0.062 | |||||
| 1 | 219 (28.6%) | 811 (27.1%) | 219 (28.6%) | 218 (28.5%) | ||
| 2 | 464 (60.6%) | 1848 (61.6%) | 0.03 | 464 (60.6%) | 450 (58.7%) | |
| 3 + 4 | 83 (10.8%) | 339 (11.3%) | 0.04 | 83 (10.8%) | 98 (12.8%) | |
| Comorbidities | ||||||
| Hypertension | 335 (43.7%) | 1510 (50.4%) | 0.13 | 335 (43.7%) | 336 (43.9%) | 0.003 |
| Diabetes mellitus | 18 (24.3%) | 723 (24.1%) | 0.003 | 186 (24.3%) | 211 (27.5%) | 0.075 |
| Coronary disease | 70 (9.1%) | 304 (10.1%) | 0.03 | 70 (9.1%) | 80 (10.4%) | 0.044 |
| Dyslipidemia | 52 (6.8%) | 197 (6.6%) | 0.01 | 52 (6.8%) | 58 (7.6%) | 0.03 |
| Smoking | 358 (46.7%) | 1011 (33.7%) | 0.28 | 358 (46.7%) | 361 (47.1%) | 0.008 |
| Anemia | 128 (16.7%) | 561 (18.7%) | 0.05 | 128 (16.7%) | 127 (16.6%) | 0.004 |
| Medication | ||||||
| NSAIDs | 113 (14.8%) | 451 (15%) | 0.01 | 113 (14.8%) | 121 (15.8%) | 0.029 |
| ACEi/ARB | 177 (23.1%) | 670 (22.4%) | 0.02 | 177 (23.1%) | 202 (26.4%) | 0.076 |
| Diuretics | 60 (7.8%) | 215 (7.2%) | 0.03 | 60 (7.8%) | 62 (8.1%) | 0.01 |
| Surgical parameter | ||||||
| Surgical level | 2.2 (1.3) | 1.6 (1.0) | 0.57 | 2.2 (1.3) | 1.7 (1.1) | 0.41 |
| Surgical time (min) | 184.3 (97.6) | 160.7 (79.8) | 0.3 | 184.3 (97.6) | 185.5 (94.6) | 0.01 |
| Anesthetic parameter | ||||||
| Hypotension * | 154 (20.1%) | 938 (31.3%) | 0.24 | 154 (20.1%) | 149 (19.5%) | 0.016 |
| Hypothermia † | 253 (33%) | 1140 (38%) | 0.1 | 253 (33%) | 254 (33.2%) | 0.002 |
| Transfusion (mL) | 88.4 (451) | 46.9 (210) | 0.2 | 88.4 (451) | 88.8 (316) | <0.001 |
| Crystalloid (mL) | 1498 (790.2) | 1167.7 (652.8) | 0.5 | 1498 (790.2) | 1498 (850) | <0.001 |
| Colloid (mL) | 242.4 (394.8) | 248 (354.1) | 0.02 | 242.4 (394.8) | 271.2 (380.8) | 0.07 |
| Blood loss (mL) | 339.7 (598.9) | 341 (422) | 0.003 | 339.7 (598.9) | 366.1 (468.7) | 0.05 |
| Ephedrine | 628 (82%) | 2379 (79%) | 0.07 | 628 (82%) | 639 (83.4%) | 0.038 |
| Phenylephrine | 470 (61.4%) | 1649 (55%) | 0.13 | 470 (61.4%) | 472 (61.6%) | 0.005 |
| Norepinephrine | 94 (12.3%) | 272 (9.1%) | 0.11 | 94 (12.3%) | 94 (12.3%) | <0.001 |
| TIVA (n = 766) | Volatile (n = 766) | p-Value | |
|---|---|---|---|
| Total AKI | 8 (1%) | 32 (4.2%) | <0.001 * |
| AKI stage 1 | 7 (0.9%) | 32 (4.2%) | |
| AKI stage 2 | 1 (0.1%) | 0 (0%) |
| Variables | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| Odd Ratio (95% CI) | p-Value | Odd Ratio (95% CI) | p-Value | |
| Age over 70 years | 1.27 (0.88–1.81) | 0.2 | ||
| Male sex (vs. female) | 1.57 (1.09–2.27) | 0.02 * | 1.85 (1.18–3.06) | <0.001 |
| BMI | 0.99 (0.99–1.01) | 0.95 | ||
| ASA physical status | ||||
| 1 | ||||
| 2 | 3.02 (1.68–5.44) | <0.001 * | ||
| 3 + 4 | 5.35 (2.73–10.47) | <0.001 * | ||
| Comorbidities | ||||
| Hypertension | 3.17 (2.11–4.75) | <0.001 * | 2.48 (1.56–3.94) | <0.001 |
| Diabetes mellitus | 2.06 (1.43–2.96) | <0.001 * | ||
| Coronary disease | 1.54 (0.92–2.56) | 0.1 | ||
| Dyslipidemia | 0.83 (0.38–1.79) | 0.63 | ||
| Smoking | 1.56 (1.1–2.23) | 0.01 * | ||
| Anemia | 2.58 (1.78–3.76) | <0.001 * | 2.66 (1.76–4.04) | <0.001 |
| Medication | ||||
| NSAIDs | 0.82 (0.48–1.4) | 0.47 | ||
| ACEi/ARB | 1.51 (1.02–2.23) | 0.04 * | ||
| Diuretics | 1.9 (1.11–3.25) | 0.02 * | ||
| Surgical parameter | ||||
| Level count | 1.17 (1.01–1.35) | 0.04 * | ||
| Surgical time >3 h | 1.5 (1.05–2.14) | 0.025 * | ||
| Anesthetic parameter | ||||
| Hypotension † | 1.58 (1.1–2.28) | 0.01 * | ||
| Hypothermia ‡ | 1.09 (0.75–1.56) | 0.66 | ||
| Transfusion | 1.00 (0.99–1.00) | 0.15 | ||
| Crystalloid | 1.00 (0.99–1.00) | 0.12 | ||
| Colloid | 1.00 (0.99–1.00) | 0.89 | ||
| Estimated blood loss | 1.00 (0.99–1.00) | 0.35 | ||
| Ephedrine | 1.02 (0.65–1.59) | 0.94 | ||
| Phenylephrine | 0.94 (0.66–1.34) | 0.72 | ||
| Norepinephrine | 1.37 (0.8–2.34) | 0.25 | ||
| Anesthetic agent: volatile (vs. TIVA) | 3.88 (1.89–7.98) | <0.001 * | 4.69 (2.24–9.83) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Oh, A.-Y.; Koo, C.-H.; Bae, Y.K.; Jeon, Y.-T. Effects of Anesthetic Technique on the Occurrence of Acute Kidney Injury after Spine Surgery: A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 5653. https://doi.org/10.3390/jcm10235653
Han J, Oh A-Y, Koo C-H, Bae YK, Jeon Y-T. Effects of Anesthetic Technique on the Occurrence of Acute Kidney Injury after Spine Surgery: A Retrospective Cohort Study. Journal of Clinical Medicine. 2021; 10(23):5653. https://doi.org/10.3390/jcm10235653
Chicago/Turabian StyleHan, Jiwon, Ah-Young Oh, Chang-Hoon Koo, Yu Kyung Bae, and Yong-Tae Jeon. 2021. "Effects of Anesthetic Technique on the Occurrence of Acute Kidney Injury after Spine Surgery: A Retrospective Cohort Study" Journal of Clinical Medicine 10, no. 23: 5653. https://doi.org/10.3390/jcm10235653
APA StyleHan, J., Oh, A.-Y., Koo, C.-H., Bae, Y. K., & Jeon, Y.-T. (2021). Effects of Anesthetic Technique on the Occurrence of Acute Kidney Injury after Spine Surgery: A Retrospective Cohort Study. Journal of Clinical Medicine, 10(23), 5653. https://doi.org/10.3390/jcm10235653





