Parameters of Glucose Homeostasis in the Recognition of the Metabolic Syndrome in Young Adults with Prader–Willi Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Data
2.3. Blood Pressure Measurements and Instrumental Examinations
2.4. Laboratory Analyses
- -
- altered 1 h PG = glycemia >155 mg/dL (>8.6 mmol/L);
- -
- impaired glucose tolerance (IGT) = 2 h PG between 140 to 199 mg/dL (7.8 mmol/L to 11.0 mmol/L);
- -
- insulin resistance, evaluated by the use of the homeostasis model assessment: HOMA-IR = (insulin (mIU/L) × glucose (mg/dL))/405 (Matthews), at time 0, 60 and 120 min during OGTT;
- -
- insulin sensitivity, evaluated by the use of the homeostasis model assessment: HOMA-S = 1/HOMA-IR [18];
- -
- insulin secretion, assessed with the insulinogenic index (IGI), which is the ratio of the changes in insulin (I) and glucose (G) concentration from 0 to 30 min (ΔI0–30/ΔG0–30) [19];
2.5. Definitions
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef]
- Hanley, A.J.; Karter, A.J.; Festa, A.; D’Agostino, R., Jr.; Wagenknecht, L.E.; Savage, P.; Tracy, R.P.; Saad, M.F.; Haffner, S. Factor analysis of metabolic syndrome using directly measured insulin sensitivity: The Insulin Resistance Atherosclerosis Study. Diabetes 2002, 51, 2642–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Sartorio, A. Indexes of adiposity and body composition in the prediction of metabolic syndrome in obese children and adolescents: Which is the best? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Tamini, S.; Cicolini, S.; Sartorio, A. The role of different indexes of adiposity and body composition for the identification of metabolic syndrome in women with obesity. J. Clin. Med. 2021, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Nathan, D.M.; Wilson, P.W.; Cupples, L.A.; Singer, D.E. Metabolic risk factors worsen continuously across the spectrum of nondiabetic glucose tolerance. The Framingham Offspring Study. Ann. Intern. Med. 1998, 128, 524–533. [Google Scholar] [CrossRef]
- Park, S.K.; Ryoo, J.H.; Oh, C.M.; Choi, J.M.; Jung, J.Y. 1-Hour and 2-Hour postload glucose level on Oral Glucose Tolerance Test and the risk of incident metabolic syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 539–549. [Google Scholar] [PubMed]
- Brambilla, P.; Crinò, A.; Bedogni, G.; Bosio, L.; Cappa, M.; Corrias, A.; Delvecchio, M.; Di Candia, S.; Gargantini, L.; Grechi, E.; et al. Genetic Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology (ISPED). Metabolic syndrome in children with Prader-Willi syndrome: The effect of obesity. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 269–276. [Google Scholar]
- Grugni, G.; Crinò, A.; Bedogni, G.; Cappa, M.; Sartorio, A.; Corrias, A.; Di Candia, S.; Gargantini, L.; Iughetti, L.; Pagano, C.; et al. Metabolic syndrome in adult patients with Prader-Willi syndrome. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1134–1140. [Google Scholar] [CrossRef]
- Butler, M.G.; Hartin, S.N.; Hossain, W.A.; Manzardo, A.M.; Kimonis, V.; Dykens, E.; Gold, J.A.; Kim, S.J.; Weisensel, N.; Tamura, R.; et al. Molecular genetic classification in Prader-Willi syndrome: A multisite cohort study. J. Med. Genet. 2019, 56, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader–Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Theodoro, M.F.; Talebizadeh, Z.; Butler, M.G. Body composition and fatness patterns in Prader–Willi syndrome: Comparison with simple obesity. Obesity 2006, 14, 1685–1690. [Google Scholar] [CrossRef]
- Fintini, D.; Grugni, G.; Bocchini, S.; Brufani, C.; Di Candia, S.; Corrias, A.; Delvecchio, M.; Salvatoni, A.; Ragusa, L.; Greggio, N.; et al. Genetic Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology (ISPED). Disorders of glucose metabolism in Prader-Willi syndrome: Results of a multicenter Italian cohort study. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 842–847. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Formoso, G.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Colao, A.; RESTARE. Prader-Willi syndrome: An uptodate on endocrine and metabolic complications. Rev. Endocr. Metab. Disord. 2019, 20, 239–250. [Google Scholar] [CrossRef]
- Crinò, A.; Grugni, G. Update on diabetes mellitus and glucose metabolism alterations in Prader-Willi Syndrome. Curr. Diabetes Rep. 2020, 20, 7. [Google Scholar] [CrossRef]
- Proffitt, J.; Osann, K.; McManus, B.; Kimonis, V.E.; Heinemann, J.; Butler, M.G.; Stevenson, D.A.; Gold, J.A. Contributing factors of mortality in Prader-Willi syndrome. Am. J. Med. Genet. A 2019, 179, 196–205. [Google Scholar] [CrossRef]
- Pellikaan, K.; Rosenberg, A.G.W.; Kattentidt-Mouravieva, A.A.; Kersseboom, R.; Bos-Roubos, A.G.; Veen-Roelofs, J.M.C.; van Wieringen, N.; Hoekstra, F.M.E.; van den Berg, S.A.A.; van der Lely, A.J.; et al. Missed diagnoses and health problems in adults with Prader-Willi syndrome: Recommendations for screening and treatment. J. Clin. Endocrinol. Metab. 2020, 105, 4671–4687. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.G.; Roche, A.F.; Martorell, R. (Eds.) Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seltzer, H.S.; Allen, E.W.; Herron, A.L., Jr.; Brennan, M.T. Insulin secretion in response to glycemic stimulus: Relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J. Clin. Investig. 1967, 46, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjaarda, L.G.; Bacha, F.; Lee, S.; Tfayli, H.; Andreatta, E.; Arslanian, S. Oral disposition index in obese youth from normal to prediabetes to diabetes: Relationship to clamp disposition index. J. Pediatr. 2012, 161, 51–57. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Obesity and Overweight. 2011. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 2 May 2021).
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome. A Joint Interim Statement of the International Diabetes Federation Task Force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: An American Heart Association/ National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef]
- Chen, C. Growth Charts of Body Mass Index (BMI) with Quantile Regression. 2005. Available online: https://www.researchgate.net/profile/Colin-Chen-4/publication/220979218_Growth_Charts_of_Body_Mass_Index_BMI_With_Quantile_Regression/links/02bfe50ef9479c0a2f000000/Growth-Charts-of-Body-Mass-Index-BMI-With-Quantile-Regression.pdf (accessed on 1 May 2021).
- Cole, T.J.; Freeman, J.V.; Preece, M.A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat. Med. 1998, 17, 407–429. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Srinivasan, S.R.; Chen, W.; Malina, R.M.; Bouchard, C.; Berenson, G.S. Body Mass Index, Waist Circumference, and Clustering of Cardiovascular Disease Risk Factors in a Biracial Sample of Children and Adolescents. Pediatrics 2004, 114, e198–e205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacoricona Alfaro, D.L.; Lemoine, P.; Ehlinger, V.; Molinas, C.; Diene, G.; Valette, M.; Pinto, G.; Coupaye, M.; Poitou-Bernert, C.; Thuilleaux, D.; et al. Causes of death in Prader-Willi syndrome: Lessons from 11 years’ experience of a national reference center. Orphanet. J. Rare Dis. 2019, 14, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, M.G.; Manzardo, A.M.; Heinemann, J.; Loker, C.; Loker, J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet. Med. 2017, 19, 635–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Fronzo, R.A.; Ferrannini, E. Insulin resistance: A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991, 14, 173–194. [Google Scholar] [CrossRef]
- Radetti, G.; Fanolla, A.; Lupi, F.; Sartorio, A.; Grugni, G. Accuracy of different indexes of body composition and adiposity in identifying metabolic syndrome in adult subjects with Prader-Willi syndrome. J. Clin. Med. 2020, 9, 1646. [Google Scholar] [CrossRef]
| Total | MetS− | MetS+ | |
|---|---|---|---|
| Clinical data | |||
| Number of subjects | 102 | 73 | 29 |
| Age (years) | 26.9 ± 7.6 | 26.5 ± 7.5 | 28.1 ± 7.9 |
| Female sex, n (%) | 53 (52%) | 38 (52%) | 15 (51.7%) |
| BMI (kg/m2) | 35.7 ± 10.7 | 33.6 ± 9.8 | 41.1 ± 11.2 * |
| WC (cm) | 106 ± 18.3 | 102.2 ± 16.8 | 115.6 ± 18.9 ° |
| WC (cm) females | 103.3 ± 19.6 (no. 53) | 99.1 ± 17.1 (no. 38) | 113.9 ± 21.9 # (no. 15) |
| WC (cm) males | 108.9 ± 16.6 (no. 49) | 105.5 ± 15.9 (no. 35) | 117.4 ± 15.7 # (no. 14) |
| SBP (mm/Hg) | 119.6 ± 8.8 | 117.0 ± 8.0 | 126.2 ± 7.3 § |
| DBP (mm/Hg) | 75.8 ± 6.5 | 74.7 ± 6.4 | 78.6 ± 6.0 * |
| Laboratory data | |||
| HDL-C (mg/dL) | 49.7 ± 12.4 | 52.8 ± 12.3 | 42.0 ± 9.2 § |
| HDL-C (mg/dL) females | 53.2 ± 12.5 (no. 53) | 56.1 ± 12.6 (no. 38) | 45.8 ± 9.1 * (no. 15) |
| HDL-C (mg/dL) males | 46.0 ± 11.3 (no. 49) | 49.2 ± 11.0 (no. 35) | 37.9 ± 7.7 * (no. 14) |
| TG (mg/dL) | 95.1 ± 43.1 | 87.1 ± 32.6 | 115.2 ± 58.2 * |
| glycemia (mg/dL) T0 | 84.6 ± 9.6 | 82.4 ± 7.7 | 90.3 ± 11.5 § |
| insulin (mIU/L) | 9.9 ± 7.3 | 8.5 ± 5.8 | 13.5 ± 9.2 ° |
| HbA1c (%) | 5.4 ± 0.4 | 5.3 ± 0.3 | 5.7 ± 0.5 § |
| Metabolic indices | |||
| glycemia (mg/dL) T60 | 137.2 ± 33.7 | 131.8 ± 30.2 | 150.9 ± 38.5 * |
| glycemia (mg/dL) T120 | 125.0 ± 32.6 | 118.3 ± 27.4 | 141.7 ± 38.7 ° |
| HOMA-IR T0 | 2.15 ± 1.70 | 1.79 ± 1.32 | 3.07 ± 2.16 ° |
| HOMA-IR T60 | 22.86 ± 25.74 | 20.27 ± 16.59 | 29.40 ± 40.25 |
| HOMA-IR T120 | 22.22 ± 20.87 | 18.95 ± 18.25 | 30.45 ± 24.82 # |
| IGI | 0.86 ± 1.62 | 0.76 ± 1.85 | 1.09 ± 0.78 |
| ODI | 0.53 ± 1.99 | 0.48 ± 2.26 | 0.66 ± 1.03 |
| Glycemic Response at 1 h | Glycemic Response at 2 h | |||
|---|---|---|---|---|
| Normal | Altered | Normal | Altered | |
| Number of subjects | 75 | 27 | 72 | 30 |
| Age (years) | 27.2 ± 7.8 | 26.2 ± 7.3 | 26.8 ± 7.2 | 27.2 ± 8.6 |
| Female/male | 37/38 | 16/11 | 36/36 | 17/13 |
| BMI (kg/m2) | 35.1 ± 10.7 | 37.4 ± 10.6 | 35.4 ± 10.8 | 36.4 ± 10.7 |
| WC (cm) | 105.0 ± 18.1 | 108.7 ± 19 | 105.7 ± 18.8 | 106.6 ± 17.6 |
| SBP (mmHg) | 118.8 ± 9.1 | 121.9 ± 7.9 | 118.6 ± 8.9 | 122.0 ± 8.5 |
| DPB (mmHg) | 75.5 ± 6.8 | 76.5 ± 5.5 | 75.6 ± 6.4 | 76.2 ± 6.7 |
| HDL-C (mg/dL) | 49.3 ± 12.5 | 50.8 ± 12.4 | 49.3 ± 12.7 | 50.7 ± 12 |
| TG (mg/dL) | 91.7 ± 44.2 | 104.7 ± 39.2 | 89.3 ± 41.4 | 108.9 ± 44.6 # |
| 1 h PG | 121.2 ± 20.5 | 181.6 ± 20.7 § | 124.1 ± 26.3 | 168.7 ± 28.6 § |
| 2 h PG | 112.8 ± 22.7 | 158.7 ± 32.2 § | 108.9 ± 20.1 | 163.4 ± 23 § |
| HbA1c (%) | 5.38 ± 0.34 | 5.59 ± 0.55 | 5.4 ± 0.4 | 5.6 ± 0.4 * |
| HOMA-IR T0 | 2.0 ± 1.5 | 2.7 ± 2.2 | 1.8 ± 1.4 | 2.9 ± 2 # |
| HOMA-IR T60 | 16.2 ± 11.9 | 41.5 ± 41 | 17.9 ± 14.6 | 34.9 ± 39.7 # |
| HOMA-IR T120 | 15.76 ± 14.48 | 40.17 ± 25.3 | 13.25 ± 8.04 | 43.74 ± 26.06 § |
| IGI | 0.89 ± 1.87 | 0.75 ± 0.42 | 0.85 ± 1.89 | 0.87 ± 0.61 |
| ODI | 0.57 ± 2.31 | 0.43 ± 0.36 | 0.60 ± 2.36 | 0.38 ± 0.26 |
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| IGI | 57.1% (47.9–66.4) | 61.1% (52.0–70.3) | 36.4% (27.3–45.4) | 78.6% (70.9–86.3) |
| ODI | 53.6% (44.2–62.9) | 70.8% $ (62.3–79.4) | 41.7% (32.4–50.9) | 79.7% (72.1–87.2) |
| HOMA-IR T0 | 69.0% (60.3–77.7) | 68.5% (59.8–77.2) | 46.5% (37.1–55.9) | 84.7% (78.0–91.5) |
| HOMA-IR T60 | 72.4% * (64.0–80.8) | 50.7% (41.3–60.1) | 36.8% (27.8–45.9) | 82.2% (75.0–89.4) |
| HOMA-IR T120 | 58.6% (49.4–67.9) | 78.1% £,& (70.3–85.5) | 51.5% (42.1–60.9) | 82.6% (75.5–89.7) |
| AUC | Cut-Off | |
|---|---|---|
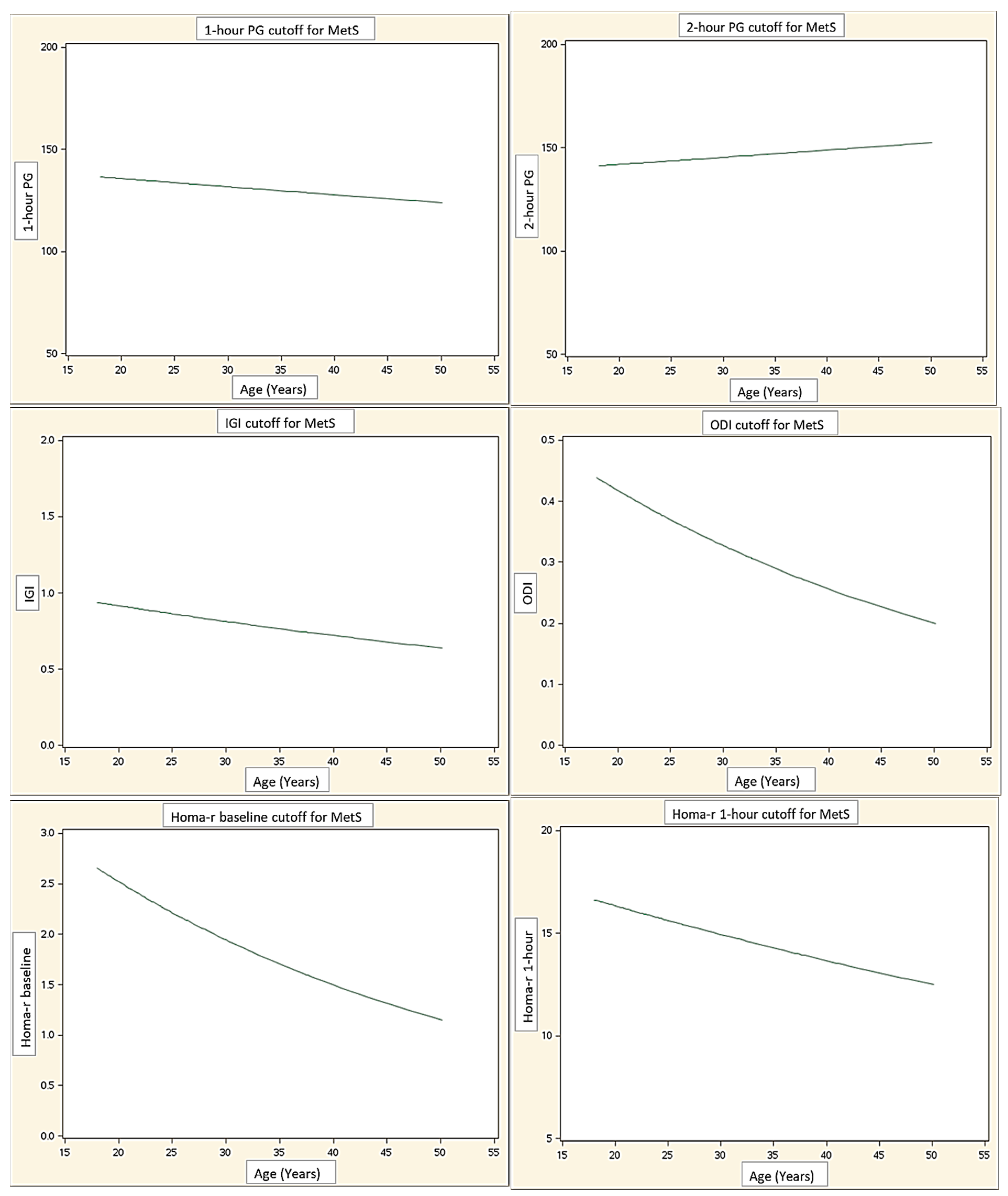
| 1 h PG | 0.65 (0.52–0.77) | 131.1 |
| 2 h PG | 0.70 (0.57–0.82) | 141.0 |
| HOMA-IR T0 | 0.69 (0.57–0.81) | 2.0 |
| HOMA-IR T60 | 0.60 (0.48–0.72) | 14.6 |
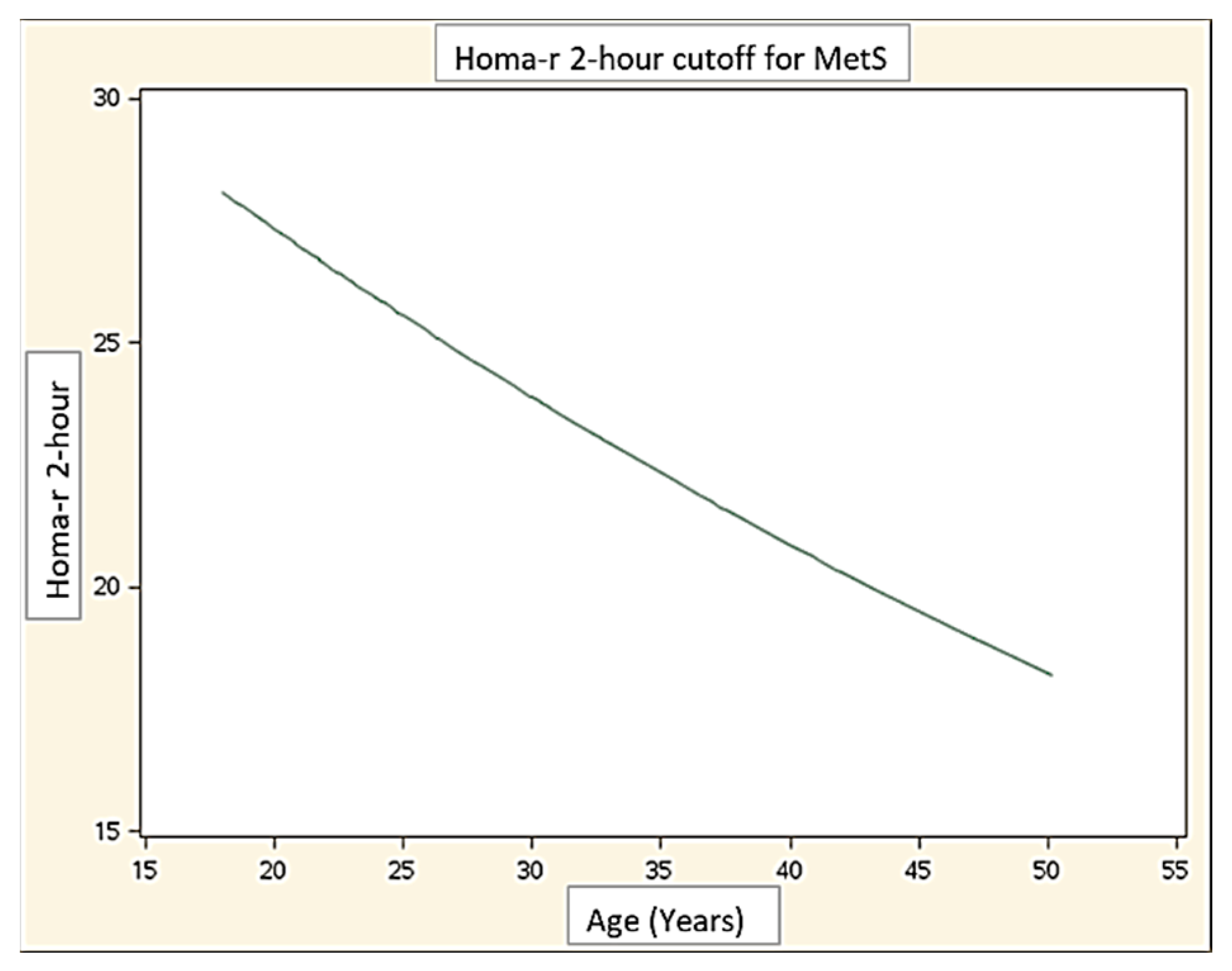
| HOMA-IR T120 | 0.68 (0.56–0.80) | 26.1 |
| IGI | 0.59 (0.47–0.71) | 0.8 |
| ODI | 0.60 (0.47–0.73) | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grugni, G.; Fanolla, A.; Lupi, F.; Longhi, S.; Saezza, A.; Sartorio, A.; Radetti, G. Parameters of Glucose Homeostasis in the Recognition of the Metabolic Syndrome in Young Adults with Prader–Willi Syndrome. J. Clin. Med. 2021, 10, 5635. https://doi.org/10.3390/jcm10235635
Grugni G, Fanolla A, Lupi F, Longhi S, Saezza A, Sartorio A, Radetti G. Parameters of Glucose Homeostasis in the Recognition of the Metabolic Syndrome in Young Adults with Prader–Willi Syndrome. Journal of Clinical Medicine. 2021; 10(23):5635. https://doi.org/10.3390/jcm10235635
Chicago/Turabian StyleGrugni, Graziano, Antonio Fanolla, Fiorenzo Lupi, Silvia Longhi, Antonella Saezza, Alessandro Sartorio, and Giorgio Radetti. 2021. "Parameters of Glucose Homeostasis in the Recognition of the Metabolic Syndrome in Young Adults with Prader–Willi Syndrome" Journal of Clinical Medicine 10, no. 23: 5635. https://doi.org/10.3390/jcm10235635
APA StyleGrugni, G., Fanolla, A., Lupi, F., Longhi, S., Saezza, A., Sartorio, A., & Radetti, G. (2021). Parameters of Glucose Homeostasis in the Recognition of the Metabolic Syndrome in Young Adults with Prader–Willi Syndrome. Journal of Clinical Medicine, 10(23), 5635. https://doi.org/10.3390/jcm10235635







