Efficacy and Safety of Different Formulations of Calcipotriol/Betamethasone Dipropionate in Psoriasis: Gel, Foam, and Ointment
Abstract
:1. Introduction
2. Pharmacodynamics of Calcipotriol/Betamethasone Dipropionate
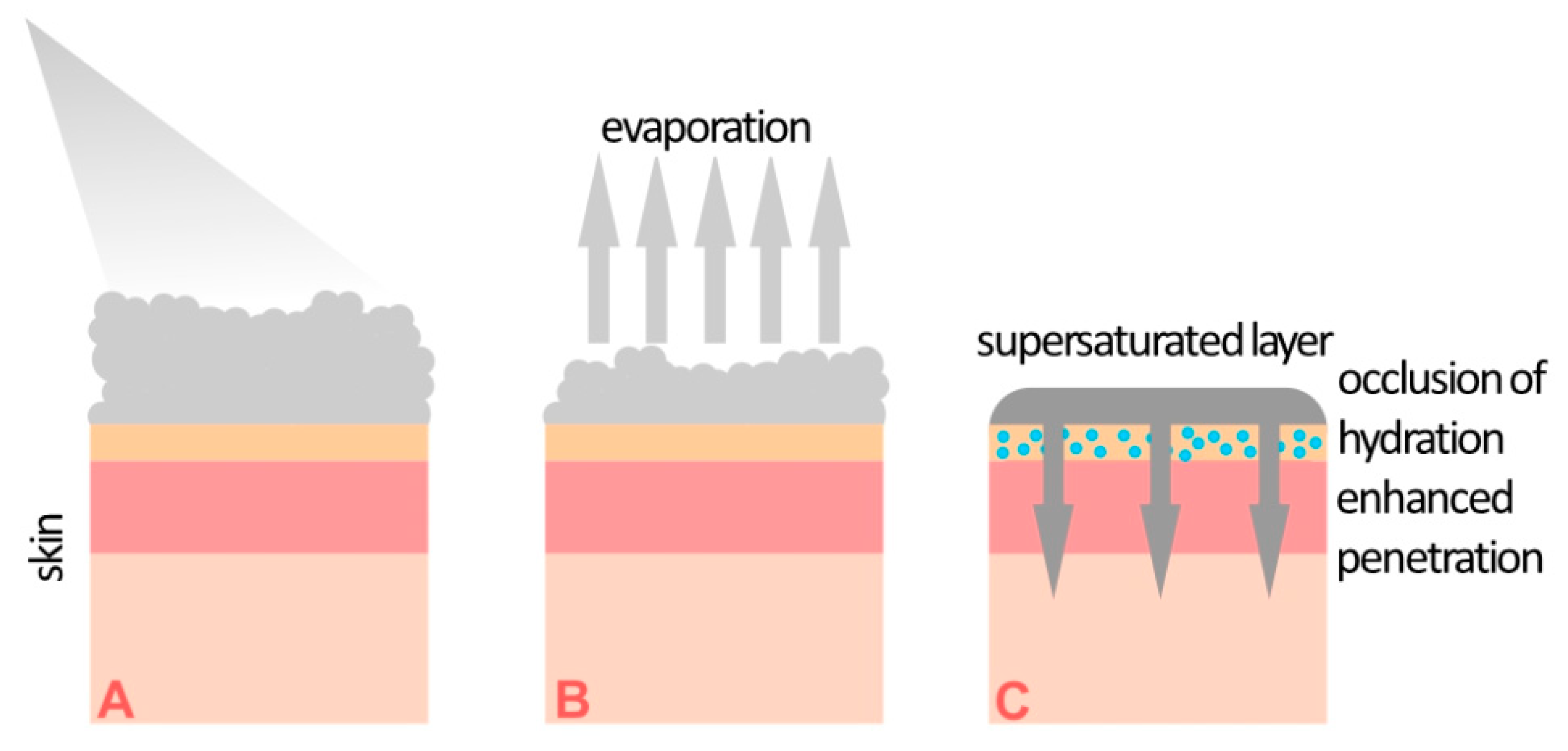
3. Supersaturated Foam Formulation of Calcipotriol/Betamethasone
4. Comparison of Foam and Ointment
5. Comparison of Foam and Gel
6. Special Locations
7. Adverse Effects
8. Calcipotriol/Betamethasone Foam as a Formulation Bridging the Gap between Topical and Systemic Treatment in Psoriasis
9. Proactive Maintenance Treatment
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Elmets, C.A.; Korman, N.J.; Prater, E.F.; Wong, E.B.; Rupani, R.N.; Kivelevitch, D.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J. Am. Acad. Dermatol. 2021, 84, 432–470. [Google Scholar] [CrossRef]
- Carrascosa, J.M.; Theng, C.; Thaci, D. Spotlight on Topical Long-Term Management of Plaque Psoriasis. Clin. Cosmet. Investig. Dermatol. 2020, 13, 495–498. [Google Scholar] [CrossRef]
- Reich, A.; Adamski, Z.; Chodorowska, G.; Kaszuba, A.; Krasowska, D.; Lesiak, A.; Maj, J.; Narbutt, J.; Osmola-Mańkowska, A.J.; Owczarczyk-Saczonek, A.; et al. Psoriasis. Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Part 1. Dermatol. Rev. Przegląd Dermatol. 2020, 107, 92–108. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Pimpinelli, N.; Ricceri, F.; Bagnoni, G.; Bartoli, L.; Bellini, M.; Brandini, L.; Caproni, M.; Castelli, A.; Fimiani, M.; et al. Treatment of psoriasis with topical agents: Recommendations from a Tuscany Consensus. Dermatol. Ther. 2017, 30. [Google Scholar] [CrossRef]
- Thaci, D.; de la Cueva, P.; Pink, A.E.; Jalili, A.; Segaert, S.; Hjuler, K.F.; Calzavara-Pinton, P. General practice recommendations for the topical treatment of psoriasis: A modified-Delphi approach. BJGP Open 2020, 4. [Google Scholar] [CrossRef]
- Kleyn, E.C.; Morsman, E.; Griffin, L.; Wu, J.J.; Cm van de Kerkhof, P.; Gulliver, W.; van der Walt, J.M.; Iversen, L. Review of international psoriasis guidelines for the treatment of psoriasis: Recommendations for topical corticosteroid treatments. J. Dermatolog. Treat. 2019, 30, 311–319. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Spotlight on calcipotriene/betamethasone dipropionate in psoriasis vulgaris of the trunk, limbs, and scalp. Am. J. Clin. Dermatol. 2011, 12, 421–424. [Google Scholar] [CrossRef] [PubMed]
- McCormack, P.L. Calcipotriol/betamethasone dipropionate: A review of its use in the treatment of psoriasis vulgaris of the trunk, limbs and scalp. Drugs 2011, 71, 709–730. [Google Scholar] [CrossRef] [PubMed]
- Stein Gold, L.; Bagel, J.; Allenby, K.; Sidgiddi, S. Betamethasone dipropionate spray 0.05% alleviates troublesome symptoms of plaque psoriasis. Cutis 2020, 105, 97–102.e101. [Google Scholar]
- Patel, N.U.; Felix, K.; Reimer, D.; Feldman, S.R. Calcipotriene/betamethasone dipropionate for the treatment of psoriasis vulgaris: An evidence-based review. Clin. Cosmet. Investig. Dermatol. 2017, 10, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Traulsen, J. Bioavailability of betamethasone dipropionate when combined with calcipotriol. Int. J. Dermatol. 2004, 43, 611–617. [Google Scholar] [CrossRef]
- Grän, F.; Kerstan, A.; Serfling, E.; Goebeler, M.; Muhammad, K. Current Developments in the Immunology of Psoriasis. Yale J. Biol. Med. 2020, 93, 97–110. [Google Scholar]
- Ogawa, K.; Okada, Y. The current landscape of psoriasis genetics in 2020. J. Dermatol. Sci. 2020, 99, 2–8. [Google Scholar] [CrossRef]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef] [Green Version]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [Green Version]
- Satake, K.; Amano, T.; Okamoto, T. Calcipotriol and betamethasone dipropionate synergistically enhances the balance between regulatory and proinflammatory T cells in a murine psoriasis model. Sci. Rep. 2019, 9, 16322. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, B.; Shear, N.H. The Evolution of Topical Formulations in Psoriasis. Skin Ther. Lett. 2018, 23, 5–9. [Google Scholar]
- Wollina, U.; Tirant, M.; Vojvodic, A.; Lotti, T. Treatment of Psoriasis: Novel Approaches to Topical Delivery. Open Access Maced. J. Med. Sci. 2019, 7, 3018–3025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, M.; Nielsen, K.T.; Schefe, L.H.; Nørremark, K.; Eriksson, A.H.; Norsgaard, H.; Pedersen, B.T.; Petersson, K. Supersaturation of Calcipotriene and Betamethasone Dipropionate in a Novel Aerosol Foam Formulation for Topical Treatment of Psoriasis Provides Enhanced Bioavailability of the Active Ingredients. Dermatol. Ther. (Heidelb) 2016, 6, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Paul, C.; Bang, B.; Lebwohl, M. Fixed combination calcipotriol plus betamethasone dipropionate aerosol foam in the treatment of psoriasis vulgaris: Rationale for development and clinical profile. Expert Opin. Pharmacother. 2017, 18, 115–121. [Google Scholar] [CrossRef]
- Puig, L.; Carretero, G. Update on Topical Treatments for Psoriasis: The Role of Calcipotriol Plus Betamethasone Dipropionate Aerosol Foam. Actas Dermosifiliogr. 2019, 110, 115–123. [Google Scholar] [CrossRef]
- Megna, M.; Cinelli, E.; Camela, E.; Fabbrocini, G. Calcipotriol/betamethasone dipropionate formulations for psoriasis: An overview of the options and efficacy data. Expert Rev. Clin. Immunol. 2020, 16, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Tyring, S.; Werschler, W.P.; Bruce, S.; Olesen, M.; Villumsen, J.; Bagel, J. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris--A randomized phase II study. J. Dermatolog. Treat. 2016, 27, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Queille-Roussel, C.; Bang, B.; Clonier, F.; Lacour, J.P. Enhanced vasoconstrictor potency of the fixed combination calcipotriol plus betamethasone dipropionate in an innovative aerosol foam formulation vs. other corticosteroid psoriasis treatments. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1951–1956. [Google Scholar] [CrossRef] [Green Version]
- Duvetorp, A.; Levin, L.A.; Engerstedt Mattsson, E.; Ryttig, L. A Cost-utility Analysis of Calcipotriol/Betamethasone Dipropionate Aerosol Foam versus Ointment for the Topical Treatment of Psoriasis Vulgaris in Sweden. Acta Derm. Venereol. 2019, 99, 393–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, C.; Stein Gold, L.; Cambazard, F.; Kalb, R.E.; Lowson, D.; Bang, B.; Griffiths, C.E. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: Randomized, controlled PSO-ABLE study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.E.; Stein Gold, L.; Cambazard, F.; Kalb, R.E.; Lowson, D.; Moller, A.; Paul, C. Greater improvement in quality of life outcomes in patients using fixed-combination calcipotriol plus betamethasone dipropionate aerosol foam versus gel: Results from the PSO-ABLE study. Eur. J. Dermatol. 2018, 28, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Papp, K.A.; Lophaven, K.W.; Skallerup, P.; Philipp, S. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: Randomized phase IIIb PSO-INSIGHTFUL study. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1876–1883. [Google Scholar] [CrossRef]
- Rigopoulos, D.; Gregoriou, S.; Daniel Iii, C.R.; Belyayeva, H.; Larios, G.; Verra, P.; Stamou, C.; Kontochristopoulos, G.; Avgerinou, G.; Katsambas, A. Treatment of nail psoriasis with a two-compound formulation of calcipotriol plus betamethasone dipropionate ointment. Dermatology 2009, 218, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Saki, N.; Hosseinpoor, S.; Heiran, A.; Mohammadi, A.; Zeraatpishe, M. Comparing the Efficacy of Triamcinolone Acetonide Iontophoresis versus Topical Calcipotriol/Betamethasone Dipropionate in Treating Nail Psoriasis: A Bilateral Controlled Clinical Trial. Dermatol. Res. Pract. 2018, 2018, 2637691. [Google Scholar] [CrossRef] [Green Version]
- Gregoriou, S.; Sidiropoulou, P.; Tsimpidakis, A.; Rompoti, N.; Tsironi, T.; Panagakis, P.; Polydorou, D.; Kostakis, P.; Rigopoulos, D. Treatment of nail psoriasis with calcipotriol/betamethasone dipropionate foam versus pulse dye laser: An unblinded, intra-patient, left-to-right prospective study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e519–e520. [Google Scholar] [CrossRef] [PubMed]
- Takama, H.; Ando, Y.; Yanagishita, T.; Ohshima, Y.; Akiyama, M.; Watanabe, D. Two cases of refractory nail psoriasis successfully treated with calcipotriol plus betamethasone dipropionate gel. J. Dermatol. 2020, 47, e211–e213. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, C.; Wang, J.; Chen, K.; Ding, Y.; Yan, G.; Lu, Q.; Li, W.; Fang, H.; Cheng, H.; et al. Comparison of safety and efficacy between calcipotriol plus betamethasone dipropionate gel and calcipotriol scalp solution as long-term treatment for scalp psoriasis in Chinese patients: A national, multicentre, prospective, randomized, active-controlled phase 4 trial. Eur. J. Dermatol. 2020, 30, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Gual, A.; Pau-Charles, I.; Molin, S. Topical treatment for scalp psoriasis: Comparison of patient preference, quality of life and efficacy for non-alcoholic mometasone emulsion versus calcipotriol/betamethasone gel in daily clinical practice. J. Dermatolog. Treat. 2016, 27, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Q.; Yang, H.; Wang, G.; Zheng, M.; Hao, F.; Gu, J.; Sun, Q.; Cui, P.; Ge, M.; et al. Calcipotriol plus betamethasone dipropionate gel compared with calcipotriol scalp solution in the treatment of scalp psoriasis: A randomized, controlled trial investigating efficacy and safety in a Chinese population. Int. J. Dermatol. 2016, 55, 106–113. [Google Scholar] [CrossRef]
- Saraceno, R.; Camplone, G.; D’Agostino, M.; De Simone, C.; Di Cesare, A.; Filosa, G.; Frascione, P.; Gabellini, M.; Lunghi, F.; Mazzotta, A.; et al. Efficacy and maintenance strategies of two-compound formulation calcipotriol and betamethasone dipropionate gel (Xamiol(R) gel) in the treatment of scalp psoriasis: Results from a study in 885 patients. J. Dermatolog. Treat. 2014, 25, 30–33. [Google Scholar] [CrossRef]
- Bottomley, J.M.; Taylor, R.S.; Ryttov, J. The effectiveness of two-compound formulation calcipotriol and betamethasone dipropionate gel in the treatment of moderately severe scalp psoriasis: A systematic review of direct and indirect evidence. Curr. Med. Res. Opin. 2011, 27, 251–268. [Google Scholar] [CrossRef]
- Petersen, B.; Lebwohl, M. Treating Scalp Psoriasis with Calcipotriene/Betamethasone Dipropionate Fixed-dose Combination Cutaneous Foam: Review of Phase 2 Data. J. Drugs Dermatol. 2020, 19, 784–786. [Google Scholar] [CrossRef]
- Schlager, J.G.; Rosumeck, S.; Werner, R.N.; Jacobs, A.; Schmitt, J.; Schlager, C.; Nast, A. Topical treatments for scalp psoriasis: Summary of a Cochrane Systematic Review. Br. J. Dermatol. 2017, 176, 604–614. [Google Scholar] [CrossRef]
- Downs, A.M. Dovobet ointment under occlusion overnight for troublesome scalp psoriasis. Acta Derm. Venereol. 2006, 86, 57–58. [Google Scholar] [CrossRef]
- Beck, K.M.; Yang, E.J.; Sanchez, I.M.; Liao, W. Treatment of Genital Psoriasis: A Systematic Review. Dermatol. Ther. 2018, 8, 509–525. [Google Scholar] [CrossRef] [Green Version]
- Amat-Samaranch, V.; Puig, L. Safety of calcipotriene and betamethasone dipropionate foam for the treatment of psoriasis. Expert Opin. Drug Saf. 2020, 19, 423–432. [Google Scholar] [CrossRef]
- Bagel, J.; Nelson, E.; Zapata, J.; Hetzel, A. Adjunctive Use of Calcipotriene/Betamethasone Dipropionate Foam in a Real-World Setting Curtails the Cost of Biologics Without Reducing Efficacy in Psoriasis. Dermatol. Ther. (Heidelb) 2020, 10, 1383–1396. [Google Scholar] [CrossRef]
- Bewley, A.P.; Shear, N.H.; Calzavara-Pinton, P.G.; Hansen, J.B.; Nyeland, M.E.; Signorovitch, J. Calcipotriol plus betamethasone dipropionate aerosol foam vs. apremilast, methotrexate, acitretin or fumaric acid esters for the treatment of plaque psoriasis: A matching-adjusted indirect comparison. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Balak, D.M.W.; Carrascosa, J.M.; Gregoriou, S.; Calzavara-Pinton, P.; Bewley, A.; Antunes, J.; Nyeland, M.E.; Viola, M.G.; Sawyer, L.M.; Becla, L. Cost per PASI-75 responder of calcipotriol plus betamethasone dipropionate cutaneous foam versus nonbiologic systemic therapies for the treatment of plaque psoriasis in seven European countries. J. Dermatolog. Treat. 2020, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bagel, J.; Nelson, E.; Riley, C.; Hetzel, A. Apremilast with Add-On Calcipotriene/Betamethasone Dipropionate for Treating Moderate to Severe Plaque Psoriasis. J. Drugs Dermatol. 2020, 19, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.; Stein Gold, L.; Teng, J.; Moore, A.; Cantrell, W.; Alonso-Llamazares, J.; Koo, J. Fixed Combination Calcipotriene and Betamethasone Dipropionate (Cal/BD) Foam for Beyond-Mild Psoriasis: A Possible Alternative to Systemic Medication. J. Drugs Dermatol. 2020, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Cho, H.H.; Kim, W.J.; Mun, J.H.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, B.S.; Kim, M.B. Efficacy and Safety of Calcipotriol/Betamethasone Dipropionate Ointment for the Treatment of Trachyonychia: An Open-Label Study. Ann. Dermatol. 2015, 27, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Lebwohl, M.; Kircik, L.; Lacour, J.P.; Liljedahl, M.; Lynde, C.; Morch, M.H.; Papp, K.A.; Perrot, J.L.; Gold, L.S.; Takhar, A.; et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial). J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stein Gold, L.; Alonso-Llamazares, J.; Lacour, J.P.; Warren, R.B.; Tyring, S.K.; Kircik, L.; Yamauchi, P.; Lebwohl, M.; Investigators, P.-L.T. PSO-LONG: Design of a Novel, 12-Month Clinical Trial of Topical, Proactive Maintenance with Twice-Weekly Cal/BD Foam in Psoriasis. Adv. Ther. 2020, 37, 4730–4753. [Google Scholar] [CrossRef] [PubMed]
- Grajdeanu, I.A.; Statescu, L.; Vata, D.; Popescu, I.A.; Porumb-Andrese, E.; Patrascu, A.I.; Taranu, T.; Crisan, M.; Solovastru, L.G. Imaging techniques in the diagnosis and monitoring of psoriasis. Exp. Ther. Med. 2019, 18, 4974–4980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errichetti, E.; Croatto, M.; Arnoldo, L.; Stinco, G. Plaque-Type Psoriasis Treated with Calcipotriene Plus Betamethasone Dipropionate Aerosol Foam: A Prospective Study on Clinical and Dermoscopic Predictor Factors in Response Achievement and Retention. Dermatol. Ther. (Heidelb) 2020, 10, 757–767. [Google Scholar] [CrossRef] [PubMed]
| Differences between Calcipotriol/Betamethasone Gel, Foam, and Ointment |
|---|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnicka, L.; Olszewska, M.; Goldust, M.; Waśkiel-Burnat, A.; Warszawik-Hendzel, O.; Dorożyński, P.; Turło, J.; Rakowska, A. Efficacy and Safety of Different Formulations of Calcipotriol/Betamethasone Dipropionate in Psoriasis: Gel, Foam, and Ointment. J. Clin. Med. 2021, 10, 5589. https://doi.org/10.3390/jcm10235589
Rudnicka L, Olszewska M, Goldust M, Waśkiel-Burnat A, Warszawik-Hendzel O, Dorożyński P, Turło J, Rakowska A. Efficacy and Safety of Different Formulations of Calcipotriol/Betamethasone Dipropionate in Psoriasis: Gel, Foam, and Ointment. Journal of Clinical Medicine. 2021; 10(23):5589. https://doi.org/10.3390/jcm10235589
Chicago/Turabian StyleRudnicka, Lidia, Małgorzata Olszewska, Mohamad Goldust, Anna Waśkiel-Burnat, Olga Warszawik-Hendzel, Przemysław Dorożyński, Jadwiga Turło, and Adriana Rakowska. 2021. "Efficacy and Safety of Different Formulations of Calcipotriol/Betamethasone Dipropionate in Psoriasis: Gel, Foam, and Ointment" Journal of Clinical Medicine 10, no. 23: 5589. https://doi.org/10.3390/jcm10235589
APA StyleRudnicka, L., Olszewska, M., Goldust, M., Waśkiel-Burnat, A., Warszawik-Hendzel, O., Dorożyński, P., Turło, J., & Rakowska, A. (2021). Efficacy and Safety of Different Formulations of Calcipotriol/Betamethasone Dipropionate in Psoriasis: Gel, Foam, and Ointment. Journal of Clinical Medicine, 10(23), 5589. https://doi.org/10.3390/jcm10235589









