Craniofacial Osteomas: From Diagnosis to Therapy
Abstract
1. Introduction and Pathogenesis
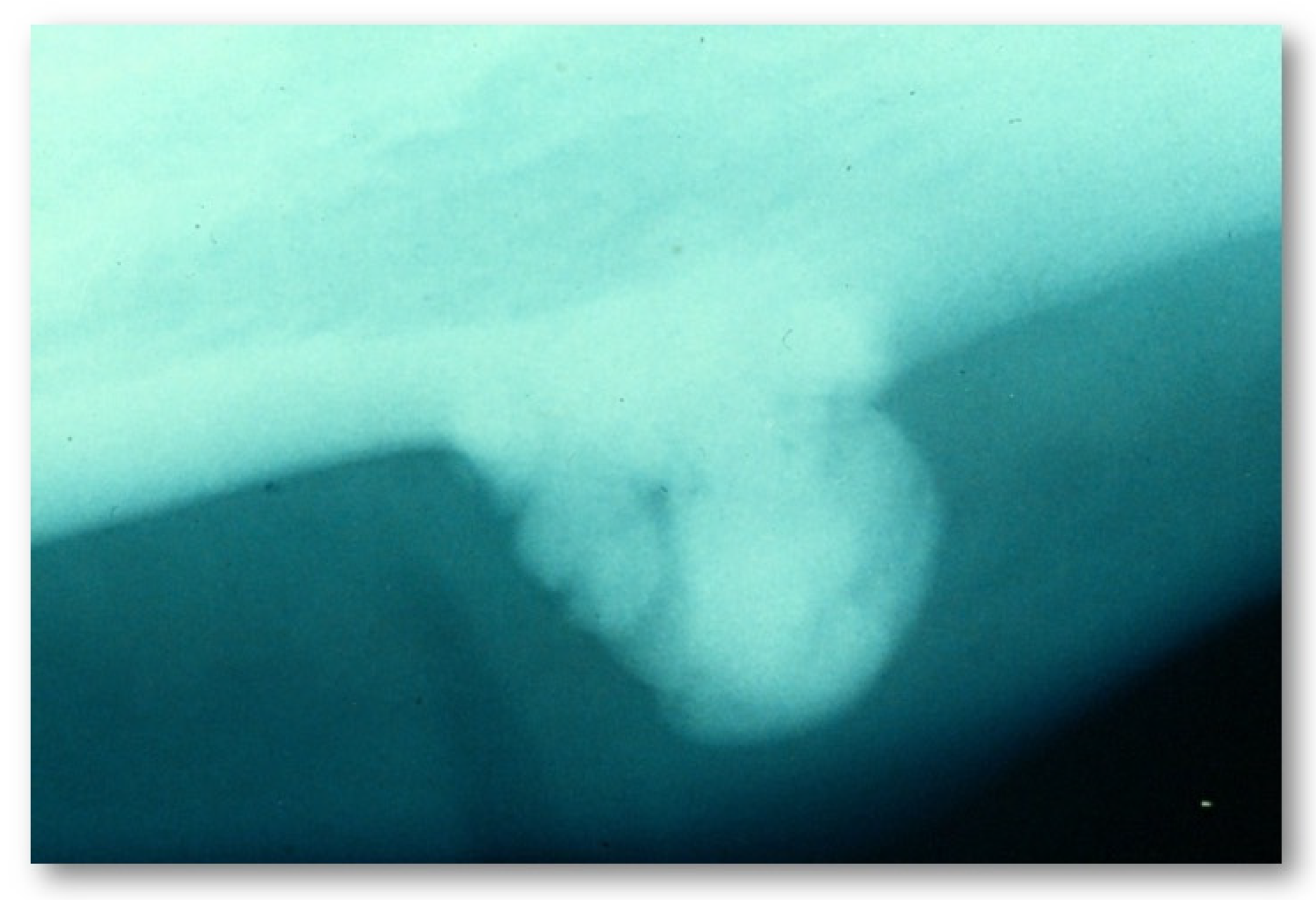
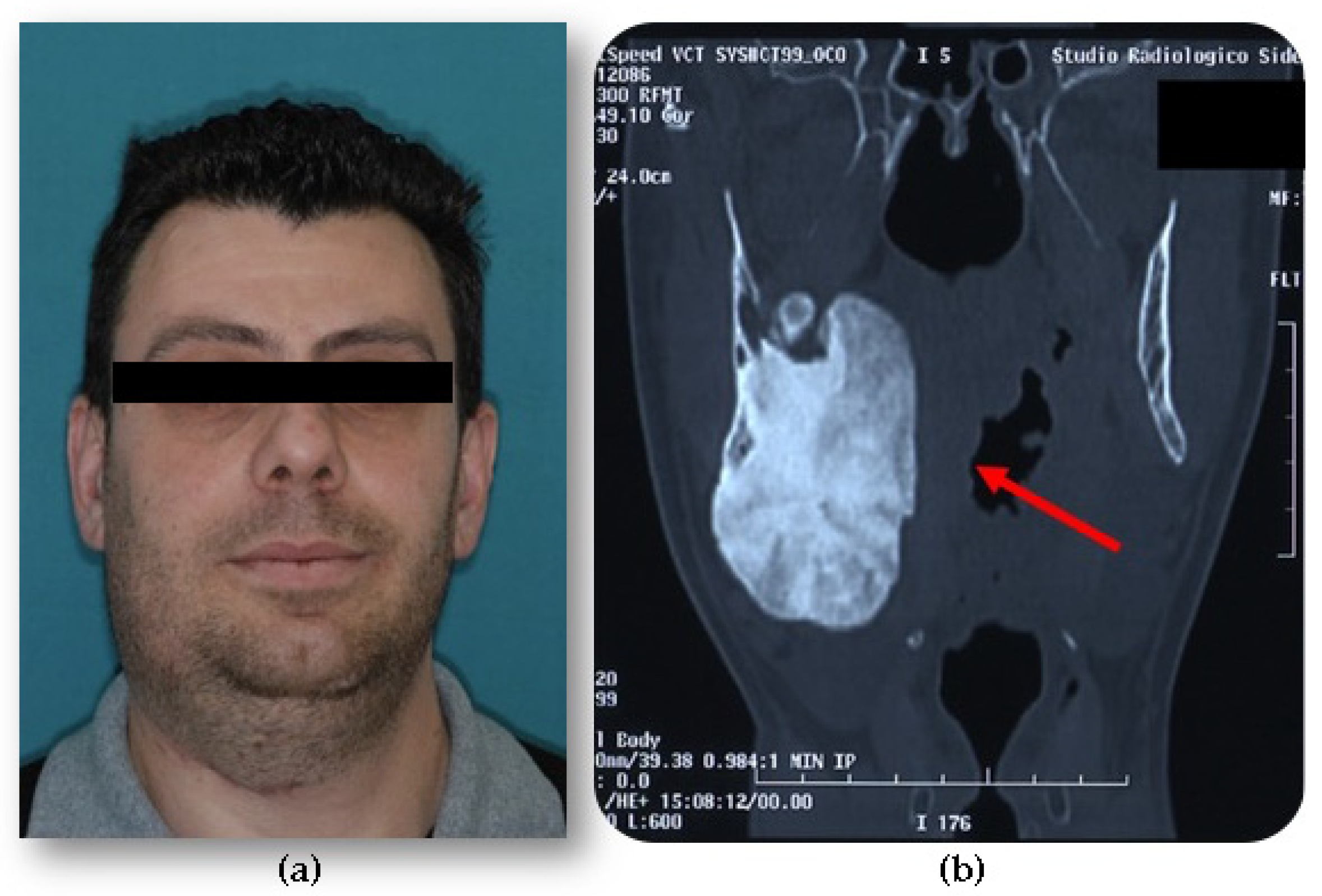
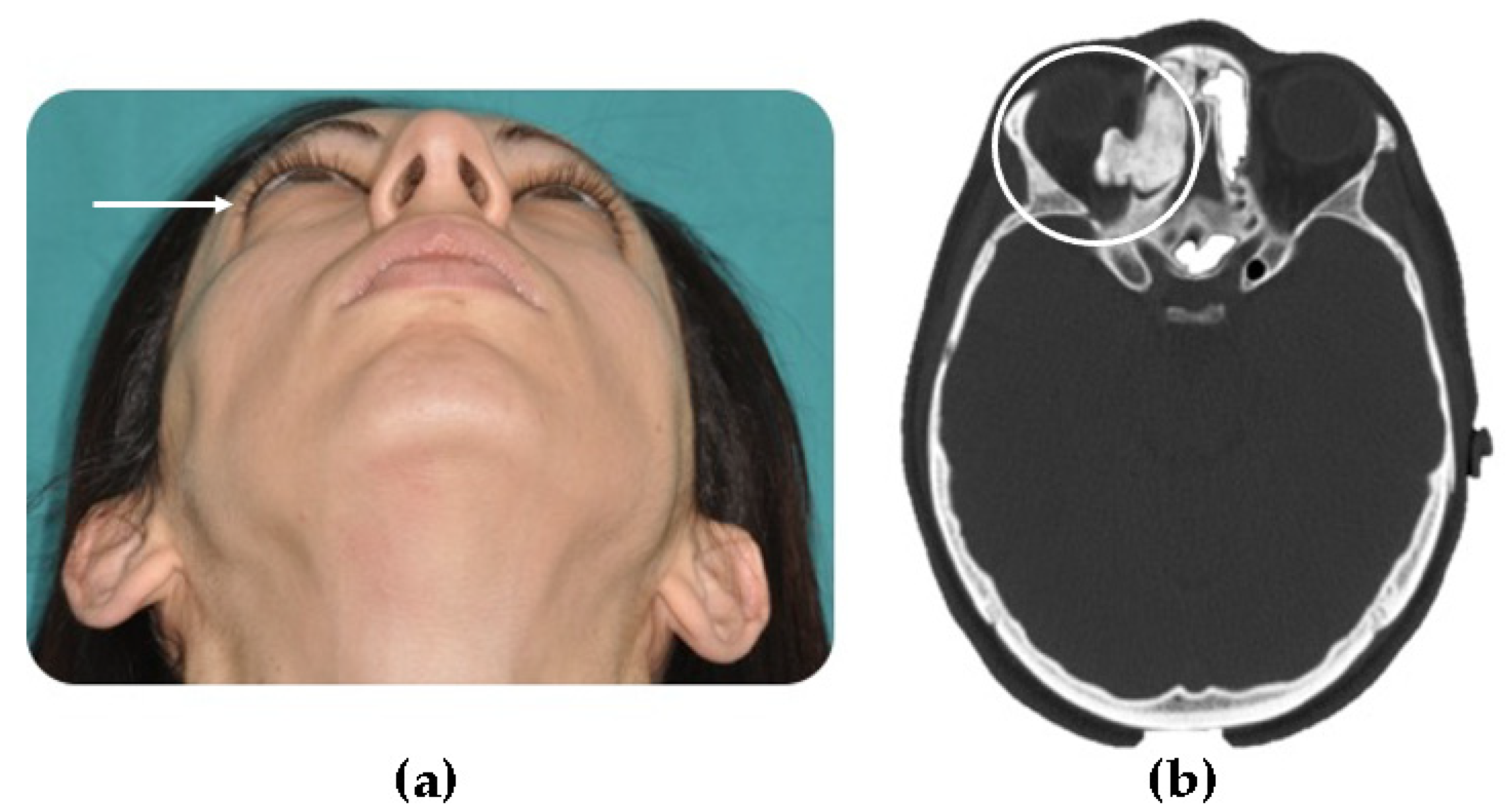
- Central, characterized by progressive endosseous development, eventually resulting in the complete replacement of the affected bone segment (Figure 1);
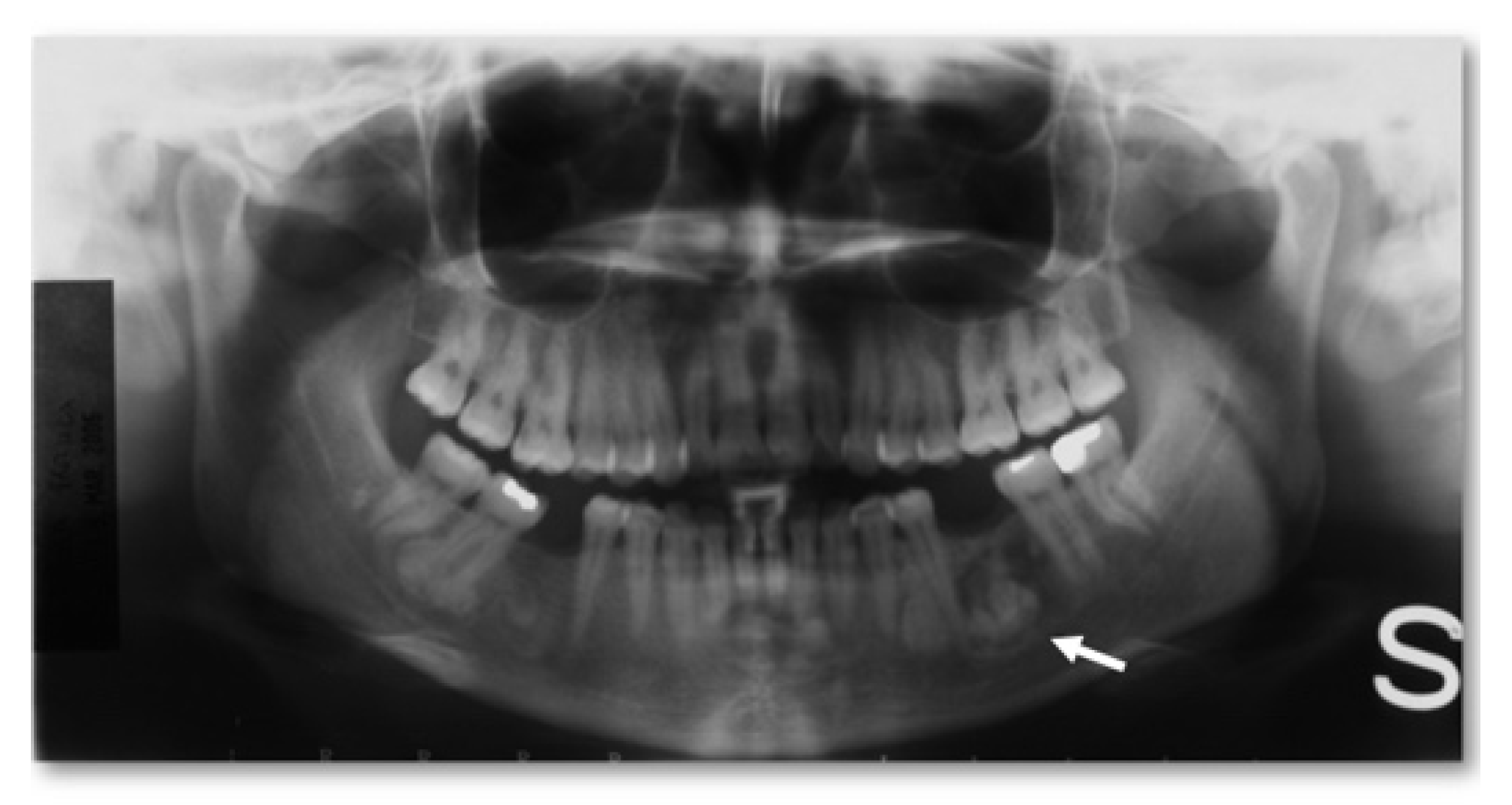
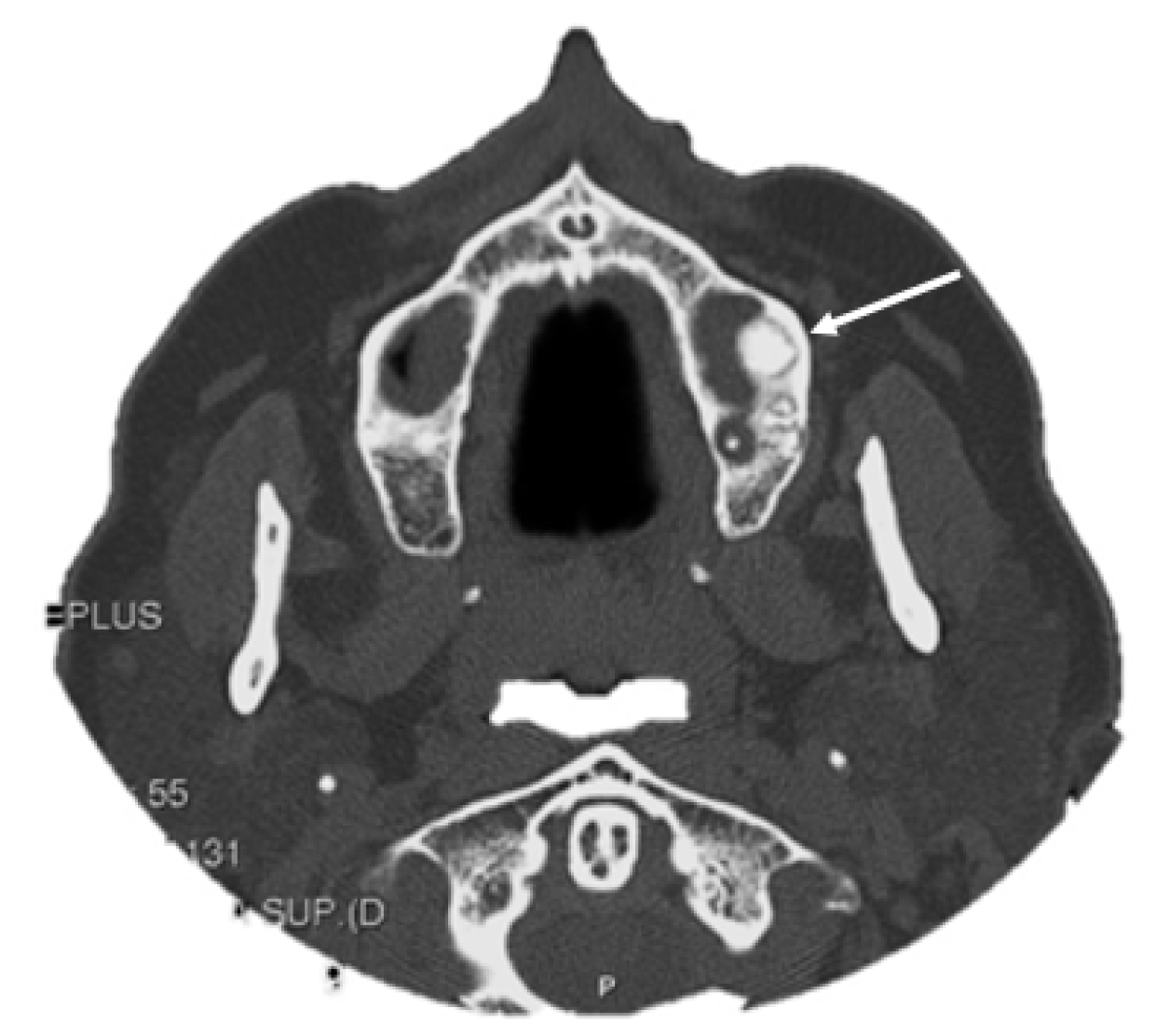
- Peripheral, consisting of periosteal development that can appear as a pedunculated mass (Figure 2);
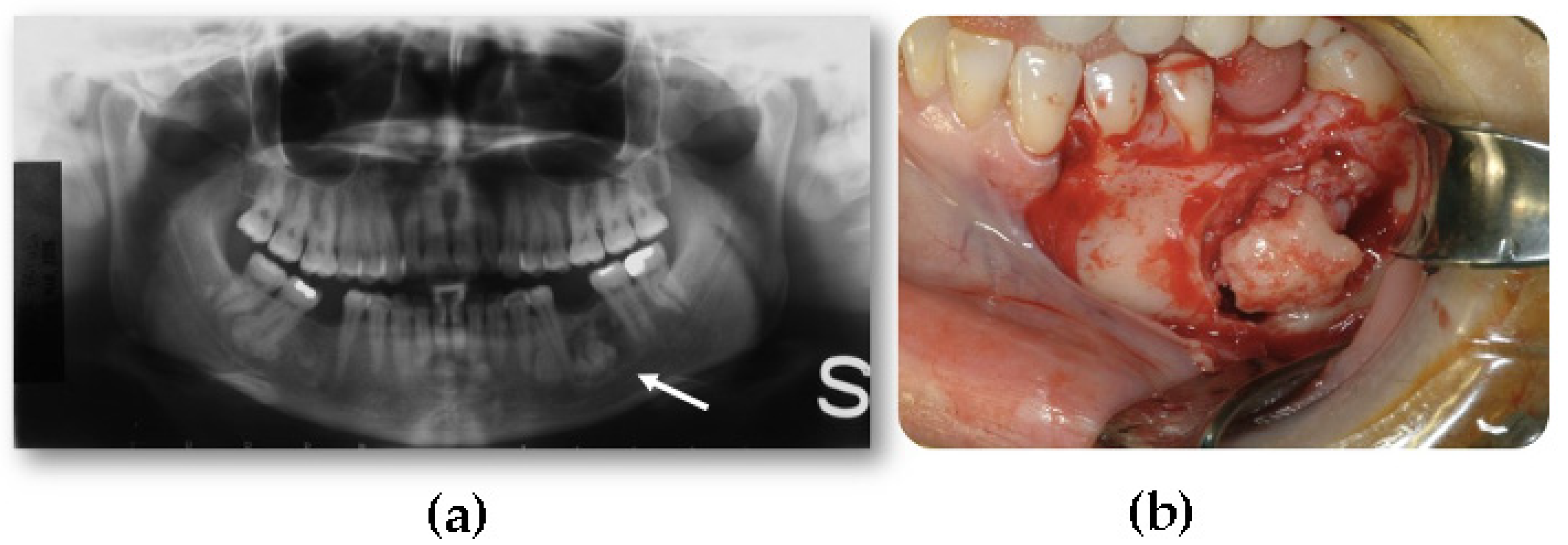
- Extraosseous, which develops within the soft tissues, particularly in the muscles [2].
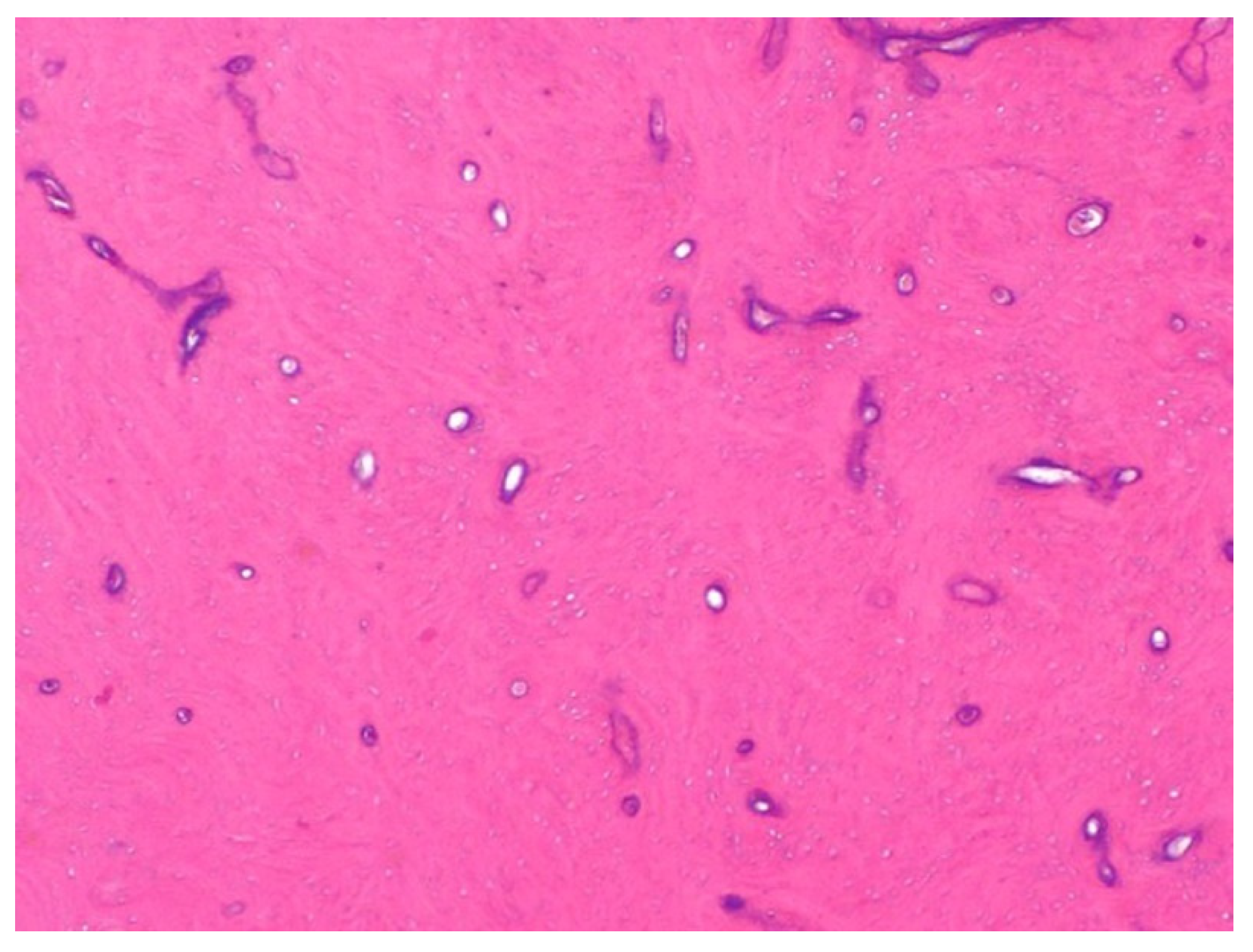
2. Histological Findings
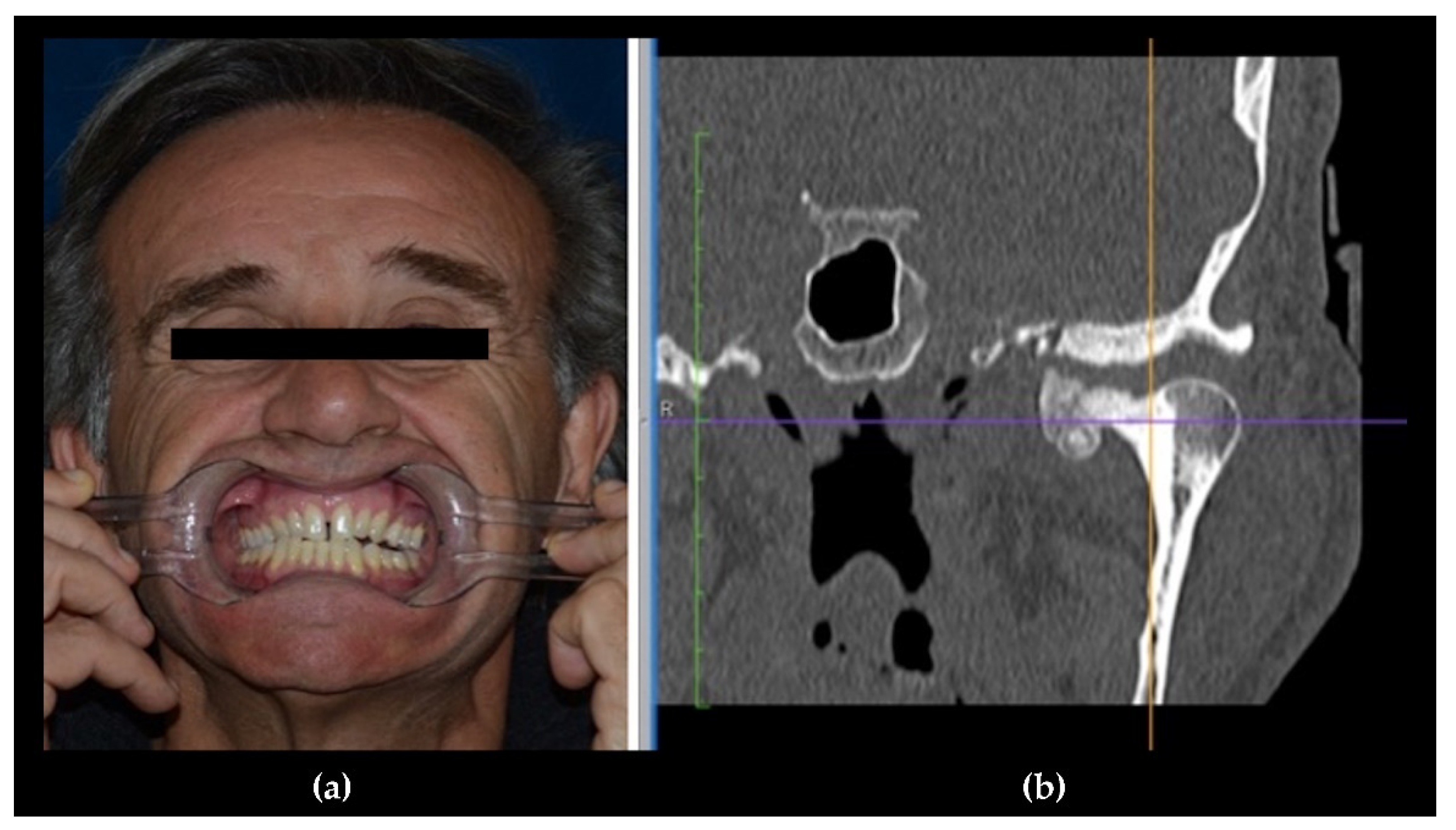
3. Clinical Features
| Author | N° Ref. | Patient Gender | Patient Age | Osteoma Localization | Clinic | Imaging | Comorbidity |
|---|---|---|---|---|---|---|---|
| Ortega Beltrá | [19] | M | 68 | Mandibula | Ankylosis of the temporomandibular joint | CT | No |
| Alkhaldi | [20] | M | 44 | Ethmoid sinus, orbital cavity, ostium of the maxillary sinus | Chronic rhinosinusitis | CT | Prior endoscopic sinus surgery |
| Dedushi | [21] | M | 61 | Frontal sinus | Headaches, generalized seizures, transient motor aphasia, regressive hemiparesis, and fluctuating blood pressure values | MRI | No |
| Ali | [22] | M | 35 | Frontal sinus | Altered Sensorium | CT, MRI | No |
| Mlouka | [23] | M | 26 | Maxillary sinus | Asymptomatic | CBCT | No |
| Öztürk | [24] | M | 15 | Frontal sinus | Frontal sinusitis | CT | No |
| Benzagmout | [25] | M | 34 | Frontoethmoidal sinus | Swelling, headaches, seizures | CT, MRI | No |
| Bagheri | [26] | F | 30 | Frontoethmoidal sinus | Orbital cellulitis | CT | No |
| Devaraja | [27] | M | 21 | Frontal sinus | Eyelid swelling and inability to open the eye | CT | No |
| Nakagawa | [28] | M | 27 | Frontoethmoidal sinus, anterior cranial fossa and orbit, frontal lobe | Headache and generalized convulsion | CT, MRI | No |
| Aksakal | [29] | M | 53 | Frontal sinus | Headache | CT | No |
| Demircan | [30] | M | 17 | Mandibular ramus | Swelling, facial asymmetry | XR, CBCT | Prior trauma |
| Azevedo | [31] | M | 30 | Nasal fossa, the bilateral ethmoidal cells, and the frontal Sinuses | Swelling | CT, MRI | Prior trauma |
| Yazici | [32] | F | 30 | Frontoethmoidal sinus, maxillary sinus, middle concha | Headache, facial pain, and blurring vision | CT | No |
| Kim | [33] | F | 39 | Zigomatic bone | Facial swelling | CT | No |
| Chen | [34] | M | 19 | Fronto-ethmoid sinus | Diplopia, proptosis | CT | No |
| Voicu | [35] | M | 38 | Frontal sinus | Frontal peri-orbital pain | XR, MRI | No |
| Hania | [36] | M | 15 | Maxillary sinus | Spontaneous epistaxis | XR, CT | No |
| Pathak | [37] | M | 45 | Fronto-ethmoid sinus | Change of behavior, forgetfulness | CT, MRI | No |
| Lee | [38] | F | 23 | External auditory canal | Aural fullness | CT | No |
| Lee | [38] | M | 19 | External auditory canal | Mild aural fullness | CT | No |
| Borissova | [39] | F | 48 | Retromastoid portion of the temporal bone | Facial swelling | CBCT | No |
| Temirbekov | [40] | F | 25 | Middle ear, mesotympanum, and hypotympanum | Hearing loss and fullness in the ear | CT | Prior unilateral otitis media |
| Canzi | [41] | F | 64 | Eustachian tube of the temporal bone | Progressive bilateral asymmetric hearing loss | CT | No |
| Falcioni | [42] | F | 36 | Middle ear, promontory, umbus | Progressive monoliteral hearing loss | CT | No |
| Lee | [43] | M | 24 | Ethmoid sinus, medial wall of the orbit | Eye pain, swelling, decreased vision, purulent drainage | CT | No |
| Saylisoy | [44] | F | 53 | Eustachian tube of the temporal bone | Intermittent otalgia and otorrhea | CT | No |
| Tan | [45] | F | 40 | Temporal bone (retromastoid) | Swelling behind the ear | CT | No |
| Nilesh | [46] | F | 65 | Mandibular condyle | Limited mouth opening | XR, CT | No |
| Ghita | [47] | F | 25 | Posterior mandible | Facial swelling | XR, CBCT | No |
| Kayaci | [48] | F | 80 | Posterolateral wall of the lesser wing of the sphenoid bone | Vision loss, pain, headache | CT | No |
| Torres | [49] | M | 21 | Posterior mandible | Facial swelling | CT | No |
| Nayak | [50] | M | 30 | Posterior mandible | Swelling in the lower left back tooth region | XR | No |
| Lazar | [51] | M | 33 | Posterior mandible | Swelling, airway deviation | CT | No |
| Guerra | [52] | M | 25 | Frontal sinus, ethmoid sinus, upper and medial orbital walls | Double vision, progressive change in the positioning of the eye | CT | Prior orbit zygomatic fracture reconstruction due to facial trauma |
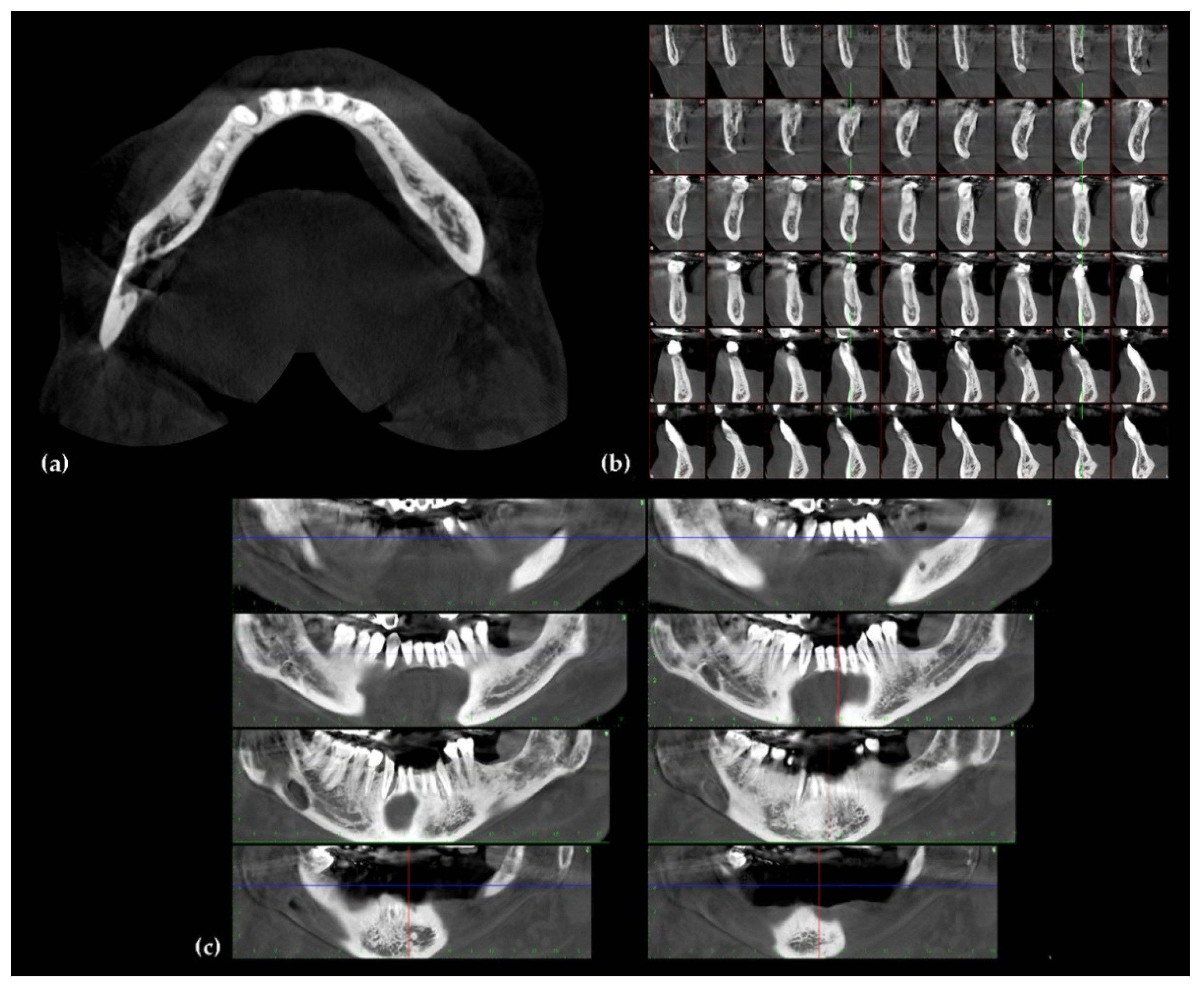
4. Imaging
5. Surgical Treatment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rappaport, J.M.; Attia, E.L. Pneumocephalus in frontal sinus osteoma: A case report. J. Otolaryngol. 1994, 23, 430–436. Available online: https://pubmed.ncbi.nlm.nih.gov/7897774/ (accessed on 24 May 2021). [PubMed]
- Bodner, L.; Gatot, A.; Sion-Vardy, N.; Fliss, D.M. Peripheral osteoma of the mandibular ascending ramus. J. Oral Maxillofac. Surg. 1998, 56, 1446–1449. [Google Scholar] [CrossRef]
- Bessho, K.; Murakami, K.I.; Iizuka, T.; Ono, T. Osteoma in mandibular condyle. Int. J. Oral Maxillofac. Surg. 1987, 16, 372–375. Available online: https://pubmed.ncbi.nlm.nih.gov/3112270/ (accessed on 24 May 2021). [CrossRef]
- Yamasoba, T.; Harada, T.; Okuno, T.; Nomura, Y. Osteoma of the middle ear: Report of a case. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 1214–1216. [Google Scholar] [CrossRef]
- Miller, N.R.; Gray, J.; Snip, R. Giant, mushroom-shaped osteoma of the orbit originating from the maxillary sinus. Am. J. Ophthalmol. 1977, 83, 587–591. [Google Scholar] [CrossRef]
- Brucoli, M.; Giarda, M.; Benech, A. Gardner syndrome: Presurgical planning and surgical management of craniomaxillofacial osteomas. J. Craniofac. Surg. 2011, 22, 946–948. Available online: https://pubmed.ncbi.nlm.nih.gov/21558892/ (accessed on 24 May 2021). [CrossRef]
- Halawi, A.M.; Maley, J.; Robinson, R.; Swenson, C.; Graham, S. Craniofacial osteoma: Clinical presentation and patterns of growth. Am. J. Rhinol. Allergy 2013, 27, 128–133. [Google Scholar] [CrossRef]
- Cutilli, B.J.; Quinn, P.D. Traumatically induced peripheral osteoma: Report of a case. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 667–669. [Google Scholar] [CrossRef]
- Larrea-Oyarbide, N.; Valmaseda-Castellón, E.; Berini-Aytés, L.; Gay-Escoda, C. Osteomas of the craniofacial region. Review of 106 cases. J. Oral Pathol. Med. 2008, 37, 38–42. Available online: https://pubmed-ncbi-nlm-nih-gov.ezproxy.unibo.it/18154576/ (accessed on 24 May 2021). [CrossRef]
- Societa’ Italiana di Chirurgia Maxillo-Facciale (SICMF). Trattato di Patologia Chirurgica Maxillo-Facciale; Minerva Medica: Torino, Italy, 2007. [Google Scholar]
- Ziccardi, V.B.; Smith, J.A.; Braun, T.W. Osteoma of the maxillary antrum. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 80, 378–379. Available online: https://pubmed.ncbi.nlm.nih.gov/8521095/ (accessed on 24 May 2021). [CrossRef]
- Loukas, M.; Hulsberg, P.; Tubbs, R.S.; Kapos, T.; Wartmann, C.T.; Shaffer, K.; Moxham, B.J. The tori of the mouth and ear: A review. Clin. Anat. 2013, 26, 953–960. [Google Scholar] [CrossRef]
- Sayan, N.B.; Üçok, C.; Karasu, H.A.; Günhan, Ö. Peripheral osteoma of the oral and maxillofacial region: A study of 35 new cases. J. Oral Maxillofac. Surg. 2002, 60, 1299–1301. [Google Scholar] [CrossRef]
- Chiu, A.G.; Schipor, I.; Cohen, N.A.; Kennedy, D.W.; Palmer, J.N. Surgical decisions in the management of frontal sinus osteomas. Am. J. Rhinol. 2005, 19, 191–197. [Google Scholar] [CrossRef]
- Gibson, T.; Walker, F.M. Large osteoma of the frontal sinus. A method of removal to minimise scarring and prevent deformity. Br. J. Plast. Surg. 1951, 4, 210–217. Available online: https://pubmed.ncbi.nlm.nih.gov/14886576/ (accessed on 26 October 2021). [CrossRef]
- Caufourier, C.; Leprovost, N.; Guillou-Jamard, M.R.; Compère, J.F.; Bénateau, H. Tumeurs bénignes ostéoformatrices du massif craniofacial. Rev. Stomatol. Chir. Maxillofac. 2009, 110, 202–208. Available online: https://pubmed.ncbi.nlm.nih.gov/19660772/ (accessed on 24 May 2021). [CrossRef]
- Nah, K.S. Osteomas of the craniofacial region. Imaging Sci. Dent. 2011, 41, 107–113. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3189534/ (accessed on 24 May 2021). [CrossRef]
- Kaplan, I.; Calderon, S.; Buchner, A. Peripheral osteoma of the mandible: A study of 10 new cases and analysis of the literature. J. Oral Maxillofac. Surg. 1994, 52, 467–470. [Google Scholar] [CrossRef]
- Beltrá, N.O.; Quiles, S.M.; Arroyo, M.M.; Rocher, F.P. Mandibular osteoma as a cause of ankylosis and progressive trismus. BMJ Case Rep. 2021, 14, e244014. [Google Scholar] [CrossRef] [PubMed]
- Alkhaldi, A.S.; Alsalamah, S.; Tatwani, T. A case of giant ethmoid sinus osteoma. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Dedushi, K.; Hyseni, F.; Musa, J.; Saliaj, K.; Vokshi, V.; Guy, A.; Bhatti, A.; Ahmetgjekaj, I.; Tahir, M.; Shatri, J. Importance of MRI in the diagnosis of a rare intracranial mucocele associated with frontal paranasal osteoma: Case report and literature review. Radiol. Case Rep. 2021, 16, 3094–3098. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Qasim, A.; Anwar, B.; Choudhary, N.; Akmal, M. Intradural extension of mucocele secondary to giant frontal sinus osteoma: Diagnostic pitfalls. Surg. Neurol. Int. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Mlouka, M.; Tlili, M.; Hamrouni, A.; Selmi, R.; Khanfir, F.; Khalfi, M.S.; Ben Amor, F. Lateral maxillary sinus floor elevation in the presence of a sinus osteoma: A case report. Clin. Case Rep. 2021, 9, e04124. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Atay, K.; Erkul, B.E.; Karademir, F. A rare case in childhood: Pott’s puffy tumor developing secondary to frontal sinus osteoma (Çocukluk Çağında Nadir Bir Olgu: Frontal Sinüs Osteomuna İkincil Pott’s Puffy Tümör Gelişimi). Turk. Arch. Pediatr. 2020, 55, 445–448. [Google Scholar] [CrossRef]
- Lakhdar, F.; Benzagmout, M.; Chakour, K.; Chaoui, M.E.F. Subdural empyema complicating a giant fronto-ethmoidal osteoma. Asian J. Neurosurg. 2020, 15, 737–740. [Google Scholar] [CrossRef]
- Bagheri, A.; Feizi, M.; Jafari, R.; Kanavi, M.R.; Raad, N. Orbital cellulitis secondary to giant Sino-orbital osteoma: A case report. Cancer Rep. 2020, 4, e1296. [Google Scholar] [CrossRef] [PubMed]
- Devaraja, K.; Pujary, K.; Pillai, S. Orbital emphysema secondary to frontal osteoma. J. Craniofacial Surg. 2020, 32, e353–e355. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, R.; Ima, H.; Yamada, M.; Nishiike, S.; Fujimoto, Y. Intradural mucocele associated with a frontoethmoidal osteoma: A case report. No Shinkei Geka 2020, 48, 691–697. (In Japanese) [Google Scholar] [CrossRef]
- Aksakal, C. Frontal recess osteoma causing severe headache. Ağrı 2018, 32, 159–161. [Google Scholar] [CrossRef]
- Demircan, S.; Işler, S.C.; Gümüşdal, A.; Genç, B. Orthognathic surgery after mandibular large-volume osteoma treatment. Case Rep. Dent. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Azevedo, C.; Lima, A.; Filipe, M.A.; Duarte, N.; Dias, L.; Marques, R. Giant post-traumatic frontoethmoid osteoma: Diagnostic, therapeutic and reconstructive approach. Turk. Arch. Otorhinolaryngol. 2020, 58, 61–64. [Google Scholar] [CrossRef]
- Yazici, D.; Kuran, G. Giant ethmoid sinus osteoma protruding into the maxillary sinus. J. Craniofacial Surg. 2020, 31, e429–e430. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, J.; Kim, B.K. Peripheral Osteoma of the Zygoma. J. Craniofac. Surg. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-J.; Chen, Y.-H. Giant craniofacial osteoma with orbital invasion. Taiwan J. Ophthalmol. 2020, 10, 144–146. [Google Scholar] [CrossRef]
- Voicu, I.P.; Bartalena, T. Frontal osteoma-induced headache revealed by conventional radiography with a false negative MRI: Röntgen strikes back. Headache J. Head Face Pain 2020, 60, 615–616. [Google Scholar] [CrossRef]
- Hania, M.; Sharif, M.O. Maxillary sinus osteoma: A case report and literature review. J. Orthod. 2020, 47, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Chaurasia, R.N.; Kumar, A.; Mishra, V.N.; Joshi, D. Frontotemporal dementia: An unusual cause. Int. J. Neurosci. 2019, 130, 736–738. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Choi, Y.J.; Choi, H.S.; Jeong, J. Spongiotic osteoma in the external auditory canal: Two cases of a rare tumor. SAGE Open Med. Case Rep. 2020, 8, 2050313X20981469. [Google Scholar] [CrossRef] [PubMed]
- Borissova, I.B.; Venturin, J.S.; Claro-Woodruff, W.I.; Shintaku, W.H. Mastoid osteoma: A rare incidental finding in an orthodontic patient. Imaging Sci. Dent. 2020, 50, 347–351. [Google Scholar] [CrossRef]
- Temirbekov, D.; Celikyurt, C. Middle ear osteoma causing Eustachian tube obstruction: A case report and literature review. J. Otol. 2020, 15, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Canzi, P.; Carlotto, E.; Manfrin, M.; Avato, I.; Nardo, M.; Simoncelli, A.M.; Pagella, F.; Benazzo, M.; Matteo, S. Isolated Eustachian tube osteoma: Common lesion in uncommon site. J. Int. Adv. Otol. 2020, 16, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Falcioni, M.; Bertoli, G.; Ciavarro, G. An uncommon cause of conductive hearing loss. Am. J. Otolaryngol. 2020, 41, 102717. [Google Scholar] [CrossRef]
- Lee, S.; Mallen, J.R.; Ehlers, W.H.; Falcone, T.E. Large ethmoid sinus osteoma presenting as vision threatening orbital abscess. Ear Nose Throat J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Saylisoy, S. Multiple osteomas of Eustachian tube causing ipsilateral otitis media. Curr. Med. Imaging 2020, 16, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.W.K.; Barco, J.B.; Rehman, M.U.; Tan, C.C. Retromastoid osteoma—A rare case report. J. Surg. Case Rep. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Nilesh, K.; Vande, A.; Reddy, S. Central compact osteoma of mandibular condyle. BMJ Case Rep. 2020, 13, e233082. [Google Scholar] [CrossRef] [PubMed]
- Ghita, I.; Brooks, J.K.; Bordener, S.L.; Emmerling, M.R.; Price, J.B.; Younis, R.H. Central compact osteoma of the mandible: Case report featuring unusual radiographic and computed tomographic presentations and brief literature review. J. Stomatol. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Kayaci, S.; Kanat, A.; Seçkin, H. Primary osteoma of the orbit with atypic facial pain: Case report and literature review. Turk. Neurosurg. 2010, 22, 389–392. [Google Scholar] [CrossRef]
- Torres, L.H.S.; Filho, J.R.T.C.; Uchôa, C.P.; Neto, R.T.A.; Santos, D.L.P.; Filho, V.A.P. Intrabuccal surgical management of a peripheral mandibular osteoma. J. Craniofacial Surg. 2020, 32, 1952–1953. [Google Scholar] [CrossRef]
- Nayak, V.; Rao, P.K.; Kini, R.; Shetty, U. Peripheral osteoma of the mandible. BMJ Case Rep. 2020, 13, e238225. [Google Scholar] [CrossRef]
- Lazar, A.; Brookes, C.C. Giant osteomas: Optimizing outcomes through virtual planning—A report of two cases and review of the literature. J. Oral Maxillofac. Surg. 2021, 79, 366–375. [Google Scholar] [CrossRef]
- Guerra, R.C.; Carvalho, P.H.D.A.; Pulino, B.D.F.B.; Passetto, M.T.; Junior, J.C.R.; Hochuli-Vieira, E. Maxillofacial manifestation of a giant dimension cranial osteoma. J. Craniofacial Surg. 2020, 31, e538–e539. [Google Scholar] [CrossRef] [PubMed]
- Gardner, E.J.; Plenk, H.P. Hereditary pattern for multiple osteomas in a family group. Am. J. Hum. Genet. 1952, 4, 31–36. Available online: https://pubmed.ncbi.nlm.nih.gov/14933371/ (accessed on 24 May 2021).
- Soni, S.; Bhargava, A. Revisiting peripheral osteoma of the mandible with case series and review of literature. Indian J. Otolaryngol. Head Neck Surg. 2012, 66, 212–218. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gundewar, S.; Kothari, D.S.; Mokal, N.J.; Ghalme, A. Osteomas of the craniofacial region: A case series and review of literature. Indian J. Plast. Surg. 2013, 46, 479–485. [Google Scholar] [CrossRef]
- Woertler, K. Benign bone tumors and tumor-like lesions: Value of cross-sectional imaging. Eur. Radiol. 2003, 13, 1820–1835. Available online: https://pubmed.ncbi.nlm.nih.gov/12700923/ (accessed on 1 August 2021). [CrossRef] [PubMed]
- Curé, J.K.; Vattoth, S.; Shah, R. Radiopaque jaw lesions: An approach to the differential diagnosis. RadioGraphics 2012, 32, 1909–1925. [Google Scholar] [CrossRef]
- McHugh, J.B.; Mukherji, S.K.; Lucas, D.R. Sino-orbital osteoma: A clinicopathologic study of 45 surgically treated cases with emphasis on tumors with osteoblastoma-like features. Arch. Pathol. Lab. Med. 2009, 133, 1587–1593. Available online: https://pubmed-ncbi-nlm-nih-gov.ezproxy.unibo.it/19792048/ (accessed on 24 May 2021). [CrossRef]
- Horikawa, F.K.; de Freitas, R.R.; Maciel, F.A.; Gonçalves, A.J. Peripheral osteoma of the maxillofacial region: A study of 10 cases. Braz. J. Otorhinolaryngol. 2012, 78, 38–43. [Google Scholar] [CrossRef]
- Angelopoulos, C.; Thomas, S.; Hechler, S.; Parissis, N.; Hlavacek, M. Comparison between digital panoramic radiography and cone-beam computed tomography for the identification of the mandibular canal as part of presurgical dental implant assessment. J. Oral Maxillofac. Surg. 2008, 66, 2130–2135. [Google Scholar] [CrossRef]
- Pauwels, R. Cone beam CT for dental and maxillofacial imaging: Dose matters: Table 1. Radiat. Prot. Dosim. 2015, 165, 156–161. [Google Scholar] [CrossRef]
- Ludlow, J.B.; Ivanovic, M. Comparative dosimetry of dental CBCT devices and 64-slice CT for oral and maxillofacial radiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Nardi, C.; Salerno, S.; Molteni, R.; Occhipinti, M.; Grazzini, G.; Norberti, N.; Cordopatri, C.; Colagrande, S. Radiation dose in non-dental cone beam CT applications: A systematic review. Radiol. Med. 2018, 123, 765–777. [Google Scholar] [CrossRef]
- Ping, Y.X.; Kun, X.B.; Feng, L.X.; Ling, L.B.; Zhang, F.; Ting, L.J. Value of multi-slice spiral CT with three-dimensional reconstruction in the diagnosis of neoplastic lesions in the jawbones. J. South. Med Univ. 2008, 28, 1700–1702. Available online: https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=150087 (accessed on 14 October 2021).
- Liang, X.; Jacobs, R.; Hassan, B.; Li, L.; Pauwels, R.; Corpas, L.; Souza, P.C.; Martens, W.; Shahbazian, M.; Alonso, A.; et al. A comparative evaluation of cone beam computed tomography (CBCT) and multi-slice CT (MSCT) Part, I. On subjective image quality. Eur. J. Radiol. 2010, 75, 265–269. Available online: https://pubmed-ncbi-nlm-nih-gov.ezproxy.unibo.it/19410409/ (accessed on 14 October 2021). [CrossRef] [PubMed]
- Weiss, R.; Read-Fuller, A. Cone beam computed tomography in oral and maxillofacial surgery: An evidence-based review. Dent. J. 2019, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, J.B.; Laster, W.S.; See, M.; Bailey, L.J.; Hershey, H.G. Accuracy of measurements of mandibular anatomy in cone beam computed tomography images. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 103, 534–542. [Google Scholar] [CrossRef]
- Bombeccari, G.P.; Candotto, V.; Gianni, A.B.; Carinci, F.; Spadari, F. Accuracy of the cone beam computed tomography in the detection of bone invasion in patients with oral cancer: A systematic review. Eurasian J. Med. 2019, 51, 298–306. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.; Casselman, J.; Swennen, G. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: A systematic review of the literature. Int. J. Oral Maxillofac. Surg. 2009, 38, 609–625. [Google Scholar] [CrossRef]
- Horner, K.; Jacobs, R.; Schulze, R. Dental CBCT equipment and performance issues. Radiat. Prot. Dosim. 2012, 153, 212–218. [Google Scholar] [CrossRef]
- Patel, S.; Brown, J.; Pimentel, T.; Kelly, R.D.; Abella, F.; Durack, C.; Pimental, T. Cone beam computed tomography in endodontics—A review of the literature. Int. Endod. J. 2019, 52, 1138–1152. [Google Scholar] [CrossRef]
- Durack, C.; Patel, S. Cone beam computed tomography in endodontics. Braz. Dent. J. 2012, 23, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, E.; Schmid, M.; Lell, M.M.; Hirschfelder, U. Cone beam computed tomography and low-dose multislice computed tomography in orthodontics and dentistry. J. Orofac. Orthop. 2014, 75, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Widmann, G.; Al-Ekrish, A.A. Ultralow dose MSCT imaging in dental implantology. Open Dent. J. 2018, 12, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, R.M.; Collin, J.; Sumi, D.V.; Araújo, L.C.; Murakoshi, R.W.; Gomes, R.L.E.; Daniel, M.M. Postoperative CT findings of orthognathic surgery and its complications: A guide for radiologists. J. Neuroradiol. 2021. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nishiyama, Y. SPECT/CT imaging in bone scintigraphy of a case of clavicular osteoma. J. Nucl. Med. Biol. 2014, 2, 73–74. [Google Scholar]
- Kitajima, K.; Futani, H.; Komoto, H.; Tsuchitani, T.; Takahashi, Y.; Tachibana, T.; Yamakado, K. Quantitative bone SPECT/CT applications for primary bone neoplasms. Hell. J. Nucl. Med. 2021, 24, 36–44. [Google Scholar]
- Enomoto, K.; Nishimura, H.; Hamada, K.; Doi, K.; Kubo, T.; Hatazawa, J. Nuclear imaging of osteoma. Clin. Nucl. Med. 2008, 33, 135–136. Available online: https://pubmed-ncbi-nlm-nih-gov.ezproxy.unibo.it/18209540/ (accessed on 24 May 2021). [CrossRef]
- Xing, Y.; Zhao, J.; Wang, T. A case of paranasal sinuses osteoma detected on bone SPECT/CT. Clin. Nucl. Med. 2011, 36, 224–226. [Google Scholar] [CrossRef]
- Kim, W.H.; Kim, D.-W.; Kim, C.G.; Kim, M.H. Additional detection of multiple osteomas in a patient with Gardner’s syndrome by bone SPECT/CT. Nucl. Med. Mol. Imaging 2013, 47, 297–298. [Google Scholar] [CrossRef][Green Version]
- Nojima, K.; Niizuma-Kosaka, F.; Nishii, Y.; Sueishi, K.; Yamakura, D.; Ikumoto, H.; Ohata, H.; Inoue, T. Multidisciplinary treatment of peripheral osteoma arising from mandibular condyle in patient presenting with facial asymmetry. Bull. Tokyo Dent. Coll. 2014, 55, 39–47. [Google Scholar] [CrossRef]
- Baykul, T.; Heybeli, N.; Oyar, O.; Doğru, H. Multiple huge osteomas of the mandible causing disfigurement related with Gardner’s syndrome: Case report. Auris Nasus Larynx 2003, 30, 447–451. [Google Scholar] [CrossRef]
- Shintaku, W.H.; Venturin, J.S.; Langlais, R.P.; Clark, G.T. Imaging modalities to access bony tumors and hyperplasic reactions of the temporomandibular joint. J. Oral Maxillofac. Surg. 2010, 68, 1911–1921. [Google Scholar] [CrossRef]
- Noyek, A.; Chapnik, J.; Kirsh, J. Radionuclide bone scan in frontal sinus osteoma. ANZ J. Surg. 1989, 59, 127–132. [Google Scholar] [CrossRef]
- Chattopadhyay, C.P.K.; Chander, M.G.M. Peripheral osteoma of the maxillofacial region diagnosis and management: A study of 106 cases. J. Maxillofac. Oral Surg. 2012, 11, 425–429. Available online: https://pubmed.ncbi.nlm.nih.gov/24293935/ (accessed on 24 May 2021). [CrossRef]
- Yudoyono, F.; Sidabutar, R.; Dahlan, R.H.; Gill, A.S.; Ompusunggu, S.E.; Arifin, M.Z. Surgical management of giant skull osteomas. Asian J. Neurosurg. 2017, 12, 408–411. [Google Scholar] [CrossRef]
- Dell’Aversana Orabona, G.; Salzano, G.; Iaconetta, G.; Piombino, P.; Ponzo, L.; Santella, A.; Astarita, F.; Solari, D.; Salzano, F.A.; Califano, L. Facial osteomas: Fourteen cases and a review of literature. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1796–1802. Available online: https://pubmed.ncbi.nlm.nih.gov/26044223/ (accessed on 24 May 2021).
- Aghabeigi, B.; Evans, A.; Crean, S.J.; Hopper, C. Simultaneous repair of an orbital floor fracture and removal of an ethmoid osteoma: Case report and review of the literature. Int. J. Oral Maxillofac. Surg. 2003, 32, 94–96. [Google Scholar] [CrossRef]
- Swanson, K.S.; Guttu, R.L.; Miller, M.E. Gigantic osteoma of the mandible: Report of a case. J. Oral Maxillofac. Surg. 1992, 50, 635–638. [Google Scholar] [CrossRef]
- Tarsitano, A.; Marchetti, C. Presentazione atipica di una sindrome delle apnee ostruttive notturne causata da un osteoma gigante della mandibola: Presentazione del caso e revisione della letteratura. Acta Otorhinolaryngol. Ital. 2013, 33, 63–66. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23620643%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3631812 (accessed on 24 May 2021).
- Longo, F.; Califano, L.; De Maria, G.; Ciccarelli, R. Solitary osteoma of the mandibular ramus: Report of a case. J. Oral Maxillofac. Surg. 2001, 59, 698–700. [Google Scholar] [CrossRef]
- Tarsitano, A.; Battaglia, S.; Ricotta, F.; Bortolani, B.; Cercenelli, L.; Marcelli, E.; Cipriani, R.; Marchetti, C. Accuracy of CAD/CAM mandibular reconstruction: A three-dimensional, fully virtual outcome evaluation method. J. Cranio-Maxillofac. Surg. 2018, 46, 1121–1125. [Google Scholar] [CrossRef]
- Ricotta, F.; Cercenelli, L.; Battaglia, S.; Bortolani, B.; Savastio, G.; Marcelli, E.; Marchetti, C.; Tarsitano, A. Navigation-guided resection of maxillary tumors: Can a new volumetric virtual planning method improve outcomes in terms of control of resection margins? J. Cranio-Maxillofac. Surg. 2018, 46, 2240–2247. Available online: https://pubmed-ncbi-nlm-nih-gov.ezproxy.unibo.it/30482714/ (accessed on 14 October 2021). [CrossRef]
- Tarsitano, A.; Ricotta, F.; Baldino, G.; Badiali, G.; Pizzigallo, A.; Ramieri, V.; Cascone, P.; Marchetti, C. Navigation-guided resection of maxillary tumours: The accuracy of computer-assisted surgery in terms of control of resection margins—A feasibility study. J. Cranio-Maxillofac. Surg. 2017, 45, 2109–2114. [Google Scholar] [CrossRef]
- Ciocca, L.; Marchetti, C.; Mazzoni, S.; Baldissara, P.; Gatto, M.R.A.; Cipriani, R.; Scotti, R.; Tarsitano, A. Accuracy of fibular sectioning and insertion into a rapid-prototyped bone plate, for mandibular reconstruction using CAD-CAM technology. J. Cranio-Maxillofac. Surg. 2015, 43, 28–33. [Google Scholar] [CrossRef]
- Tarsitano, A.; Ciocca, L.; Scotti, R.; Marchetti, C. Morphological results of customized microvascular mandibular reconstruction: A comparative study. J. Cranio-Maxillofac. Surg. 2016, 44, 697–702. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarsitano, A.; Ricotta, F.; Spinnato, P.; Chiesa, A.M.; Di Carlo, M.; Parmeggiani, A.; Miceli, M.; Facchini, G. Craniofacial Osteomas: From Diagnosis to Therapy. J. Clin. Med. 2021, 10, 5584. https://doi.org/10.3390/jcm10235584
Tarsitano A, Ricotta F, Spinnato P, Chiesa AM, Di Carlo M, Parmeggiani A, Miceli M, Facchini G. Craniofacial Osteomas: From Diagnosis to Therapy. Journal of Clinical Medicine. 2021; 10(23):5584. https://doi.org/10.3390/jcm10235584
Chicago/Turabian StyleTarsitano, Achille, Francesco Ricotta, Paolo Spinnato, Anna Maria Chiesa, Maddalena Di Carlo, Anna Parmeggiani, Marco Miceli, and Giancarlo Facchini. 2021. "Craniofacial Osteomas: From Diagnosis to Therapy" Journal of Clinical Medicine 10, no. 23: 5584. https://doi.org/10.3390/jcm10235584
APA StyleTarsitano, A., Ricotta, F., Spinnato, P., Chiesa, A. M., Di Carlo, M., Parmeggiani, A., Miceli, M., & Facchini, G. (2021). Craniofacial Osteomas: From Diagnosis to Therapy. Journal of Clinical Medicine, 10(23), 5584. https://doi.org/10.3390/jcm10235584








