Management of Osteoblastoma and Giant Osteoid Osteoma with Percutaneous Thermoablation Techniques
Abstract
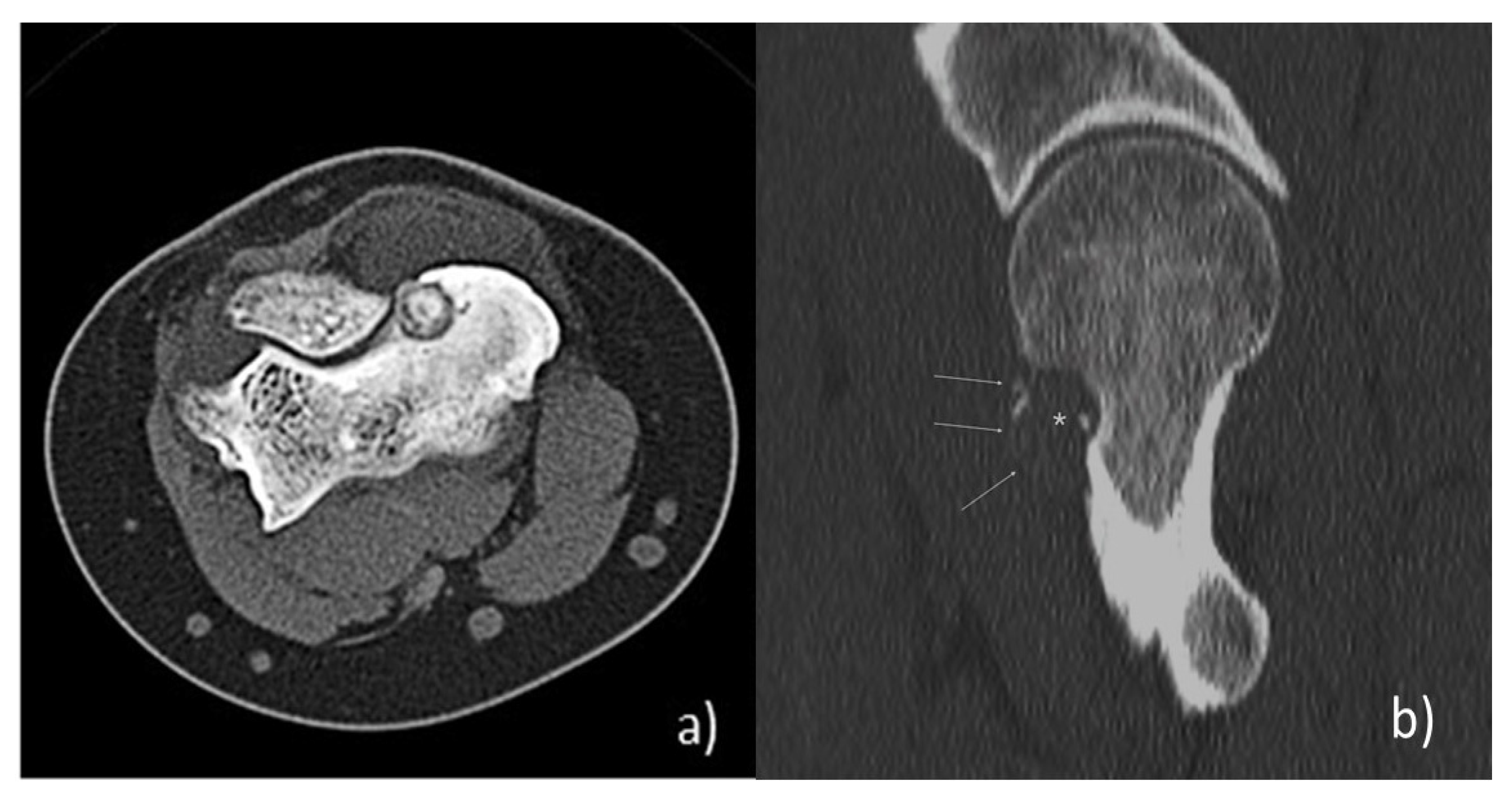
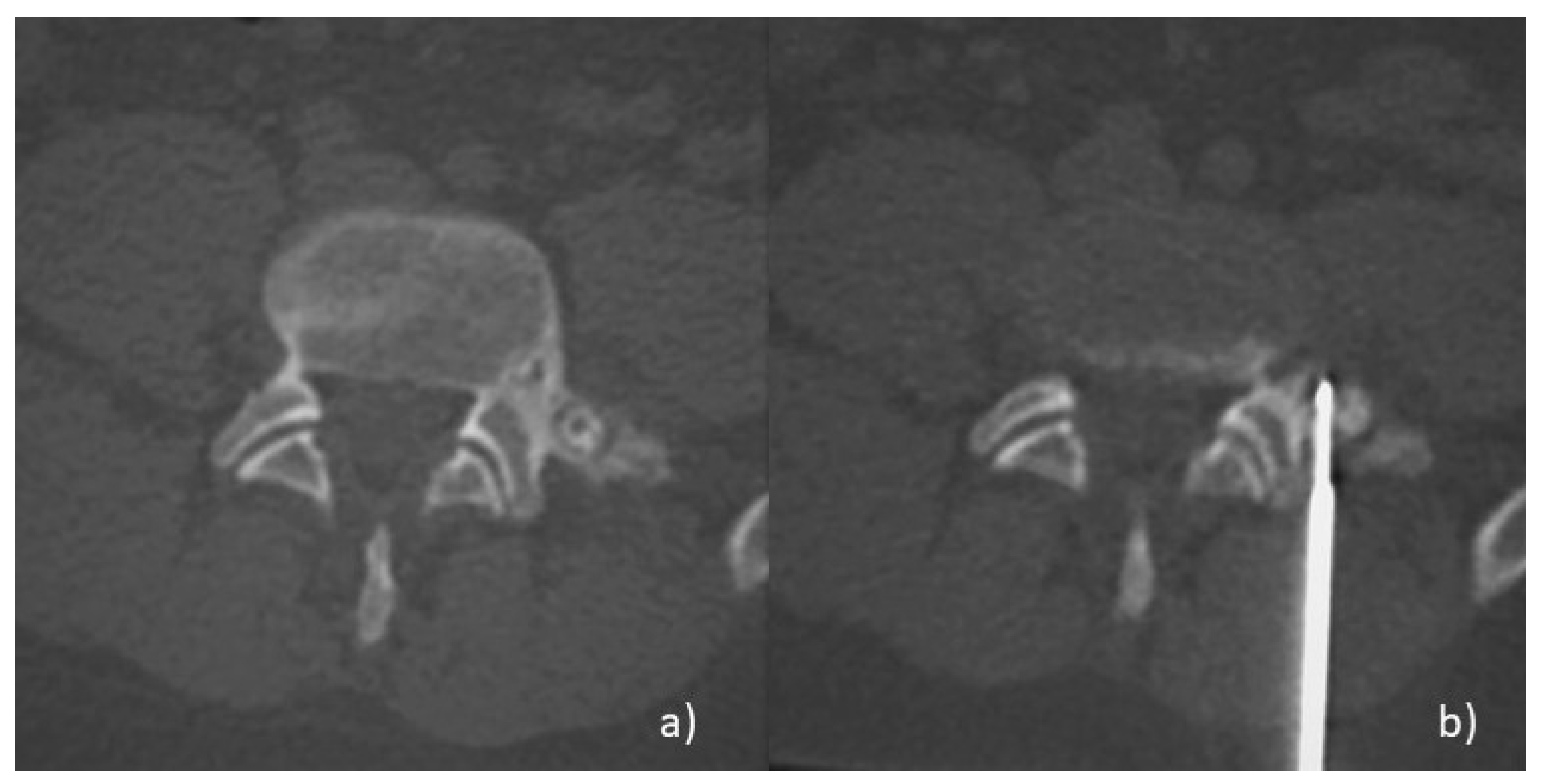
:1. Introduction: Clinical, Pathological, and Imaging Findings
2. Treatment
2.1. Literature Research
2.2. Surgical Treatment
2.3. Radiofrequency Ablation (RFA)
2.3.1. Thermoablation: Physical Aspects
2.3.2. Thermoablation: Technical Aspects
2.3.3. Clinical Aspects
2.4. Cryoablation (CA)
2.4.1. Thermoablation: Physical Aspects
2.4.2. Thermoablation: Technical Aspects
2.4.3. Clinical Aspects
2.5. Other Thermoablation Techniques
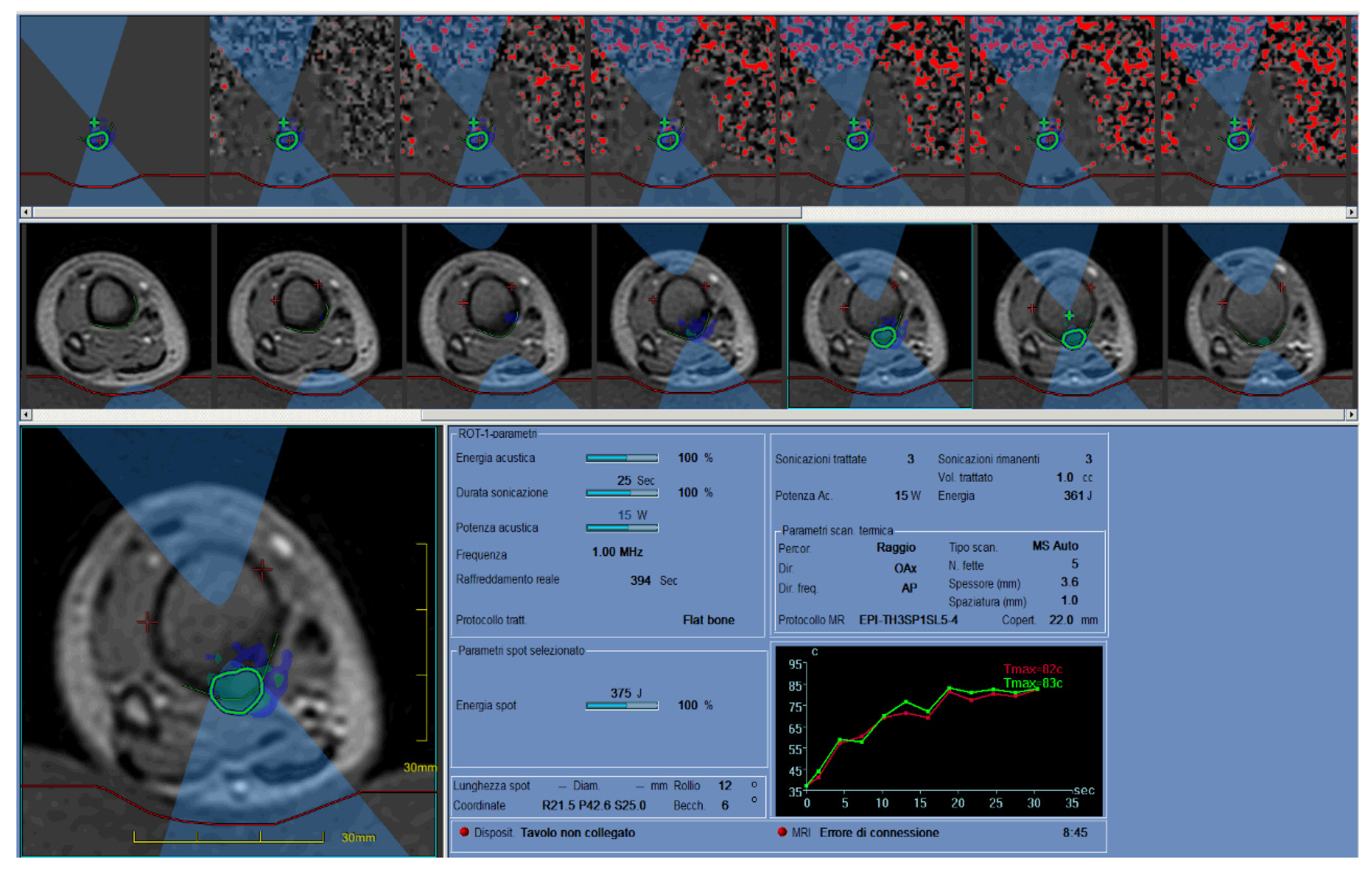
2.6. MRgFUS
2.6.1. Thermoablation: Physical and Technical Aspects
2.6.2. Clinical Aspects
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenspan, A. Benign bone-forming lesions: Osteoma, osteoid osteoma, and osteoblastoma—Clinical, imaging, pathologic, and differential considerations. Skelet. Radiol. 1993, 22, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.R. Osteoblastoma. Arch. Pathol. Lab. Med. 2010, 134, 1460–1466. [Google Scholar] [CrossRef]
- Arrigoni, F.; Barile, A.; Zugaro, L.; Fascetti, E.; Zappia, M.; Brunese, L.; Masciocchi, C. CT-guided radiofrequency ablation of spinal osteoblastoma: Treatment and long-term follow-up. Int. J. Hyperth. 2018, 34, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Xie, Y.; Yan, F.; Deng, Z.; Lei, J.; Cai, L. Diagnostic and Management Options of Osteoblastoma in the Spine. Med. Sci. Monit. 2019, 25, 1362–1372. [Google Scholar] [CrossRef]
- Masciocchi, C.; Zugaro, L.; Arrigoni, F.; Gravina, G.L.; Mariani, S.; La Marra, A.; Zoccali, C.; Flamini, S.; Barile, A. Radiofrequency ablation versus magnetic resonance guided focused ultrasound surgery for minimally invasive treatment of osteoid osteoma: A propensity score matching study. Eur. Radiol. 2016, 26, 2472–2481. [Google Scholar] [CrossRef]
- Kroon, H.M.; Schurmans, J. Osteoblastoma: Clinical and radiologic findings in 98 new cases. Radiology 1990, 175, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.; van Rijswijk, C.S.P.; Villagrán, J.M.; Rehnitz, C.; Muto, M.; von Falck, C.; Gielen, J.; Thierfelder, K.M.; Weber, M.A. European multicentre study on technical success and long-term clinical outcome of radiofrequency ablation for the treatment of spinal osteoid osteomas and osteoblastomas. Neuroradiology 2019, 61, 935–942, Correction to 2019, 61, 943. [Google Scholar] [CrossRef]
- Masciocchi, C.; Arrigoni, F.; Marra, A.; Mariani, S.; Zugaro, L.; Barile, A. Treatment of focal benign lesions of the bone: MRgFUS and RFA. Br. J. Radiol. 2016, 89, 20150356. [Google Scholar] [CrossRef] [Green Version]
- Rehnitz, C.; Sprengel, S.D.; Lehner, B.; Ludwig, K.; Omlor, G.; Merle, C.; Kauczor, H.-U.; Ewerbeck, V.; Weber, M.-A. CT-guided radiofrequency ablation of osteoid osteoma and osteoblastoma: Clinical success and long-term follow up in 77 patients. Eur. J. Radiol. 2012, 81, 3426–3434. [Google Scholar] [CrossRef]
- Rosenthal, D.I.; Hornicek, F.J.; Torriani, M.; Gebhardt, M.C.; Mankin, H.J. Osteoid osteoma: Percutaneous treatment with radiofrequency energy. Radiology 2003, 229, 171–175. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Auloge, P.; De Marini, P.; Boatta, E.; Koch, G.; Dalili, D. Spinal Tumor Ablation: Indications, Techniques, and Clinical Management. Tech. Vasc. Interv. Radiol. 2020, 23, 100677. [Google Scholar] [CrossRef] [PubMed]
- Pourfeizi, H.H.; Tabrizi, A.; Bazavar, M.; Sales, J.G. Clinical findings and results of surgical resection of thoracolumbar osteoid osteoma. Asian Spine J. 2014, 8, 150–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanem, I. The management of osteoid osteoma: Updates and controversies. Curr. Opin. Pediatr. 2006, 18, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Sprengel, S.D.; Omlor, G.W.; Lehner, B.; Wiedenhöfer, B.; Kauczor, H.-U.; Rehnitz, C. Clinical long-term outcome, technical success, and cost analysis of radiofrequency ablation for the treatment of osteoblastomas and spinal osteoid osteomas in comparison to open surgical resection. Skelet. Radiol. 2015, 44, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Lindner, N.J.; Ozaki, T.; Roedl, R.; Gosheger, G.; Winkelmann, W.; Wörtler, K. Percutaneous radiofrequency ablation in osteoid osteoma. J. Bone Jt. Surg. Ser. B 2001, 83, 391–396. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Bosaily, A.E.-S.; Brown, L.C.; Gabe, R.; Kaplan, R.S.; Parmar, M.K. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Natali, G.L.; Paolantonio, G.; Fruhwirth, R.; Alvaro, G.; Parapatt, G.K.; Toma, P.; Rollo, M. Paediatric musculoskeletal interventional radiology. Br. J. Radiol. 2016, 89, 20150369. [Google Scholar] [CrossRef] [Green Version]
- Rybak, L.D. Fire and Ice: Thermal Ablation of Musculoskeletal Tumors. Radiol. Clin. N. Am. 2009, 47, 455–469. [Google Scholar] [CrossRef]
- Wallace, A.N.; Tomasian, A.; Chang, R.O.; Jennings, J.W. Treatment of Osteoid Osteomas Using a Navigational Bipolar Radiofrequency Ablation System. Cardiovasc. Interv. Radiol. 2016, 768–772. [Google Scholar] [CrossRef]
- Tomasian, X.A.; Wallace, X.A.N.; Jennings, X.J.W. Benign Spine Lesions: Advances in Techniques for Minimally Invasive Percutaneous Treatment. AJNR Am. J. Neuroradiol. 2017, 38, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Arrigoni, F.; Izzo, A.; Bruno, F.; Zugaro, L.; Arrigoni, G.; Vacca, F.; Barile, A.; Masciocchi, C. Could anaesthesia be a key factor for the good outcome of bone ablation procedures? A retrospective analysis of a musculoskeletal interventional centre. Br. J. Radiol. 2021, 94, 20200937. [Google Scholar] [CrossRef]
- Dupuy, D.E.; Hong, R.; Oliver, B.; Goldberg, S.N. Radiofrequency Ablation of Spinal Tumors: Temperature Distribution in the Spinal Canal. AJR Am. J. Roentgenol. 2000, 175, 1263–1266. [Google Scholar] [CrossRef] [PubMed]
- Cazzato, R.L.; Auloge, P.; Dalili, D.; De Marini, P.; Di Marco, A. Percutaneous Image-Guided Cryoablation of Osteoblastoma. AJR Am. J. Roentgenol. 2019, 213, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.K.; Tutton, S.; Mazioti, A.; Kelekis, A. Percutaneous image-guided ablation of bone and soft tissue tumours: A review of available techniques and protective measures. Insights Imaging 2014, 5, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oc, Y.; Kilinc, B.E.; Cennet, S.; Boyacioglu, M.M.; Ertugrul, R.; Varol, A. Complications of Computer Tomography Assisted Radiofrequency Ablation in the Treatment of Osteoid Osteoma. Biomed. Res. Int. 2019, 2019, 4376851. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Bo, S.; Liang, H.; Hui, J.; Yuan, S.; Liu, C. Percutaneous radiofrequency ablation for spinal osteoid osteoma and osteoblastoma. Eur. Spine J. 2017, 26, 1884–1892. [Google Scholar] [CrossRef]
- Donkol, R.H.; Al-Nammi, A.; Moghazi, K. Efficacy of percutaneous radiofrequency ablation of osteoid osteoma in children. Pediatr. Radiol. 2008, 38, 180–185. [Google Scholar] [CrossRef]
- Baal, J.D.; Pai, J.S.; Chen, W.C.; Joseph, G.B.; O’Donnell, R.J.; Link, T.M. Factors Associated with Osteoid Osteoma Recurrence after CT-Guided Radiofrequency Ablation. J. Vasc. Interv. Radiol. 2019, 30, 744–751. [Google Scholar] [CrossRef]
- Poullain, F.; Mattei, J.C.; Rochwerger, A.; Bouvier, C.; Chagnaud, C.; Champsaur, P.; Le Corroller, T. Percutaneous cryoablation of osteoblastoma in the proximal femur. Skelet. Radiol. 2020, 49, 1467–1471. [Google Scholar] [CrossRef]
- Koch, G.; Cazzato, R.L.; Gilkison, A.; Caudrelier, J.; Garnon, J.; Gangi, A. Percutaneous Treatments of Benign Bone Tumors. Semin. Intervent. Radiol. 2018, 35, 324–332. [Google Scholar] [CrossRef]
- Tsoumakidou, G.; Koch, G.; Caudrelier, J.; Garnon, J. Image-Guided Spinal Ablation: A Review. Cardiovasc. Intervent. Radiol. 2016, 39, 1229–1238. [Google Scholar] [CrossRef]
- Cazzato, R.L.; Garnon, J.; Ramamurthy, N.; Koch, G.; Tsoumakidou, G.; Caudrelier, J.; Arrigoni, F.; Zugaro, L.; Barile, A.; Masciocchi, C.; et al. Percutaneous image-guided cryoablation: Current applications and results in the oncologic field. Med. Oncol. 2016, 33, 140. [Google Scholar] [CrossRef]
- Kumasaka, S.; Miyazaki, M.; Tsushima, Y. CT-Guided Percutaneous Cryoablation of an Aggressive Osteoblastoma: A Case Report. J. Vasc. Interv. Radiol. 2012, 26, 1746–1748. [Google Scholar] [CrossRef]
- Gangi, A.; Basile, A.; Buy, X.; Alizadeh, H.; Sauer, B.; Bierry, G. Radiofrequency and Laser Ablation of Spinal Lesions. Semin. Ultrasound. CT MR 2005, 26, 89–97, Erratum in 2005, 26, 304. [Google Scholar] [CrossRef]
- Gangi, A.; Alizadeh, H.; Wong, L.; Buy, X.; Dietemann, J.L.; Roy, C. Osteoid osteoma: Percutaneous laser ablation and follow-up in 114 patients. Radiology 2007, 242, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Waynberger, É.; Druon, J. Interstitial laser photocoagulation for the treatment of osteoid osteoma: Retrospective study on 35 cases. Diagn. Interv. Imaging 2013, 94, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Arrigoni, F.; Gregori, L.M.; Zugaro, L.; Barile, A.; Masciocchi, C. MRgFUS in the treatment of MSK lesions: A review based on the experience of the University of L’Aquila, Italy. Trans. Cancer Res. 2014, 3, 442–448. [Google Scholar] [CrossRef]
- Arrigoni, F.; Napoli, A.; Bazzocchi, A.; Zugaro, L.; Scipione, R.; Bruno, F.; Palumbo, P.; Anzidei, M.; Mercatelli, D.; Gravina, G.L.; et al. Magnetic-resonance-guided focused ultrasound treatment of non-spinal osteoid osteoma in children: Multicentre experience. Pediatr. Radiol. 2019, 49, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, F.; Bruno, F.; Palumbo, P.; Zugaro, L.; Zoccali, C.; Barile, A.; Masciocchi, C. Magnetic resonance-guided focused ultrasound surgery treatment of non-spinal intra-articular osteoblastoma: Feasibility, safety, and outcomes in a single-center retrospective analysis. Int. J. Hyperth. 2019, 36, 768–775. [Google Scholar] [CrossRef]
- Napoli, A.; Mastantuono, M.; Marincola, B.C.; Anzidei, M.; Zaccagna, F.; Moreschini, O.; Passariello, R.; Catalano, C. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology 2013, 267, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Temple, M.J.; Waspe, A.C.; Amaral, J.G.; Napoli, A.; LeBlang, S.; Ghanouni, P.; Bucknor, M.D.; Campbell, F.; Drake, J.M. Establishing a clinical service for the treatment of osteoid osteoma using magnetic resonance-guided focused ultrasound: Overview and guidelines. J. Ther. Ultrasound 2016, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesfin, A.; Boriani, S.; Gambarotti, M.; Bandiera, S.; Gasbarrini, A. Can Osteoblastoma Evolve to Malignancy? A Challenge in the Decision-Making Process of a Benign Spine Tumor. World Neurosurg. 2020, 136, 150–156. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzo, A.; Zugaro, L.; Fascetti, E.; Bruno, F.; Zoccali, C.; Arrigoni, F. Management of Osteoblastoma and Giant Osteoid Osteoma with Percutaneous Thermoablation Techniques. J. Clin. Med. 2021, 10, 5717. https://doi.org/10.3390/jcm10245717
Izzo A, Zugaro L, Fascetti E, Bruno F, Zoccali C, Arrigoni F. Management of Osteoblastoma and Giant Osteoid Osteoma with Percutaneous Thermoablation Techniques. Journal of Clinical Medicine. 2021; 10(24):5717. https://doi.org/10.3390/jcm10245717
Chicago/Turabian StyleIzzo, Antonio, Luigi Zugaro, Eva Fascetti, Federico Bruno, Carmine Zoccali, and Francesco Arrigoni. 2021. "Management of Osteoblastoma and Giant Osteoid Osteoma with Percutaneous Thermoablation Techniques" Journal of Clinical Medicine 10, no. 24: 5717. https://doi.org/10.3390/jcm10245717
APA StyleIzzo, A., Zugaro, L., Fascetti, E., Bruno, F., Zoccali, C., & Arrigoni, F. (2021). Management of Osteoblastoma and Giant Osteoid Osteoma with Percutaneous Thermoablation Techniques. Journal of Clinical Medicine, 10(24), 5717. https://doi.org/10.3390/jcm10245717