Arterial Hypertension in Aortic Valve Stenosis: A Critical Update
Abstract
1. Introduction
2. Epidemiology
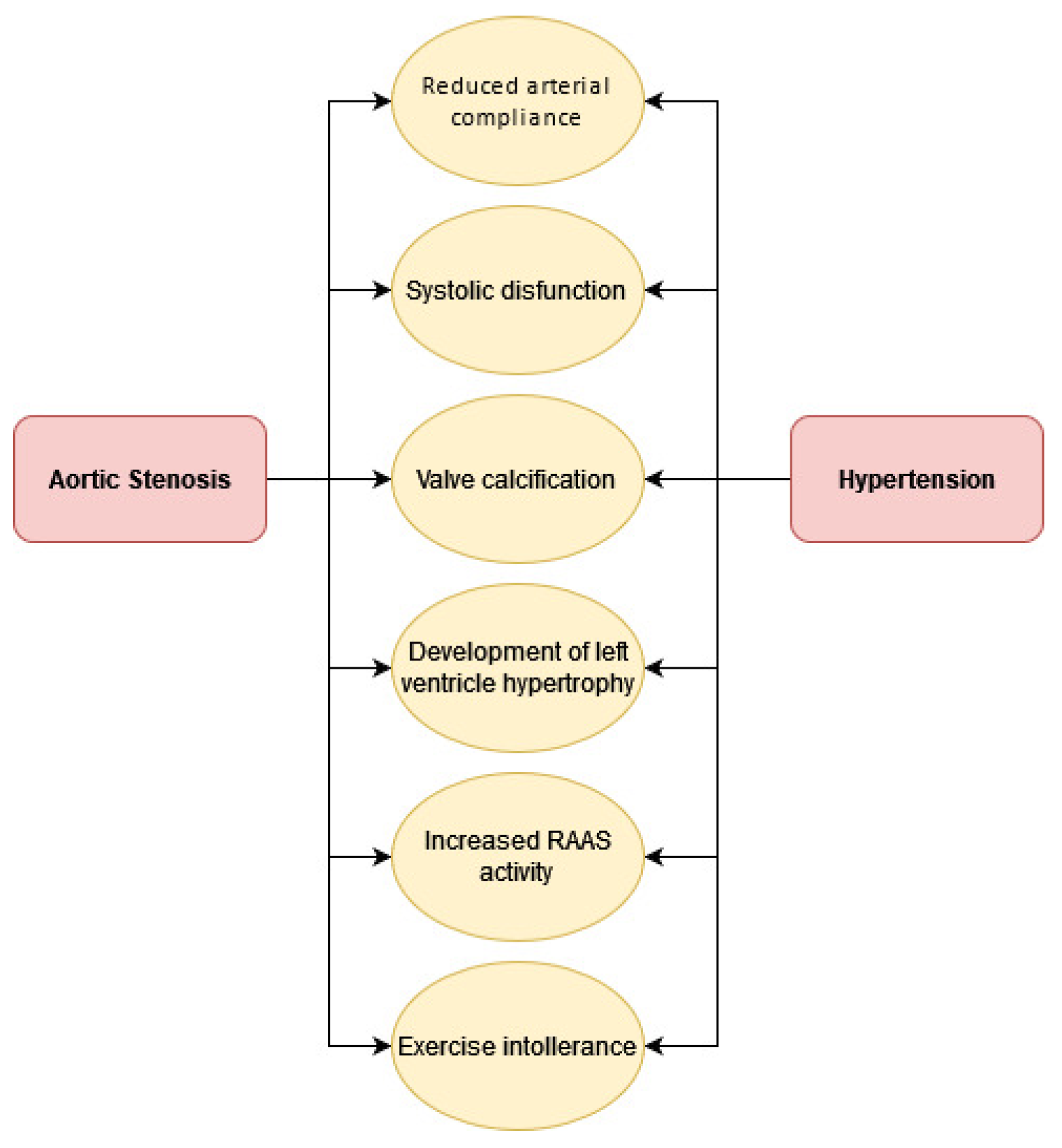
3. Pathophysiology
4. Combined Effects of Aortic Stenosis and Hypertension
5. Challenges in Diagnosis
6. Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; de Bonis, M.; de Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2021, ehab395. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Tastet, L.; Capoulade, R.; Clavel, M.-A.; LaRose, É.; Shen, M.; Dahou, A.; Arsenault, M.; Mathieu, P.; Bédard, É.; Dumesnil, J.G.; et al. Systolic hypertension and progression of aortic valve calcification in patients with aortic stenosis: Results from the PROGRESSA study. Eur. Hear. J. Cardiovasc. Imaging 2016, 18, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kadem, L.; Dumesnil, J.G.; Rieu, R.; Durand, L.-G.; Garcia, D.; Pibarot, P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart 2005, 91, 354–361. [Google Scholar] [CrossRef]
- Mancusi, C.; de Simone, G.; Hitij, J.B.; Sudano, I.; Mahfoud, F.; Parati, G.; Kahan, T.; Barbato, E.; Pierard, L.A.; Garbi, M.; et al. Management of patients with combined arterial hypertension and aortic valve stenosis: A consensus document from the Council on Hypertension and Council on Valvular Heart Disease of the European Society of Cardiology, the European Association of Cardiovascular Imaging (EACVI), and the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 242–250. [Google Scholar] [CrossRef]
- Blais, S.; Meloche-Dumas, L.; Fournier, A.; Dallaire, F.; Dahdah, N. Long-term risk factors for dilatation of the proximal aorta in a large cohort of children with bicuspid aortic valve. Circ. Cardiovasc. Imaging 2020, 13, e009675. [Google Scholar] [CrossRef]
- Grattan, M.; Prince, A.; Ruman, R.; Morgan, C.; Petrovic, M.; Hauck, A.; Young, L.; Franco-Cereceda, A.; Loeys, B.; Mohamed, S.A.; et al. Redictors of bicuspid aortic valve-associated aortopathy in childhood: A report from the MIBAVA Consortium. Circ. Cardiovasc. Imaging 2020, 13, e009717. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular health study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Rahimi, K.; Mohseni, H.; Kiran, A.; Tran, J.; Nazarzadeh, M.; Rahimian, F.; Woodward, M.; Dwyer, T.; MacMahon, S.; Otto, C.M. Elevated blood pressure and risk of aortic valve disease: A cohort analysis of 5.4 million UK adults. Eur. Heart. J. 2018, 39, 3596–3603. [Google Scholar] [CrossRef]
- Linhartova, K.; Filipovsky, J.; Čerbák, R.; Sterbakova, G.; Hanišová, I.; Beránek, V. Severe aortic stenosis and its association with hypertension: Analysis of clinical and echocardiographic parameters. Blood Press. 2007, 16, 122–128. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Lindroos, M.; Kupari, M.; Valvanne, J.; Strandberg, T.; Heikkilä, J.; Tilvis, R. Factors associated with calcific aortic valve degeneration in the elderly. Eur. Heart J. 1994, 15, 865–870. [Google Scholar] [CrossRef]
- Cuniberti, L.A.; Stutzbach, P.G.; Guevara, E.; Yannarelli, G.G.; Laguens, R.P.; Favaloro, R.R. Development of mild aortic valve stenosis in a rabbit model of hypertension. J. Am. Coll. Cardiol. 2006, 47, 2303–2309. [Google Scholar] [CrossRef][Green Version]
- Kaden, J.J.; Dempfle, C.-E.; Grobholz, R.; Tran, H.-T.; Kılıç, R.; Sarıkoç, A.; Brueckmann, M.; Vahl, C.; Hagl, S.; Haase, K.K.; et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 2003, 170, 205–211. [Google Scholar] [CrossRef]
- Satta, J.; Melkko, J.; Pöllänen, R.; Tuukkanen, J.; Pääkkö, P.; Ohtonen, P.; Mennander, A.; Soini, Y. Progression of human aortic valve stenosis is associated with tenascin-C expression. J. Am. Coll. Cardiol. 2002, 39, 96–101. [Google Scholar] [CrossRef]
- Mackie, E.; Scott-Burden, T.; Hahn, A.W.; Kern, F.; Bernhardt, J.; Regenass, S.; Weller, A.; Bühler, F.R. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am. J. Pathol. 1992, 141, 377–388. [Google Scholar]
- Touyz, R.M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance? Hypertension 2004, 44, 248–252. [Google Scholar] [CrossRef]
- Liberman, M.; Bassi, E.; Martinatti, M.K.; Lario, F.C.; Wosniak, J.; Pomerantzeff, P.M.; Laurindo, F.R. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arter. Thromb. Vasc. Biol. 2008, 28, 463–470. [Google Scholar] [CrossRef]
- O’Brien, K.D.; Shavelle, D.M.; Caulfield, M.T.; McDonald, T.O.; Olin-Lewis, K.; Otto, C.; Probstfield, J.L. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation 2002, 106, 2224–2230. [Google Scholar] [CrossRef]
- Helske, S.; Syväranta, S.; Kupari, M.; Lappalainen, J.; Laine, M.; Lommi, J.; Turto, H.; Mäyränpää, M.; Werkkala, K.; Kovanen, P.T.; et al. Possible role for mast cell-derived cathepsin G in the adverse remodelling of stenotic aortic valves. Eur. Hear. J. 2006, 27, 1495–1504. [Google Scholar] [CrossRef]
- Ngo, D.T.; Heresztyn, T.; Mishra, K.; Marwick, T.H.; Horowitz, J.D. Aortic stenosis is associated with elevated plasma levels of asymmetric dimethylarginine (ADMA). Nitric Oxide 2007, 16, 197–201. [Google Scholar] [CrossRef]
- Tsakiris, A.; Doumas, M.; Nearchos, N.; Mavrokefalos, A.; Mpatakis, N.; Skoufas, P. Aortic calcification is associated with age and sex but not left ventricular mass in essential hypertension. J. Clin. Hypertens. 2004, 6, 65–70. [Google Scholar] [CrossRef]
- Helske, S.; Lindstedt, K.A.; Laine, M.; Mäyränpää, M.; Werkkala, K.; Lommi, J.; Turto, H.; Kupari, M.; Kovanen, P.T. Induction of local angiotensin II-producing systems in stenotic aortic valves. J. Am. Coll. Cardiol. 2004, 44, 1859–1866. [Google Scholar] [CrossRef]
- Antonini-Canterin, F.; Huang, G.; Cervesato, E.; Faggiano, P.; Pavan, D.; Piazza, R.; Nicolosi, G.L. Symptomatic aortic stenosis: Does systemic hypertension play an additional role? Hypertension 2003, 41, 1268–1272. [Google Scholar] [CrossRef]
- Aldrugh, S.; Valle, J.E.; Parker, M.W.; Harrington, C.M.; Aurigemma, G.P. Prevalence of left ventricular hypertrophy caused by systemic hypertension preceding the development of severe aortic stenosis. Am. J. Cardiol. 2021, 150, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Lembo, M.; Esposito, R.; Santoro, C.; Iudice, F.L.; Schiano-Lomoriello, V.; Fazio, V.; Grimaldi, M.G.; Trimarco, B.; de Simone, G.; Galderisi, M. Three-dimensional echocardiographic ventricular mass/end-diastolic volume ratio in native hypertensive patients: Relation between stroke volume and geometry. J. Hypertens. 2018, 36, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Rieck, Å.E.; Cramariuc, D.; Boman, K.; Gohlke-Bärwolf, C.; Staal, E.M.; Lønnebakken, M.T.; Rossebø, A.B.; Gerdts, E. Hypertension in aortic stenosis: Implications for left ventricular structure and cardiovascular events. Hypertension 2012, 60, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Briand, M.; Dumesnil, J.G.; Kadem, L.; Tongue, A.G.; Rieu, R.; Garcia, D.; Pibarot, P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J. Am. Coll. Cardiol. 2005, 46, 291–298. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Assessment of aortic stenosis severity: Check the valve but don’t forget the arteries! Heart 2007, 93, 780–782. [Google Scholar] [CrossRef]
- Eleid, M.F.; Nishimura, R.A.; Sorajja, P.; Borlaug, B.A. Systemic hypertension in low-gradient severe aortic stenosis with preserved ejection fraction. Circulation 2013, 128, 1349–1353. [Google Scholar] [CrossRef]
- Miyazaki, S.; Daimon, M.; Miyazaki, T.; Onishi, Y.; Koiso, Y.; Nishizaki, Y.; Ichikawa, R.; Chiang, S.-J.; Makinae, H.; Suzuki, H.; et al. Global longitudinal strain in relation to the severity of aortic stenosis: A two-dimensional speckle-tracking study. Echocardiography 2011, 28, 703–708. [Google Scholar] [CrossRef]
- Lindman, B.; Liu, Q.; Cupps, B.P.; Woodard, P.K.; Novak, E.; Vatterott, A.M.; Bs, D.J.K.; Bs, K.K.; Pasque, M.K. Heterogeneity of systolic dysfunction in patients with severe aortic stenosis and preserved ejection fraction. J. Card. Surg. 2017, 32, 454–461. [Google Scholar] [CrossRef]
- Tadic, M.; Cuspidi, C.; Pencic, B.; Ivanovic, B.; Grassi, G.; Kocijancic, V.; Celic, V. The impact of arterial hypertension on left ventricular strain in patients with aortic stenosis and preserved ejection fraction. J. Hypertens. 2019, 37, 747–753. [Google Scholar] [CrossRef]
- Ng, A.; Prihadi, E.A.; Antoni, M.L.; Bertini, M.; Ewe, S.H.; Marsan, N.A.; Leung, D.Y.; Delgado, V.; Bax, J.J. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur. Hear. J. Cardiovasc. Imaging 2018, 19, 859–867. [Google Scholar] [CrossRef]
- Fries, B.; Liu, D.; Gaudron, P.; Hu, K.; Nordbeck, P.; Ertl, G.; Weidemann, F.; Herrmann, S. Role of global longitudinal strain in the prediction of outcome in patients with severe aortic valve stenosis. Am. J. Cardiol. 2017, 120, 640–647. [Google Scholar] [CrossRef]
- Kusunose, K.; Goodman, A.; Parikh, R.; Barr, T.; Agarwal, S.; Popovic, Z.; Grimm, R.A.; Griffin, B.P.; Desai, M.Y. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ. Cardiovasc. Imaging 2014, 7, 938–945. [Google Scholar] [CrossRef]
- Huded, C.P.; Kusunose, K.; Shahid, F.; Goodman, A.L.; Alashi, A.; Grimm, R.A.; Gillinov, A.M.; Johnston, D.R.; Rodriguez, L.L.; Popović, Z.B.; et al. Novel echocardiographic parameters in patients with aortic stenosis and preserved left ventricular systolic function undergoing surgical aortic valve replacement. Am. J. Cardiol. 2018, 122, 284–293. [Google Scholar] [CrossRef]
- Liga, R.; Gimelli, A.; De Carlo, M.; Marzullo, P.; Pedrinelli, R.; Petronio, A.S. Cardiac sympathetic dysfunction in left ventricular hypertrophy caused by arterial hypertension and degenerative aortic stenosis. J. Nucl. Cardiol. 2020. [Google Scholar] [CrossRef]
- Saeed, S.; Mancia, G.; Rajani, R.; Parkin, D.; Chambers, J.B. Hypertension in aortic stenosis: Relationship with revealed symptoms and functional measures on treadmill exercise. J. Hypertens. 2019, 37, 2209–2215. [Google Scholar] [CrossRef]
- Saeed, S.; Mancia, G.; Rajani, R.; Seifert, R.; Parkin, D.; Chambers, J.B. Exercise treadmill testing in moderate or severe aortic stenosis: The left ventricular correlates of an exaggerated blood pressure rise. J. Am. Hear. Assoc. 2018, 7, e010735. [Google Scholar] [CrossRef]
- Rajani, R.; Rimington, H.; Chambers, J.B. Treadmill exercise in apparently asymptomatic patients with moderate or severe aortic stenosis: Relationship between cardiac index and revealed symptoms. Heart 2010, 96, 689–695. [Google Scholar] [CrossRef]
- Chambers, J.B.; Rajani, R.; Parkin, D.; Saeed, S. Rapid early rise in heart rate on treadmill exercise in patients with asymptomatic moderate or severe aortic stenosis: A new prognostic marker? Open Heart 2019, 6, e000950. [Google Scholar] [CrossRef]
- Lancellotti, P.; Magne, J. Valvuloarterial impedance in aortic stenosis: Look at the load, but do not forget the flow. Eur. J. Echocardiogr. 2011, 12, 354–357. [Google Scholar] [CrossRef]
- Chambers, J. Can high blood pressure mask severe aortic stenosis? J. Heart Valve Dis. 1999, 8, 277–278. [Google Scholar]
- Pibarot, P.; Dumesnil, J.G. New concepts in valvular hemodynamics: Implications for diagnosis and treatment of aortic stenosis. Can. J. Cardiol. 2007, 23, 40B–47B. [Google Scholar] [CrossRef]
- Cramariuc, D.; Cioffi, G.; Rieck, Å.E.; Devereux, R.B.; Staal, E.M.; Ray, S.; Wachtell, K.; Gerdts, E. Low-flow aortic stenosis in asymptomatic patients: Valvular–arterial impedance and systolic function from the SEAS substudy. JACC Cardiovasc. Imaging 2009, 2, 390–399. [Google Scholar] [CrossRef]
- Hachicha, Z.; Dumesnil, J.G.; Pibarot, P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 1003–1011. [Google Scholar] [CrossRef]
- Ito, H.; Mizumoto, T.; Shomura, Y.; Sawada, Y.; Kajiyama, K.; Shimpo, H. The impact of global left ventricular afterload on left ventricular reverse remodeling after aortic valve replacement. J. Card. Surg. 2017, 32, 530–536. [Google Scholar] [CrossRef]
- Otto, C.M. Valvular aortic stenosis: Disease severity and timing of intervention. J. Am. Coll. Cardiol. 2006, 47, 2141–2151. [Google Scholar] [CrossRef]
- Little, S.H.; Chan, K.-L.; Burwash, I.G. Impact of blood pressure on the Doppler echocardiographic assessment of severity of aortic stenosis. Heart 2006, 93, 848–855. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Improving assessment of aortic stenosis. J. Am. Coll. Cardiol. 2012, 60, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, O.W.; Sajadieh, A.; Sabbah, M.; Greve, A.M.; Olsen, M.H.; Boman, K.; Nienaber, C.A.; Kesäniemi, Y.A.; Pedersen, T.R.; Willenheimer, R.; et al. Assessing optimal blood pressure in patients with asymptomatic aortic valve stenosis: The simvastatin ezetimibe in aortic stenosis study (SEAS). Circulation 2016, 134, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Nadir, M.A.; Wei, L.; Elder, D.H.; Libianto, R.; Lim, T.K.; Pauriah, M.; Pringle, S.D.; Doney, A.D.; Choy, A.-M.; Struthers, A.D.; et al. Impact of renin-angiotensin system blockade therapy on outcome in aortic stenosis. J. Am. Coll. Cardiol. 2011, 58, 570–576. [Google Scholar] [CrossRef]
- Sen, J.; Chung, E.; Neil, C.; Marwick, T. Antihypertensive therapies in moderate or severe aortic stenosis: A systematic review and meta-analysis. BMJ Open 2020, 10, e036960. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, A.; Venkatesan, S.; Subramaniam, T.; Jagannathan, V.; Elangovan, S.; Alagesan, R.; Gnanavelu, G.; Dorairajan, S.; Krishna, B.; Chockalingam, V. Safety and efficacy of angiotensin-converting enzyme inhibitors in symptomatic severe aortic stenosis: Symptomatic cardiac obstruction–pilot study of enalapril in aortic stenosis (SCOPE-AS). Am. Heart J. 2004, 147, 740. [Google Scholar] [CrossRef]
- Goel, S.S.; Aksoy, O.; Gupta, S.; Houghtaling, P.L.; Tuzcu, E.M.; Marwick, T.; Mihaljevic, T.; Svensson, L.; Blackstone, E.H.; Griffin, B.P.; et al. Renin-angiotensin system blockade therapy after surgical aortic valve replacement for severe aortic stenosis: A cohort study. Ann. Intern. Med. 2014, 161, 699–710. [Google Scholar] [CrossRef]
- Magne, J.; Guinot, B.; Le Guyader, A.; Bégot, E.; Marsaud, J.-P.; Mohty, D.; Aboyans, V. Relation between renin-angiotensin system blockers and survival following isolated aortic valve replacement for aortic stenosis. Am. J. Cardiol. 2018, 121, 455–460. [Google Scholar] [CrossRef]
- Inohara, T.; Manandhar, P.; Kosinski, A.S.; Matsouaka, R.A.; Kohsaka, S.; Mentz, R.J.; Thourani, V.H.; Carroll, J.D.; Kirtane, A.J.; Bavaria, J.E.; et al. Association of renin-angiotensin inhibitor treatment with mortality and heart failure readmission in patients with transcatheter aortic valve replacement. JAMA 2018, 320, 2231–2241. [Google Scholar] [CrossRef]
- Ochiai, T.; Saito, S.; Yamanaka, F.; Shishido, K.; Tanaka, Y.; Yamabe, T.; Shirai, S.; Tada, N.; Araki, M.; Naganuma, T.; et al. Renin–angiotensin system blockade therapy after transcatheter aortic valve implantation. Heart 2017, 104, 644–651. [Google Scholar] [CrossRef]
- Rodriguez-Gabella, T.; Catalá, P.; Muñoz-García, A.J.; Nombela-Franco, L.; Del Valle, R.; Gutiérrez, E.; Regueiro, A.; Jimenez-Diaz, V.A.; Ribeiro, H.B.; Rivero, F.; et al. Renin-angiotensin system inhibition following transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2019, 74, 631–641. [Google Scholar] [CrossRef]
- Helske, S.; Kupari, M.; Lindstedt, K.A.; Kovanen, P.T. Aortic valve stenosis: An active atheroinflammatory process. Curr. Opin. Lipidol. 2007, 18, 483–491. [Google Scholar] [CrossRef]
- Côté, N.; Couture, C.; Pibarot, P.; Després, J.-P.; Mathieu, P. Angiotensin receptor blockers are associated with a lower remodelling score of stenotic aortic valves. Eur. J. Clin. Investig. 2011, 41, 1172–1179. [Google Scholar] [CrossRef]
- Capoulade, R.; Clavel, M.-A.; Mathieu, P.; Côté, N.; Dumesnil, J.G.; Arsenault, M.; Bédard, É.; Pibarot, P. Impact of hypertension and renin-angiotensin system inhibitors in aortic stenosis. Eur. J. Clin. Investig. 2013, 43, 1262–1272. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamamoto, H.; Takeuchi, M.; Kisanuki, A.; Akasaka, T.; Ohte, N.; Hirano, Y.; Yoshida, K.; Nakatani, S.; Takeda, Y.; et al. Risk factors for progression of degenerative aortic valve disease in the Japanese—The Japanese aortic stenosis study (JASS) prospective analysis. Circ. J. 2015, 79, 2050–2057. [Google Scholar] [CrossRef]
- Ardehali, R.; Leeper, N.J.; Wilson, A.M.; Heidenreich, P. The effect of angiotensin-converting enzyme inhibitors and statins on the progression of aortic sclerosis and mortality. J. Heart Valve Dis. 2012, 21, 337–343. [Google Scholar]
- Danilczyk, U.; Penninger, J. Angiotensin-converting enzyme ii in the heart and the kidney. Circ. Res. 2006, 98, 463–471. [Google Scholar] [CrossRef]
- Dalsgaard, M.; Iversen, K.; Kjaergaard, J.; Grande, P.; Goetze, J.P.; Clemmensen, P.; Hassager, C. Short-term hemodynamic effect of angiotensin-converting enzyme inhibition in patients with severe aortic stenosis: A placebo-controlled, randomized study. Am. Heart J. 2014, 167, 226–234. [Google Scholar] [CrossRef]
- Bang, C.N.; Greve, A.M.; Køber, L.; Rossebø, A.B.; Ray, S.; Boman, K.; Nienaber, C.A.; Devereux, R.B.; Wachtell, K. Renin–angiotensin system inhibition is not associated with increased sudden cardiac death, cardiovascular mortality or all-cause mortality in patients with aortic stenosis. Int. J. Cardiol. 2014, 175, 492–498. [Google Scholar] [CrossRef]
- Bull, S.; Loudon, M.; Francis, J.M.; Joseph, J.; Gerry, S.; Karamitsos, T.; Prendergast, B.D.; Banning, A.; Neubauer, S.; Myerson, S.G. A prospective, double-blind, randomized controlled trial of the angiotensin-converting enzyme inhibitor ramipril in aortic stenosis (RIAS trial). Eur. Hear. J. Cardiovasc. Imaging 2015, 16, 834–841. [Google Scholar] [CrossRef]
- Helske-Suihko, S.; Laine, M.; Lommi, J.; Kaartinen, M.; Werkkala, K.; Kovanen, P.T.; Kupari, M. Is blockade of the renin-angiotensin system able to reverse the structural and functional remodeling of the left ventricle in severe aortic stenosis? J. Cardiovasc. Pharmacol. 2015, 65, 233–240. [Google Scholar] [CrossRef]
- O’Brien, K.D.; Probstfield, J.L.; Caulfield, M.T.; Nasir, K.; Takasu, J.; Shavelle, D.M.; Wu, A.H.; Zhao, X.-Q.; Budoff, M.J. Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch. Intern. Med. 2005, 165, 858–862. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Tsujino, T.; Naito, Y.; Ezumi, A.; Lee-Kawabata, M.; Nakao, S.; Goda, A.; Sakata, Y.; Yamamoto, K.; Daimon, T.; et al. Administration of angiotensin-converting enzyme inhibitors is associated with slow progression of mild aortic stenosis in Japanese patients. Heart Vessel. 2011, 26, 252–257. [Google Scholar] [CrossRef]
- Witherow, F.N.; Helmy, A.; Webb, D.J.; Fox, K.; Newby, D.E. Bradykinin contributes to the vasodilator effects of chronic angiotensin-converting enzyme inhibition in patients with heart failure. Circulation 2001, 104, 2177–2181. [Google Scholar] [CrossRef]
- Nikolaidis, L.A.; Doverspike, A.; Huerbin, R.; Hentosz, T.; Shannon, R.P. Angiotensin-converting enzyme inhibitors improve coronary flow reserve in dilated cardiomyopathy by a bradykinin-mediated, nitric oxide-dependent mechanism. Circulation 2002, 105, 2785–2790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamoto, D.; Takai, S.; Miyazaki, M. Prediction of interaction mode between a typical ACE inhibitor and MMP-9 active site. Biochem. Biophys. Res. Commun. 2007, 354, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.I. Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin–angiotensin system. Circulation 2004, 109, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hansson, N.H.; Sörensen, J.; Harms, H.J.; Kim, W.Y.; Nielsen, R.; Tolbod, L.P.; Frøkiær, J.; Bouchelouche, K.; Dodt, K.K.; Sihm, I.; et al. Metoprolol reduces hemodynamic and metabolic overload in asymptomatic aortic valve stenosis patients: A randomized trial. Circ. Cardiovasc. Imaging 2017, 10, e006557. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Temporelli, P.L.; Cicoira, M.; Gaibazzi, N.; Cioffi, G.; Nistri, S.; Magatelli, M.; Tavazzi, L.; Faggiano, P. Beta-blockers can improve survival in medically-treated patients with severe symptomatic aortic stenosis. Int. J. Cardiol. 2015, 190, 15–17. [Google Scholar] [CrossRef]
- Bang, C.N.; Greve, A.M.; Rossebø, A.B.; Ray, S.; Egstrup, K.; Boman, K.; Nienaber, C.; Okin, P.M.; Devereux, R.B.; Wachtell, K. Antihypertensive treatment with β-blockade in patients with asymptomatic aortic stenosis and association with cardiovascular events. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Dumonteil, N.; Vaccaro, A.; Despas, F.; Labrunee, M.; Marcheix, B.; Lambert, E.; Esler, M.; Carrie, D.; Senard, J.-M.; Galinier, M.; et al. Transcatheter aortic valve implantation reduces sympathetic activity and normalizes arterial spontaneous baroreflex in patients with aortic stenosis. JACC Cardiovasc. Interv. 2013, 6, 1195–1202. [Google Scholar] [CrossRef]
- Claveau, D.; Gossack, A.; Friedland, S.N.; Afilalo, J.; Rudski, L. Complications associated with nitrate use in patients presenting with acute pulmonary edema and concomitant moderate or severe aortic stenosis. Ann. Emerg. Med. 2015, 66, 355–362. [Google Scholar] [CrossRef]
- Khot, U.N.; Novaro, G.M.; Popovic, Z.; Mills, R.M.; Thomas, J.D.; Tuzcu, E.M.; Hammer, D.; Nissen, S.E.; Francis, G.S. Nitroprusside in critically ill patients with left ventricular dysfunction and aortic stenosis. N. Engl. J. Med. 2003, 348, 1756–1763. [Google Scholar] [CrossRef]
- Stewart, R.A.; Kerr, A.J.; Cowan, B.R.; Young, A.A.; Occleshaw, C.; Richards, A.M.; Edwards, C.; Whalley, G.; Freidlander, D.; Williams, M.; et al. A randomized trial of the aldosterone-receptor antagonist eplerenone in asymptomatic moderate-severe aortic stenosis. Am. Heart J. 2008, 156, 348–355. [Google Scholar] [CrossRef]
- Takimoto, E.; Champion, H.C.; Li, M.; Belardi, D.; Ren, S.; Rodriguez, E.R.; Bedja, D.; Gabrielson, K.L.; Wang, Y.; Kass, D.A. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 2005, 11, 214–222. [Google Scholar] [CrossRef]
- Lepore, J.J.; Maroo, A.; Bigatello, L.M.; Dec, G.W.; Zapol, W.M.; Bloch, K.D.; Semigran, M.J. Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension: Combined administration with inhaled nitric oxide. Chest 2005, 127, 1647–1653. [Google Scholar] [CrossRef]
- Lewis, G.D.; Shah, R.; Shahzad, K.; Camuso, J.M.; Pappagianopoulos, P.P.; Hung, J.; Tawakol, A.; Gerszten, R.E.; Systrom, D.; Bloch, K.D.; et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 2007, 116, 1555–1562. [Google Scholar] [CrossRef]
- Lindman, B.R.; Zajarias, A.; Madrazo, J.A.; Shah, J.; Gage, B.F.; Novak, E.; Johnson, S.N.; Chakinala, M.M.; Hohn, T.A.; Saghir, M.; et al. Effects of phosphodiesterase type 5 inhibition on systemic and pulmonary hemodynamics and ventricular function in patients with severe symptomatic aortic stenosis. Circulation 2012, 125, 2353–2362. [Google Scholar] [CrossRef]
- Bermejo, J.; Yotti, R.; García-Orta, R.; Sánchez-Fernández, P.L.; Castaño, M.; Segovia-Cubero, J.; Escribano-Subías, P.; San Román, J.A.; Borrás, X.; Alonso-Gómez, A.; et al. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: A multicenter, double-blind, randomized clinical. Eur. Heart J. 2018, 39, 1255–1264. [Google Scholar] [CrossRef]
- Saeed, S.; Mancia, G.; Rajani, R.; Parkin, D.; Chambers, J.B. Antihypertensive treatment with calcium channel blockers in patients with moderate or severe aortic stenosis: Relationship with all-cause mortality. Int. J. Cardiol. 2020, 298, 122–125. [Google Scholar] [CrossRef]
- Sutanto, H.; Dobrev, D.; Heijman, J. Angiotensin receptor-neprilysin inhibitor (ARNI) and cardiac arrhythmias. Int. J. Mol. Sci. 2021, 22, 8994. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Q.; Wang, Q.; Zhang, Q.; Zheng, Y.; Wang, L.; Jin, Q. Protection of sacubitril/valsartan against pathological cardiac remodeling by inhibiting the NLRP3 inflammasome after relief of pressure overload in mice. Cardiovasc. Drugs Ther. 2020, 34, 629–640. [Google Scholar] [CrossRef]
- Desai, A.S.; Solomon, S.D.; Shah, A.M.; Claggett, B.L.; Fang, J.C.; Izzo, J.; McCague, K.; Abbas, C.A.; Rocha, R.; Mitchell, G.F.; et al. Effect of sacubitril-valsartan vs. enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: A randomized clinical trial. JAMA 2019, 322, 1077–1084. [Google Scholar] [CrossRef]
- Amat-Santos, I.J.; Sánchez-Luna, J.P.; Abu-Assi, E.; Viu, M.M.; Cruz-Gonzalez, I.; Nombela-Franco, L.; García, A.J.M.; Blas, S.G.; Hernandez, J.M.T.; Romaguera, R.; et al. Rationale and design of the dapagliflozin after transcatheter aortic valve implantation (DapaTAVI) randomized trial. Eur. J. Heart Fail. 2021. [Google Scholar] [CrossRef] [PubMed]

| Trial, Author Year | Design | Sample Size | Medication or Class | Follow-Up | Results |
|---|---|---|---|---|---|
| Khot, U. et al., 2003 [82] | prospective | 25 | Nitroprussiate | 24 h | Nitroprusside improves heart function in patients with decompensated heart failure due to severe left ventricular systolic dysfunction and severe aortic stenosis. |
| SCOPE-AS, Chockalingam et al., 2004 [55] | randomized double-blind | 52 | Enalapril 2.5 mg bis in die titrated up to 10 mg bis in die vs. placebo | 12 weeks | NYHA class, Borg index and 6 min walking test improvement. |
| O’Brien et al., 2005 [71] | retrospective | 123 | ACE-inhibitors | 2.6 ± 1.8 years | Less calcification of the aorta on CT. |
| Ralph A H Stewart et al., 2008 [83] | randomized | 65 | Eplerenone 100 mg/die | 19 months | In patients with moderate–severe aortic stenosis, eplerenone does not slow down the onset of ventricular dysfunction, does not reduce the mass of the left ventricle and does not reduce the progression to valve stenosis. |
| Nadir et al., 2011 [53] | retrospective | 2117 | RAAS blockers | 4.2 years | Lower frequency of mortality and cardiovascular events. |
| Lindman BR et al., 2012 [87] | open-label | 22 | Sildenafil 40 mg or 80 mg | A single dose of Sildenafil is safe and well-tolerated in patients with symptomatic severe aortic stenosis. It also improves stroke volume and reduces pre- and postload. | |
| Eleid MF et al., 2013 [30] | prospective | 24 | Nitroprussiate | Nitroprusside is safe in patients with low-flow LG AS. | |
| Capoulade et al., 2013 [63] | retrospective | 338 | RAAS blockers | 6.2 ± 2.4 years | Angiotensin II receptor blocker I, but not ACE-I, was associated with slower progression of AS and lower mortality. |
| Dalsgaard et al., 2014 [67] | randomized | 44 | Trandolapril up to 2 mg/die | 3 days | Blood pressure, peripheral resistance and left ventricular end-systolic volume were significantly reduced. |
| Goel et al., 2014 [56] | retrospective | 1752 | RAAS blockers | 5.8 years | Better long-term survival after aortic valve replacement. |
| Bang et al., 2014 [68] | prospective | 1873 | RAAS blockers | 4.3 ± 0.9 years | Slowed progression of the ventricular mass. |
| RIAS, 2015 [69] | randomized double-blind | 100 | Ramipril 10 mg vs. placebo | 1 year | Improved systolic function, decreased left ventricular mass and slight reduction in left ventricular mass with Ramipril. |
| Helske-Suishko et al., 2015 [70] | randomized | 51 | Candesartan | 5 months | No improvement. |
| Yamamoto et al., 2015 [64] | prospective | 359 | No intervention | 3 years | Angiotensin II receptor blockers were associated with a smaller decrease in the indexed valve area in patients with AS jet velocity <2 m/s. |
| Claveau et al., 2015 [81] | retrospective | 195 | Nitrates | When nitroglycerin was used for acute pulmonary edema in patients with moderate and severe aortic stenosis, the risk of clinically detected hypotension as an adverse event was comparable to patients without aortic stenosis. | |
| Bang et al., 2017 [79] | prospective | 1873 | Beta-blockers | 4.3 ± 0.9 years | Lower mortality. |
| Magne et al., 2018 [57] | retrospective | 508 | RAAS blockers | 4.8 ± 2.7 years | Better long-term survival after valve replacement. |
| Inohara et al., 2018 [58] | retrospective | 21312 | RAAS blockers | 1 year | Lower mortality and lower risk of rehospitalization 1 year after TAVI. |
| Ochiai et al., 2018 [59] | retrospective | 1215 | RAAS blockers | 1.1 years | Lower mortality and greater reduction in ventricular mass 1 year after TAVI. |
| SIOVAC 2018 [88] | randomized | 200 | Sildenafil | 6 months | Worst clinical outcome of patients treated with Sildenafil compared to placebo. |
| Rodriguez-Gabella et al., 2019 [60] | retrospective | 2785 | RAAS blockers | 3 years | Reduced cardiovascular mortality at 1 and 3 years after TAVI. |
| Saeed et al., 2020 [89] | retrospective | 314 | Calcium channel blocker | 2.9 ± 2.9 years | Sevenfold increased risk of all-cause mortality. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basile, C.; Fucile, I.; Lembo, M.; Manzi, M.V.; Ilardi, F.; Franzone, A.; Mancusi, C. Arterial Hypertension in Aortic Valve Stenosis: A Critical Update. J. Clin. Med. 2021, 10, 5553. https://doi.org/10.3390/jcm10235553
Basile C, Fucile I, Lembo M, Manzi MV, Ilardi F, Franzone A, Mancusi C. Arterial Hypertension in Aortic Valve Stenosis: A Critical Update. Journal of Clinical Medicine. 2021; 10(23):5553. https://doi.org/10.3390/jcm10235553
Chicago/Turabian StyleBasile, Christian, Ilaria Fucile, Maria Lembo, Maria Virginia Manzi, Federica Ilardi, Anna Franzone, and Costantino Mancusi. 2021. "Arterial Hypertension in Aortic Valve Stenosis: A Critical Update" Journal of Clinical Medicine 10, no. 23: 5553. https://doi.org/10.3390/jcm10235553
APA StyleBasile, C., Fucile, I., Lembo, M., Manzi, M. V., Ilardi, F., Franzone, A., & Mancusi, C. (2021). Arterial Hypertension in Aortic Valve Stenosis: A Critical Update. Journal of Clinical Medicine, 10(23), 5553. https://doi.org/10.3390/jcm10235553








