Central Sleep Apnea Is Associated with an Abnormal P-Wave Terminal Force in Lead V1 in Patients with Acute Myocardial Infarction Independent from Ventricular Function
Abstract
:1. Introduction
2. Materials and Methods
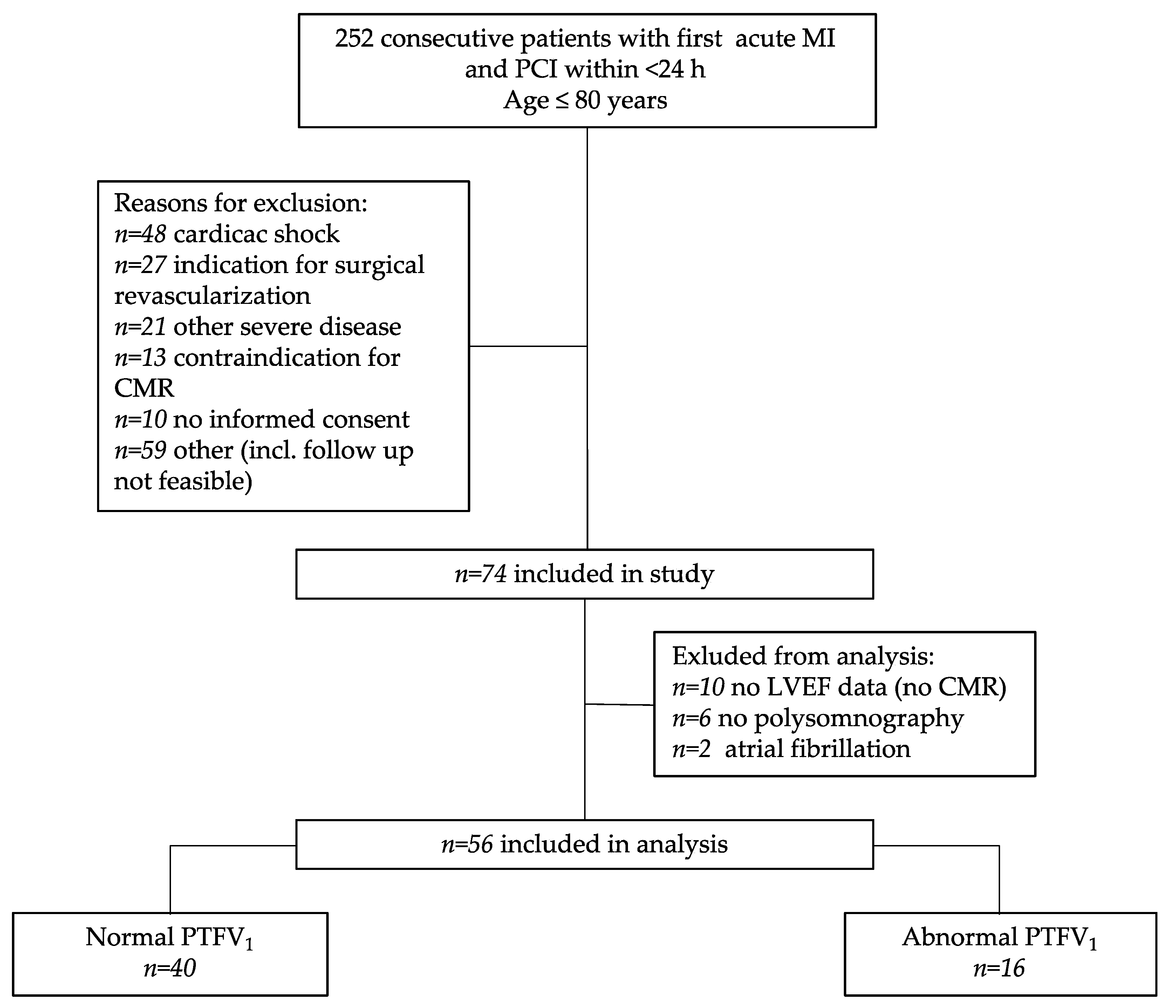
2.1. Study Approval and Design
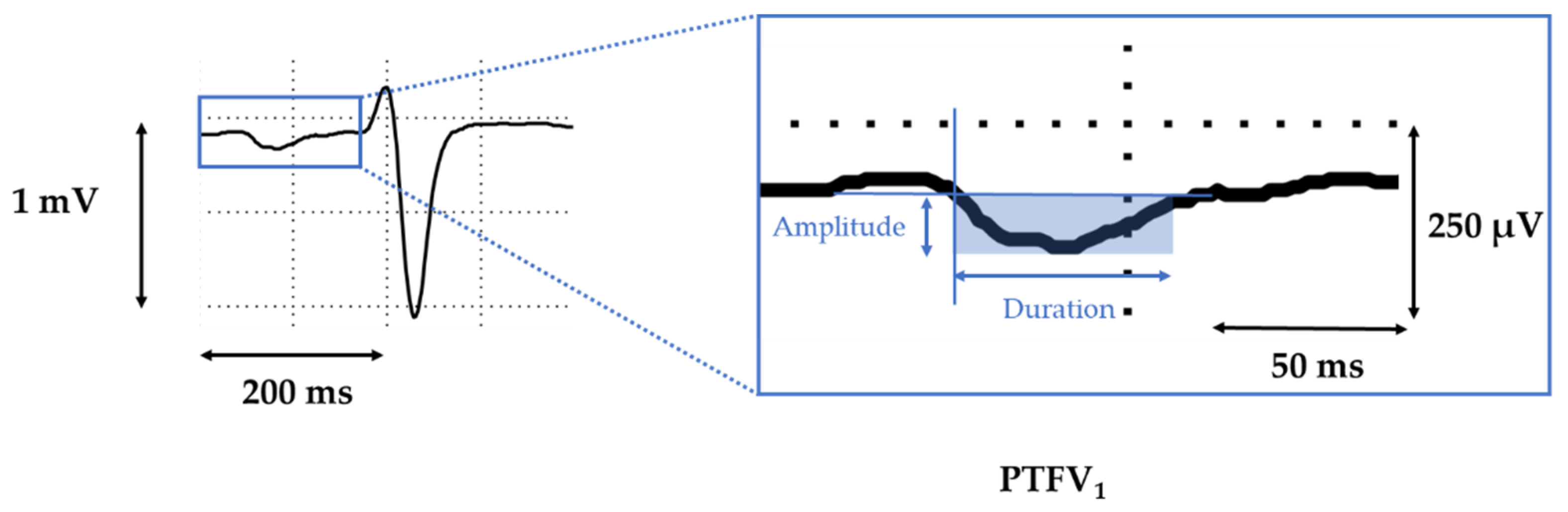
2.2. Electrocardiography
2.3. Polysomnography
2.4. Cardiovascular Magnetic Resonance
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Central Sleep Apnea Is Independently Associated with Abnormal PTFV1
3.3. PTFV1 as a Diagnostic Marker for Predicting Sleep-Disordered Breathing
4. Discussion
4.1. Possible Mechanisms for an Abnormal PTFV1 in SDB
4.2. PTFV1 as a Diagnostic Marker for SDB and SDB-Related Arrhythmias
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Inter-Observer Reproducibility | |||
|---|---|---|---|
| ICC | CIlower | CIupper | |
| PTFV1 | 0.888 | 0.647 | 0.951 |
References
- Uchôa, C.H.G.; Danzi-Soares, N.D.J.; Nunes, F.S.; de Souza, A.A.L.; Nerbass, F.B.; Pedrosa, R.P.; César, L.A.M.; Lorenzi-Filho, G.; Drager, L.F. Impact of OSA on cardiovascular events aft er coronary artery bypass surgery. Chest 2015, 147, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, O.; Lamp, B.; Faber, L.; Teschler, H.; Horstkotte, D.; Töpfer, V. Sleep-disordered breathing in patients with symptomatic heart failure. A contemporary study of prevalence in and characteristics of 700 patients. Eur. J. Hear. Fail. 2007, 9, 251–257. [Google Scholar] [CrossRef]
- Buchner, S.; Greimel, T.; Hetzenecker, A.; Luchner, A.; Hamer, O.W.; Debl, K.; Poschenrieder, F.; Fellner, C.; Riegger, G.A.; Pfeifer, M.; et al. Natural course of sleep-disordered breathing after acute myocardial infarction. Eur. Respir. J. 2012, 40, 1173–1179. [Google Scholar] [CrossRef]
- Lebek, S.; Pichler, K.; Reuthner, K.; Trum, M.; Tafelmeier, M.; Mustroph, J.; Camboni, D.; Rupprecht, L.; Schmid, C.; Maier, L.S.; et al. Enhanced CaMKII-Dependent Late INa Induces Atrial Proarrhythmic Activity in Patients with Sleep-Disordered Breathing. Circ. Res. 2020, 126, 603–615. [Google Scholar] [CrossRef]
- Mehra, R.; Benjamin, E.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef]
- Tung, P.; Anter, E. Atrial fibrillation and sleep apnea: Considerations for a dual epidemic. J. Atr. Fibrillation 2016, 8, 84–90. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Sánchez-De-La-Torre, M.; Sánchez-De-La-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- Traaen, G.M.; Aakerøy, L.; Hunt, T.-E.; Øverland, B.; Bendz, C.; Sande, L.Ø.; Aakhus, S.; Fagerland, M.W.; Steinshamn, S.; Anfinsen, O.-G.; et al. Effect of Continuous Positive Airway Pressure on Arrhythmia in Atrial Fibrillation and Sleep Apnea: A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Woehrle, H.; Wegscheider, K.; Angermann, C.; d’Ortho, M.-P.; Erdmann, E.; Lévy, P.; Simonds, A.K.; Somers, V.K.; Zannad, F.; et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N. Engl. J. Med. 2015, 373, 1095–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrisjr, J.J.; Estesjr, E.H.; Whalen, R.E.; Thompsonjr, H.K.; Mcintosh, H.D. P-Wave Analysis in Valvular Heart Disease. Circulation 1964, 29, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Eranti, A.; Aro, A.L.; Kerola, T.; Anttonen, O.; Rissanen, H.A.; Tikkanen, J.T.; Junttila, M.J.; Kenttä, T.V.; Knekt, P.; Huikuri, H.V. Prevalence and prognostic significance of abnormal P terminal force in lead V1 of the ECG in the general population. Circ. Arrhythmia Electrophysiol. 2014, 7, 1116–1121. [Google Scholar] [CrossRef] [Green Version]
- Kamel, H.; Hunter, M.; Moon, Y.P.; Yaghi, S.; Cheung, K.; Di Tullio, M.R.; Okin, P.M.; Sacco, R.L.; Soliman, E.Z.; Elkind, M.S. Electrocardiographic left atrial abnormality and risk of stroke: Northern manhattan study. Stroke 2015, 46, 3208–3212. [Google Scholar] [CrossRef] [Green Version]
- Goda, T.; Sugiyama, Y.; Ohara, N.; Ikegami, T.; Watanabe, K.; Kobayashi, J.; Takahashi, D. P-Wave Terminal Force in Lead V1 Predicts Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tamura, A.; Torigoe, K.; Kawano, Y.; Shinozaki, K.; Kotoku, M.; Kadota, J. Abnormal P-wave terminal force in lead V1 is associated with cardiac death or hospitalization for heart failure in prior myocardial infarction. Hear. Vessel. 2013, 28, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-Dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ. Res. 2010, 106, 1134–1144. [Google Scholar] [CrossRef] [Green Version]
- Rossi, V.A.; Stradling, J.R.; Kohler, M. Effects of obstructive sleep apnoea on heart rhythm. Eur. Respir. J. 2012, 41, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM manual for the scoring of sleep and associated events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [Green Version]
- Lebek, S.; Wester, M.; Pec, J.; Poschenrieder, F.; Tafelmeier, M.; Fisser, C.; Provaznik, Z.; Schopka, S.; Debl, K.; Schmid, C.; et al. Abnormal P-wave terminal force in lead V 1 is a marker for atrial electrical dysfunction but not structural remodelling. ESC Heart Fail. 2021, 8, 4055–4066. [Google Scholar] [CrossRef]
- Ludka, O.; Stepanova, R.; Vyskocilova, M.; Galkova, L.; Mikolaskova, M.; Belehrad, M.; Kostalova, J.; Mihalova, Z.; Drozdova, A.; Hlasensky, J.; et al. Sleep apnea prevalence in acute myocardial infarction - The Sleep Apnea in Post-acute Myocardial Infarction Patients (SAPAMI) Study. Int. J. Cardiol. 2014, 176, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Florés, M.; de Batlle, J.; Sánchez-De-La-Torre, A.; Sánchez-De-La-Torre, M.; Aldomá, A.; Worner, F.; Galera, E.; Seminario, A.; Torres, G.; Dalmases, M.; et al. Central sleep apnoea is related to the severity and short-term prognosis of acute coronary syndrome. PLOS ONE 2016, 11, e0167031. [Google Scholar] [CrossRef] [Green Version]
- Lanfranchi, P.A.; Somers, V.K.; Braghiroli, A.; Corrà, U.; Eleuteri, E.; Giannuzzi, P. Prevalence and Implications for Arrhythmic Risk. Circulation 2003, 107, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghi, S.; Kamel, H.; Elkind, M.S.V. Atrial cardiopathy: A mechanism of cryptogenic stroke. Expert Rev. Cardiovasc. Ther. 2017, 15, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Okin, P.M.; Elkind, M.S.V.; Iadecola, C. Atrial Fibrillation and Mechanisms of Stroke: Time for a New Model. Stroke 2016, 47, 895–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaghi, S.; Boehme, A.K.; Hazan, R.; Hod, E.A.; Canaan, A.; Andrews, H.F.; Kamel, H.; Marshall, R.S.; Elkind, M.S. Atrial Cardiopathy and Cryptogenic Stroke: A Cross-sectional Pilot Study. J. Stroke Cerebrovasc. Dis. 2016, 25, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, H.; Longstreth, J.W.; Tirschwell, D.L.; Kronmal, R.A.; Broderick, J.P.; Palesch, Y.Y.; Meinzer, C.; Dillon, C.; Ewing, I.; Spilker, J.A.; et al. The AtRial Cardiopathy and Antithrombotic Drugs In prevention after cryptogenic stroke randomized trial: Rationale and methods. Int. J. Stroke 2019, 14, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Ruff, H.M.; Weber, S.L.; Bellmann, S.; Sowa, T.; Schulte, T.; Anderson, M.E.; Grandi, E.; Bers, D.; Backs, J.; et al. Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late INa augmentation leading to cellular Na and Ca overload. Circ. Res. 2011, 108, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, J.R.; Joiner, M.-L.A.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef] [Green Version]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent Hypoxemia and OSA Implications for Comorbidities. Chest 2015, 147, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Varadarajan, V.; Ambale-Venkatesh, B.; Hong, S.Y.; Habibi, M.; Ashikaga, H.; Wu, C.O.; Chen, L.Y.; Heckbert, S.R.; Bluemke, D.A.; Lima, J.A.C. Association of Longitudinal Changes in NT-proBNP With Changes in Left Atrial Volume and Function: MESA. Am. J. Hypertens. 2021, 34, 626–635. [Google Scholar] [CrossRef]
- Petersson, R.; Berge, H.M.; Gjerdalen, G.F.; Carlson, J.; Holmqvist, F.; Steine, K.; Platonov, P.G. P-wave morphology is unaffected by atrial size: A study in healthy athletes. Ann. Noninvasive Electrocardiol. 2014, 19, 366–373. [Google Scholar] [CrossRef]
- Tsao, C.W.; Josephson, M.E.; Hauser, T.H.; O'Halloran, T.D.; Agarwal, A.; Manning, W.J.; Yeon, S.B. Accuracy of electrocardiographic criteria for atrial enlargement: Validation with cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.; Redline, S.; Yaggi, H.K.; Wu, R.; Zhao, C.G.; Ostfeld, R.; Menegus, M.; Tracy, D.; Brush, E.; Appel, W.D.; et al. Obstructive sleep apnea and acute myocardial infarction severity: Ischemic preconditioning? Sleep Breath. 2013, 17, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Buchner, S.; Satzl, A.; Debl, K.; Hetzenecker, A.; Luchner, A.; Husser, O.; Hamer, O.W.; Poschenrieder, F.; Fellner, C.; Zeman, F.; et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur. Hear. J. 2014, 35, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-H.; Khoo, S.-M.; Chan, Y.Y.M.; Wong, H.-B.; Low, A.F.; Phua, Q.-H.; Richards, A.M.; Tan, H.-C.; Yeo, T.-C. Severe obstructive sleep apnea and outcomes following myocardial infarction. J. Clin. Sleep Med. 2011, 7, 616–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, L.C.L.; Souza, A.C.; Garcia, G.; Sabino, M.; Brito, M.; Maraux, M.; Rabelo, M.M.N.; Esteves, J.P. Obstructive sleep apnea affects hospital outcomes of patients with non-ST-elevation acute coronary syndromes. Sleep 2012, 35, 1241–1245. [Google Scholar] [CrossRef]
- Lebek, S.; Plößl, A.; Baier, M.; Mustroph, J.; Tarnowski, D.; Lücht, C.; Schopka, S.; Flörchinger, B.; Schmid, C.; Zausig, Y.; et al. The novel CaMKII inhibitor GS-680 reduces diastolic SR Ca leak and prevents CaMKII-dependent pro-arrhythmic activity. J. Mol. Cell. Cardiol. 2018, 118, 159–168. [Google Scholar] [CrossRef]
| Normal PTFV1 (n = 40) | Abnormal PTFV1 (n = 16) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | ||
| Age | [years] | 53.88 | ±9.87 | 57.88 | ±9.63 | 0.174 T |
| BMI | [kg*m−2] | 28.52 | ±3.06 | 28.82 | ±3.99 | 0.771 T |
| Male | [n, %] | 34 (85%) | n.a. | 11 (68.8%) | n.a. | 0.263 Chi |
| Arterial hypertension | [n, %] | 19 (47.5%) | n.a. | 9 (60%) | n.a. | 0.409 F |
| Diabetes mellitus | [n, %] | 6 (15%) | n.a. | 3 (20%) | n.a. | 0.692 F |
| Hypercholesterolemia | [n, %] | 12 (30%) | n.a. | 5 (33.3%) | n.a. | 1.000 F |
| LDL-cholesterol | [mg*dL−1] | 136.53 | ±35.33 | 111.57 | ±23.4 | 0.018 T |
| Smoking | [n, %] | 30 (75%) | n.a. | 11 (73.3%) | n.a. | 1.000 F |
| SDB | [n, %] | 17 (42.5%) | n.a. | 13 (86.7%) | n.a. | 0.003Chi |
| STEMI | [n, %] | 37 (92.5%) | n.a. | 11 (68.8%) | n.a. | 0.035F |
| CK max | [U*L−1] | 1993.49 | ±1393.21 | 2232.07 | ±1588.63 | 0.590 T |
| Troponin I max | [ng*mL−1] | 29.11 | ±66.63 | 40.26 | ±90.6 | 0.638 T |
| NT-proBNP at discharge | [pg*mL−1] | 774.47 | ±835.61 | 2201.19 | ±1390.37 | 0.002W |
| eGFR | [mL*min−1*1, 73 m−2] | 95.16 | ±16.53 | 83.63 | ±28.03 | 0.152 W |
| Resting heart rate | [min−1] | 75.46 | ±12.13 | 75.33 | ±22.14 | 0.983 W |
| Systolic blood pressure | [mmHg] | 127.43 | ±22.79 | 127.67 | ±17.65 | 0.971 T |
| Diastolic blood pressure | [mmHg] | 78.43 | ±12.95 | 75.8 | ±11.38 | 0.493 T |
| LV EF | [%] | 48.93 | ±7.45 | 43.15 | ±11.51 | 0.035T |
| RV EF | [%] | 58.25 | ±8.98 | 59 | ±11.24 | 0.808 T |
| TAPSE | [mm] | 20.12 | ±6.01 | 19.99 | ±4.33 | 0.943 T |
| Systolic LA area | [cm2] | 25.9 | ±4.19 | 24.67 | ±3.53 | 0.369 T |
| Diastolic LA area | [cm2] | 18.11 | ±3.03 | 18.44 | ±3.82 | 0.764 T |
| LA FAC | [%] | 32.56 | ±8.41 | 30.75 | ±11.45 | 0.574 T |
| ACEi/ARB at discharge | [n, %] | 38 (97.4%) | n.a. | 15 (100%) | n.a. | 1.000 F |
| ACEi/ARB at admission | [n, %] | 4 (10%) | n.a. | 1 (6.7%) | n.a. | 1.000 F |
| β-Blocker at discharge | [n, %] | 37 (97.4%) | n.a. | 14 (93.3%) | n.a. | 0.490 F |
| β-Blocker at admission | [n, %] | 1 (2.5%) | n.a. | 1 (6.7%) | n.a. | 0.475 F |
| Loop diuretics at discharge | [n, %] | 14 (36.8%) | n.a. | 8 (53.3%) | n.a. | 0.272 Chi |
| Loop diuretics at admission | [n, %] | 0 | n.a. | 0 | n.a. | n.a. |
| MRA at discharge | [n, %] | 16 (42.1%) | n.a. | 10 (66.7%) | n.a. | 0.107 Chi |
| MRA at admission | [n, %] | 0 | n.a. | 0 | n.a. | n.a. |
| Normal PTFV1 (n = 40) | Abnormal PTFV1 (n = 16) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | ||
| SDB | [n, %] | 17 (42.5%) | n.a. | 13 (86.7%) | n.a. | 0.003Chi |
| -OSA | [n, %] | 10 (25.6%) | n.a. | 6 (40%) | n.a. | 0.333 F |
| -CSA | [n, %] | 7 (17.9%) | n.a. | 7 (46.7%) | n.a. | 0.043F |
| AHI | [h-1] | 14.64 | ±13.91 | 36.14 | ±24.87 | <0.001T |
| oAHI | [h-1] | 8.10 | ±8.16 | 12.82 | ±10.43 | 0.084 T |
| cAHI | [h-1] | 6.75 | ±9.55 | 23.32 | ±27.03 | 0.034W |
| ODI | [h-1] | 11.39 | ±9.88 | 28.77 | ±23.69 | 0.018W |
| SaO2 mean | % | 93.18 | ±2.26 | 93.00 | ±1.73 | 0.783 T |
| SaO2 min | % | 85.74 | ±5.87 | 82.20 | ±6.09 | 0.055 T |
| Sleep efficiency | % | 72.15 | ±16.25 | 69.95 | ±12.77 | 0.653 T |
| REM | % | 16.07 | ±6.17 | 14.13 | ±7.23 | 0.327 T |
| ESS | 7.32 | ±4.57 | 5.75 | ±2.60 | 0.147 W | |
| Univariate Linear Regression Analysis with PTFV1 | ||||
|---|---|---|---|---|
| PTFV1 [µV*ms] | B | 95% CI | R2 (adj.) | p Value |
| ODI [h-1] | 68.116 | 35.992 to 100.240 | 0.268 | <0.001 |
| AHI [h-1] | 48.845 | 22.644 to 75.045 | 0.197 | <0.001 |
| cAHI [h-1] | 46.810 | 15.375 to 78.246 | 0.128 | 0.004 |
| oAHI [h-1] | 56.127 | −7.940 to 120.194 | 0.039 | 0.085 |
| NT-proBNP at discharge [pg/mL] | 0.628 | 0.109 to 1.148 | 0.097 | 0.019 |
| LV EF [%] | −60.863 | −132.377 to 10.651 | 0.036 | 0.094 |
| Age [y] | 47.404 | −9.450 to 104.258 | 0.032 | 0.100 |
| eGFR [mL*min−1* 1,73 m−2] | −23.099 | −51.033 to 4.845 | 0.032 | 0.103 |
| RR sys [mmHg] | 19.564 | −8.311 to 47.438 | 0.018 | 0.165 |
| BMI [kg/m2] | 87.580 | −88.285 to 263.446 | < 0.001 | 0.322 |
| Trop I max [ng/mL] | 3.950 | −4.516 to 12.416 | −0.002 | 0.353 |
| Systolic LA area | −71.394 | −239.042 to 96.255 | −0.006 | 0.395 |
| CK max [U/l] | 0.132 | −0.276 to 0.540 | −0.011 | 0.518 |
| Smoking | 402.290 | −943.843 to 1748.422 | −0.012 | 0.551 |
| Male sex | −303.380 | −1741.292 to 1134.532 | −0.015 | 0.674 |
| Diabetes mellitus | 251.791 | −1296.227 to 1799.809 | −0.017 | 0.745 |
| LA FAC [%] | −10.620 | −85.278 to 64.038 | −0.022 | 0.775 |
| Model 1 (with ODI) Multiple Linear Regression Analysis R2 = 0.408 (adj. R2 = 0.317); p = 0.002 | Model 2 (with AHI) Multiple Linear Regression Analysis R2 = 0.330 (adj. R2 = 0.227); p = 0.012 | Model 3 (with cAHI) Multiple Linear Regression Analysis R2 = 0.256 (adj. R2 = 0.186); p = 0.014 | ||||
|---|---|---|---|---|---|---|
| PTFV1 [µV*ms] | B * [95% CI] | P # | B * [95% CI] | P # | B * [95% CI] | P # |
| ODI [h-1] | 65.619 [29.717 to 101.522] | 0.001 | ||||
| AHI [h-1] | 45.170 [11.903 to 78.437] | 0.009 | ||||
| cAHI [h-1] | 45.172 [11.905 to 78.440] | 0.009 | ||||
| oAHI [h-1] | −20.049 [−87.368 to 47.269] | 0.550 | −7.992 [−79.286 to 63.303] | 0.822 | 37.178 [−27.626 to 101.983] | 0.253 |
| NT-proBNP at discharge [pg/mL] | 0.375 [−0.139 to 0.888] | 0.148 | 0.402 [−0.146 to 0.950] | 0.146 | 0.402 [−0.146 to 0.950] | 0.146 |
| LV EF [%] | −60.432 [−128.593 to 7.729] | 0.081 | −50.472 [−122.825 to 21.882] | 0.166 | −50.472 [−122.825 to 21.881] | 0.166 |
| Age [y] | −24.189 [−101.905 to 53.527] | 0.533 | −40.920 [−123.645 to 41.806] | 0.323 | −40.917 [−123.642 to 41.808] | 0.323 |
| eGFR [mL*min−1* 1,73 m−2] | −24.263 [−58.700 to 10.175] | 0.162 | −30.712 [−67.833 to 6.409] | 0.102 | −30.710 [−67.831 to 6.411] | 0.102 |
| Univariate Linear Regression Analysis with AHI | Model 1 Multiple Linear Regression Analysis R2 = 0.326 (adj. R2 = 0.213); p = 0.021 | Model 2 Multiple Linear Regression Analysis R2 = 0.351 (adj. R2 = 0.245); p = 0.010 | |||||
|---|---|---|---|---|---|---|---|
| AHI [h-1] | B [95% CI] | p Value | R2 (adj.) | B [95% CI] | p Value | B [95% CI] | p Value |
| PTFV1 [µV*ms] | 0.004 [0.002 to 0.07] | <0.001 | 0.197 | 0.004 [0.001 to 0.07] | 0.024 | ||
| Abnormal PTFV1 | 21.495 [10.872 to 32.118] | <0.001 | 0.223 | 21.209 [4.452 to 37.966] | 0.015 | ||
| BMI [kg/m2] | 1.807 [0.231 to 3.382] | 0.025 | 0.074 | 1.737 [−0.156 to 3.630] | 0.071 | 1.500 [−0.296 to 3.295] | 0.099 |
| NT-proBNP at discharge [pg/mL] | 0.005 [<0.001 to 0.009] | 0.062 | 0.053 | 0.002 [−0.004 to 0.007] | 0.487 | 0.001 [−0.007 to 0.006] | 0.823 |
| Systolic LA area | 0.876 [−0.187 to 1.940] | 0.104 | 0.038 | −0.048 [−1.187 to 1.091] | 0.932 | 0.255 [−0.815 to 1.325] | 0.632 |
| Smoking | −8.729 [−20.929 to 3.471] | 0.157 | 0.019 | −7.268 [−21.764 to7.227] | 0.316 | −9.148 [−23.060 to 4.765] | 0.191 |
| LV EF [%] | −0.443 [−1.075 to 0.188] | 0.164 | 0.020 | −0.125 [−0.832 to 0.582] | 0.723 | −0.148 [−0.798 to 0.501] | 0.646 |
| Male sex | 7.269 [−6.631 to 21.169] | 0.299 | 0.002 | ||||
| LA FAC [%] | −0.299 [−0.985 to 0.386] | 0.384 | −0.005 | ||||
| Age [y] | 0.218 [−0.342 to 0.778] | 0.438 | −0.007 | ||||
| RR sys [mmHg] | 0.073 [−0.182 to 0.328] | 0.570 | −0.013 | ||||
| Trop I max [ng/mL] | 0.012 [−0.068 to 0.092] | 0.768 | −0.019 | ||||
| eGFR [mL*min−1* 1,73 m−2] | −0.030 [−0.294 to 0.234] | 0.822 | −0.018 | ||||
| CK max [U/l] | <0.001 [−0.005 to 0.005] | 0.917 | −0.021 | ||||
| Diabetes mellitus | 0.416 [−14.225 to 15.057] | 0.955 | −0.019 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pec, J.; Wester, M.; Fisser, C.; Debl, K.; Hamer, O.W.; Poschenrieder, F.; Buchner, S.; Maier, L.S.; Arzt, M.; Wagner, S. Central Sleep Apnea Is Associated with an Abnormal P-Wave Terminal Force in Lead V1 in Patients with Acute Myocardial Infarction Independent from Ventricular Function. J. Clin. Med. 2021, 10, 5555. https://doi.org/10.3390/jcm10235555
Pec J, Wester M, Fisser C, Debl K, Hamer OW, Poschenrieder F, Buchner S, Maier LS, Arzt M, Wagner S. Central Sleep Apnea Is Associated with an Abnormal P-Wave Terminal Force in Lead V1 in Patients with Acute Myocardial Infarction Independent from Ventricular Function. Journal of Clinical Medicine. 2021; 10(23):5555. https://doi.org/10.3390/jcm10235555
Chicago/Turabian StylePec, Jan, Michael Wester, Christoph Fisser, Kurt Debl, Okka W. Hamer, Florian Poschenrieder, Stefan Buchner, Lars S. Maier, Michael Arzt, and Stefan Wagner. 2021. "Central Sleep Apnea Is Associated with an Abnormal P-Wave Terminal Force in Lead V1 in Patients with Acute Myocardial Infarction Independent from Ventricular Function" Journal of Clinical Medicine 10, no. 23: 5555. https://doi.org/10.3390/jcm10235555
APA StylePec, J., Wester, M., Fisser, C., Debl, K., Hamer, O. W., Poschenrieder, F., Buchner, S., Maier, L. S., Arzt, M., & Wagner, S. (2021). Central Sleep Apnea Is Associated with an Abnormal P-Wave Terminal Force in Lead V1 in Patients with Acute Myocardial Infarction Independent from Ventricular Function. Journal of Clinical Medicine, 10(23), 5555. https://doi.org/10.3390/jcm10235555





