Combination Therapy with Nusinersen and Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy Type I
Abstract
1. Introduction
2. Materials and Methods
3. Results
- ✓
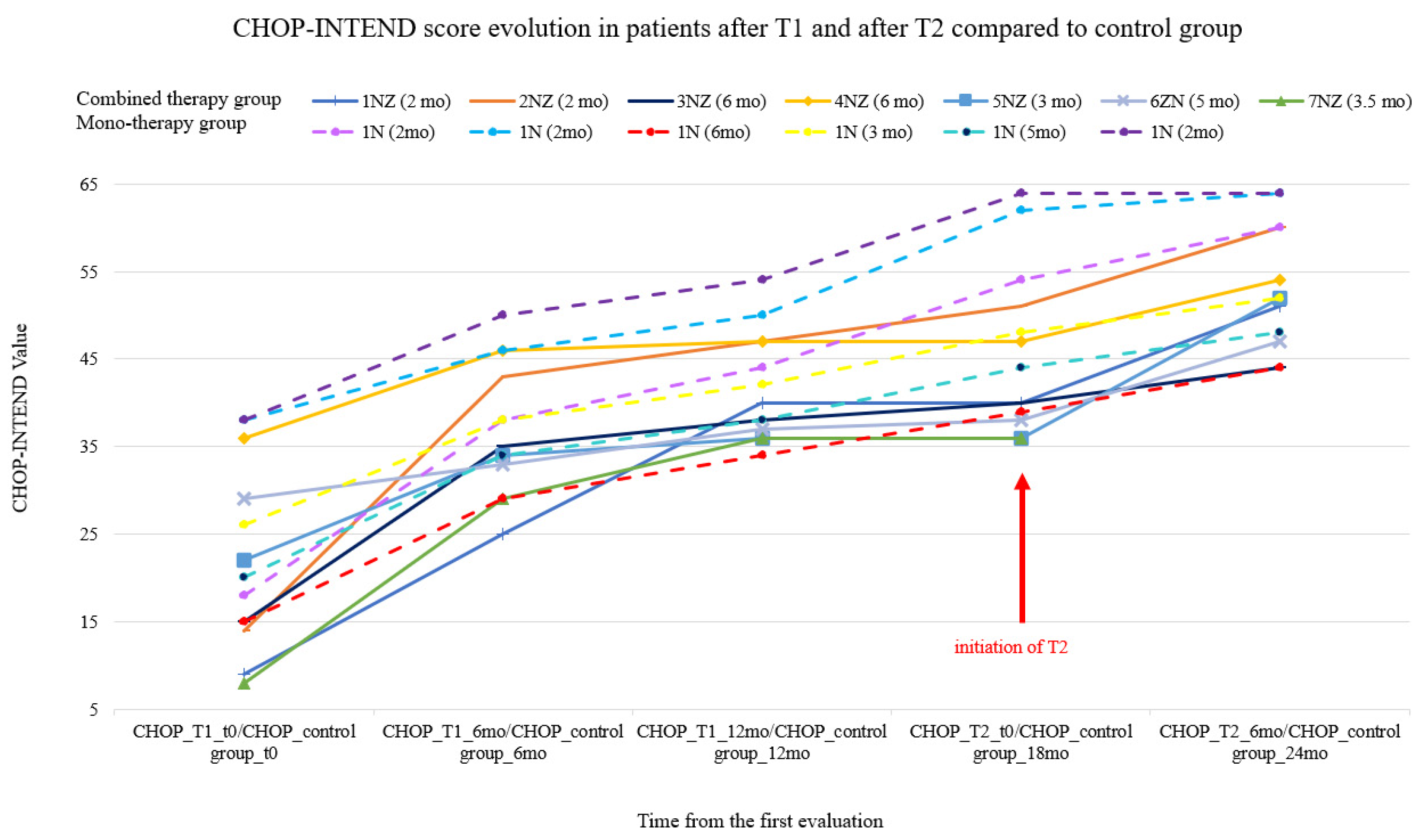
- Higher improvements in CHOP-INTEND scores 6 months after T1 compared to the same period after T2. Calculated medians for CHOP-INTEND: before T1—15 points, 6 months later it increased to 35 points; before T2—40 points, 6 months later it increased to 52 points.
- ✓
- Ventilation hours were not modified 6 months after T1, the median being 16 for both evaluations. However, before T2, median for the ventilation hours dropped to 12 and after T2 to 8 h/day.
- ✓
- Median for cough assist number of sessions was 4 before T1, 3 after 6 months from T1. Before T2, median was 3, and 6 months later it decreased to 1 time per day.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SMN1 Gene Survival of Motor Neuron 1, Telomeric. Available online: https://medlineplus.gov/genetics/gene/smn1/#location (accessed on 17 May 2021).
- Spinal Muscular Atrophy. Available online: https://ghr.nlm.nih.gov/condition/spinal-muscular-atrophy#statistics (accessed on 17 May 2021).
- Vyas, S.; Bechade, C.; Riveau, B.; Downward, J.; Triller, A. Involvement of survival motor neuron (SMN) protein in cell death. Hum. Mol. Genet. 2002, 11, 2751–2764. [Google Scholar] [CrossRef][Green Version]
- Tisdale, S.; Pellizzoni, L. Disease mechanisms and therapeutic approaches in spinal muscular atrophy. J. Neurosci. 2015, 35, 8691–8700. [Google Scholar] [CrossRef]
- Peeters, K.; Chamova, T.; Jordanova, A. Clinical and genetic diversity of SMN1-negative proximal spinal muscular atrophies. Brain 2014, 137, 2879–2896. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—A literature review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef]
- Farrar, M.A.; Kiernan, M.C. The genetics of spinal muscular atrophy: Progress and challenges. Neurotherapeutics 2015, 12, 290–302. [Google Scholar] [CrossRef]
- Appendix 7 Clinical Features, Epidemiology, Natural History, and Management of Spinal Muscular Atrophy. Clinical Review Report: Nusinersen (Spinraza); Indication: Treatment of patients with 5q SMA; Biogen Canada Inc.: Toronto, ON, Canada, 2018.
- Le, T.T.; Pham, L.T.; Butchbach, M.E.R.; Zhang, H.L.; Monani, U.R.; Coovert, D.D.; Gavrilina, T.O.; Xing, L.; Bassell, G.J.; Burghes, A.H. SMNΔ7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 2005, 14, 845–857. [Google Scholar] [CrossRef]
- Lorson, C.L.; Hahnen, E.; Androphy, E.J.; Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. USA 1999, 96, 6307–6311. [Google Scholar] [CrossRef]
- Cartegni, L.; Krainer, A.R. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 2002, 30, 377–384. [Google Scholar] [CrossRef]
- Coovert, D.D.; Le, T.T.; McAndrew, P.E.; Strasswimmer, J.; Crawford, T.O.; Mendell, J.R.; Coulson, S.E.; Androphy, E.J.; Prior, T.W.; Burghes, A.H. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997, 6, 1205–1214. [Google Scholar] [CrossRef]
- Mailman, M.D.; Heinz, J.W.; Papp, A.C.; Snyder, P.J.; Sedra, M.S.; Wirth, B.; Burghes, A.H.; Prior, T.W. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet. Med. 2002, 4, 20–26. [Google Scholar] [CrossRef]
- Butchbach, M.E.R. Copy number variations in the survival motor neuron genes: Implications for spinal muscular atrophy and other neurodegenerative diseases. Front. Mol. Biosci. 2016, 3, 7. [Google Scholar] [CrossRef]
- Muscular Distrophy Association. Available online: https://www.mda.org/disease/spinal-muscular-atrophy/types (accessed on 17 May 2021).
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal Muscular Atrophy: Mutations, Testing, and Clinical Relevance. Appl. Clin. Genet. 2021, 14, 11. [Google Scholar] [CrossRef]
- Dubowitz, V. Very severe spinal muscular atrophy (SMA type 0): An expanding clinical phenotype. Eur. J. Paediatr. Neurol. 1999, 3, 49–51. Available online: http://europepmc.org/abstract/MED/10700538 (accessed on 17 May 2021). [CrossRef]
- Peredo, D.E.; Hannibal, M.C. The Floppy Infant. Pediatr. Rev. 2009, 30, e66–e76. Available online: http://pedsinreview.aappublications.org/content/30/9/e66.abstract Pediatr Neurol (accessed on 17 May 2021). [CrossRef]
- Al-Zaidy, S.A.; Mendell, J.R. From Clinical Trials to Clinical Practice: Practical Considerations for Gene Replacement Therapy in SMA Type 1. Pediatr. Neurol. 2019, 100, 3–11. Available online: https://www.sciencedirect.com/science/article/pii/S0887899418311639 (accessed on 17 May 2021). [CrossRef]
- Finkel, R.S.; Mercuri, E.; Meyer, O.H.; Simonds, A.K.; Schroth, M.K.; Graham, R.J.; Kirschner, J.; Iannaccone, S.T.; Crawford, T.O.; Woods, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 2018, 28, 197–207. Available online: https://www.sciencedirect.com/science/article/pii/S0960896617312907 (accessed on 17 May 2021). [CrossRef]
- Mercuri, E.; Finkel, R.S.; Muntoni, F.; Wirth, B.; Montes, J.; Main, M.; Mazzone, E.S.; Vitale, M.; Snyder, B.; Quijano-Roy, S.; et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018, 28, 103–115. Available online: https://www.sciencedirect.com/science/article/pii/S0960896617312841 (accessed on 17 May 2021). [CrossRef]
- U.S. Food & Drug Administration. Available online: https://www.fda.gov (accessed on 17 May 2021).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en (accessed on 17 May 2021).
- Gidaro, T.; Servais, L. Nusinersen treatment of spinal muscular atrophy: Current knowledge and existing gaps. Dev. Med. Child. Neurol. 2019, 61, 19–24. [Google Scholar] [CrossRef]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in type 1 spinal muscular atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef]
- Waldrop, M.A.; Kolb, S.J. Current Treatment Options in Neurology—SMA Therapeutics. Curr. Treat. Options Neurol. 2019, 21, 25. [Google Scholar] [CrossRef]
- Summarry of Product INN Nusinersen. Available online: https://www.ema.europa.eu/en/documents/product-information/spinraza-epar-product-information_en.pdf (accessed on 17 May 2021).
- AHFS Approved Spinraza. Available online: https://www.ahfsdruginformation.com/spinraza-nusinersen-approved/ (accessed on 17 May 2021).
- EMA Nusinersen Approved. Available online: https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu312976 (accessed on 17 May 2021).
- Hua, Y.; Sahashi, K.; Hung, G.; Rigo, F.; Passini, M.A.; Bennett, C.F.; Krainer, A.R. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev. 2010, 24, 1634–1644. [Google Scholar] [CrossRef]
- Healthcare Information SAFETY-NUSINERSEN. Available online: https://www.spinraza.com/en_us/home/taking-spinraza/dosing.html (accessed on 17 May 2021).
- Intrathecal Administration with SPINRAZA® (Nusinersen). Available online: https://www.spinraza.com/content/dam/commercial/spinraza/caregiver/en_us/pdf/SPZ-US-0603-Intrathecal-Discussion-Tool.pdf (accessed on 17 May 2021).
- Figueiredo, M. Motor Function Improved after Spinraza. Available online: https://smanewstoday.com/2020/06/24/spinraza-improves-motor-function-after-1-year-children-sma-type-1-2-real-life-study-france/ (accessed on 17 May 2021).
- SMA NEWS TODAY-SPINRAZA. Available online: https://smanewstoday.com/spinraza-nusinersen/ (accessed on 17 May 2021).
- LoMauro, A.; Mastella, C.; Alberti, K.; Masson, R.; Aliverti, A.; Baranello, G. Effect of nusinersen on respiratory muscle function in different subtypes of type 1 spinal muscular atrophy. Am. J. Respir. Crit. Care Med. 2019, 200, 1547–1550. [Google Scholar] [CrossRef]
- Darras, B.T.; Farrar, M.A.; Mercuri, E.; Finkel, R.S.; Foster, R.; Hughes, S.G.; Bhan, I.; Farwell, W.; Gheuens, S. An Integrated Safety Analysis of Infants and Children with Symptomatic Spinal Muscular Atrophy (SMA) Treated with Nusinersen in Seven Clinical Trials. CNS Drugs 2019, 33, 919–932. [Google Scholar] [CrossRef]
- Mahajan, R. Onasemnogene abeparvovec for spinal muscular atrophy: The costlier drug ever. Int. J. Appl. Basic Med. Res. 2019, 9, 127. [Google Scholar] [CrossRef]
- Chand, D.; Mohr, F.; McMillan, H.; Tukov, F.F.; Montgomery, K.; Kleyn, A.; Sun, R.; Tauscher-Wisniewski, S.; Kaufmann, P.; Kullak-Ublick, G. Hepatotoxicity following administration of onasemnogene abeparvovec (AVXS-101) for the treatment of spinal muscular atrophy. J. Hepatol. 2021, 74, 560–566. Available online: https://www.sciencedirect.com/science/article/pii/S016882782033748X (accessed on 17 May 2021). [CrossRef]
- Chand, D.H.; Zaidman, C.; Arya, K.; Millner, R.; Farrar, M.A.; Mackie, F.E.; Goedeker, N.L.; Dharnidharka, V.R.; Dandamudi, R.; Reyna, S.P. Thrombotic Microangiopathy Following Onasemnogene Abeparvovec for Spinal Muscular Atrophy: A Case Series. J. Pediatr. 2021, 231, 265–268. Available online: https://www.sciencedirect.com/science/article/pii/S0022347620314669 (accessed on 17 May 2021). [CrossRef]
- FDA. Statement on Data Accuracy Issues with Recently Approved Gene Therapy. 2019. Available online: https://www.fda.gov/news-events/press-announcements/statement-data-accuracy-issues-recently-approved-gene-therapy (accessed on 17 May 2021).
- AVEXIS EC APPROVAL-Day 1. Available online: https://www.novartis.com/news/media-releases/avexis-receives-ec-approval-and-activates-%22day-one%22-access-program-zolgensma-only-gene-therapy-spinal-muscular-atrophy-sma (accessed on 17 May 2021).
- Clear for Use AVEXIS. Available online: https://www.novartis.com/news/media-releases/avexis-receives-ec-approval-and-activates-day-one-access-program-zolgensma-only-gene-therapy-spinal-muscular-atrophy-sma (accessed on 17 May 2021).
- MAP AVEXIS. Available online: https://smanewstoday.com/2019/12/28/novartis-global-access-program-for-zolgensma/ (accessed on 17 May 2021).
- Company News HQ. Novartis. Novartis Gene Therapies Recommits to Global Man-Aged Access Program for 2021. Available online: https://www.companynewshq.com/company-news/pharmaceutical-company-news/novartis-gene-therapies-recommits-to-global-managed-access-program-for-2021/ (accessed on 17 May 2021).
- National Health Insurance House. PRESS RELEASE–Romania Has Started Treating Patients with Spinal Amyotrophy with the Drug Nusinersen. Available online: http://www.cnas.ro/post/type/local/comunicat-romania-a-inceput-tratarea-pacientilor-cu-amiotrofie-spinala-cu-medicamentul-nusinersen.html (accessed on 17 May 2021).
- Harada, Y.; Rao, V.K.; Arya, K.; Kuntz, N.L.; DiDonato, C.J.; Napchan-Pomerantz, G.; Agarwal, A.; Stefans, V.M.; Katsuno, M.; Veerapandiyan, A. Combination molecular therapies for type 1 spinal muscular atrophy. Muscle Nerve 2020, 62, 550–554. [Google Scholar] [CrossRef]
- MLPA. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/multiplex-ligation-dependent-probe-amplification (accessed on 17 May 2021).
- Wang, C.H.; Finkel, R.S.; Bertini, E.S.; Schroth, M.; Simonds, A.; Wong, B.; Aloysius, A.; Morrison, L.; Main, M.; Crawford, T.O.; et al. Consensus statement for standard of care in spinal muscular atrophy. J. Child. Neurol. 2007, 22, 1027–1049. [Google Scholar] [CrossRef]
- Glanzman, A.M.; Mazzone, E.; Main, M.; Pelliccioni, M.; Wood, J.; Swoboda, K.J.; Scott, C.; Pane, M.; Messina, S.; Bertini, E.; et al. The Children’s Hospital of Philadelphia infant test of neuromuscular disorders (CHOP INTEND): Test development and reliability. Neuromuscul. Disord. 2010, 20, 155–161. [Google Scholar] [CrossRef]
- CHOP INTEND Score Sheet. Available online: http://www.musculardystrophyuk.org/wp-content/uploads/2017/06/CHOP-INTEND_Score-Sheet.pdf (accessed on 17 May 2021).
- Lemoine, T.J.; Swoboda, K.J.; Bratton, S.L.; Holubkov, R.; Mundorff, M.; Srivastava, R. Spinal muscular atrophy type 1: Are proactive respiratory interventions associated with longer survival? Pediatr. Crit Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2012, 13, e161. [Google Scholar] [CrossRef]
- Bach, J.R. POINT: Is Noninvasive Ventilation Always the Most Appropriate Manner of Long-term Ventilation for Infants With Spinal Muscular Atrophy Type 1? Yes, Almost Always. Chest 2017, 151, 962–965. [Google Scholar] [CrossRef]
- Schroth, M.K. Special considerations in the respiratory management of spinal muscular atrophy. Pediatrics 2009, 123 (Suppl. S4), S245–S249. [Google Scholar] [CrossRef]
- O’Sullivan, R.; Carrier, J.; Cranney, H.; Hemming, R. The Impact of Lung Volume Recruitment on Pulmonary Function in Progressive Childhood Onset Neuromuscular Disease: A Systematic Review. Arch. Phys. Med. Rehabil. 2020, 102, 976–983. [Google Scholar] [CrossRef]
- Sumner, C.J.; Darras, B.T.; Muntoni, F.; Crawford, T.O.; Finkel, R.S.; Mercuri, E.; de Vivo, D.C.; Oskoui, M.; Tizzano, E.; Ryan, M.M.; et al. Association of Phosphorylated Neurofilament Heavy Chain (pNF-H) Levels With Motor Function Achievement in Individuals With Spinal Muscular Atrophy (SMA) Treated With Nusinersen (S27.009). Neurology 2019, 92 (Suppl. S15), S27.009. Available online: http://n.neurology.org/content/92/15_Supplement/S27.009.abstract (accessed on 17 May 2021).
- Dabbous, O.; Maru, B.; Jansen, J.P.; Lorenzi, M.; Cloutier, M.; Guérin, A.; Pivneva, I.; Wu, E.Q.; Arjunji, R.; Feltner, D.; et al. Survival, motor function, and motor milestones: Comparison of AVXS-101 relative to nusinersen for the treatment of infants with spinal muscular atrophy type 1. Adv. Ther. 2019, 36, 1164–1176. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.-L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. Available online: https://www.sciencedirect.com/science/article/pii/S0960896619311277 (accessed on 17 May 2021). [CrossRef]
- Zolgensma Presymptomatics, SPRINT. Available online: https://www.novartis.com/news/media-releases/avexis-presented-robust-data-aan-demonstrating-efficacy-zolgensma-broad-spectrum-spinal-muscular-atrophy-sma-patients (accessed on 17 May 2021).
- Wadman, M. Newborn screening urged for fatal neurological disorder. Science 2018, 360, 1385. [Google Scholar] [CrossRef]
- Arnold, W.D.; Porensky, P.N.; McGovern, V.L.; Iyer, C.C.; Duque, S.; Li, X.; Meyer, K.; Schmelzer, L.; Kaspar, B.K.; Kolb, S.J.; et al. Electrophysiological biomarkers in spinal muscular atrophy: Proof of concept. Ann. Clin. Transl. Neurol. 2014, 1, 34. [Google Scholar] [CrossRef]
| Patient | 1NZ | 2NZ | 3NZ | 4NZ | 5NZ | 6ZN | 7NZ |
|---|---|---|---|---|---|---|---|
| Gender | M | M | F | M | M | F | F |
| Age at symptom onset (months) | 1 | 1 | 4.5 | 4.5 | 2 | 2 | 0.5 |
| Age at T1 (months) | 2 | 2 | 6 | 6 | 3 | 5 | 3.5 |
| Side effects after T1 | no side effects | no side effects | no side effects | no side effects | no side effects | liver enzymes slightly increased; inappetence | no side effects |
| Age at T2 (months) | 9.5 | 12 | 12 | 12 | 14 | 23 | 19 |
| Side effects after T2 | AST, ALT higher 2.5-fold from normal; fever | AST, ALT slightly higher; fever; vomit | AST, ALT slightly higher; vomit | AST, ALT slightly higher | AST, ALT higher 3.5-fold from normal; fever | no side effects | severe platelet level drop; AST, ALT higher 10-fold from normal; fever; vomit; bleeding; anemia; respiratory arrest; renal failure |
| Duration of steroid (months) | 3 | 2 | 2 | 2 | 5 | 2 | 2.5 |
| Ventilation hours before T1 | 16 | 16 | 16 | 0 | 16 | 14 | 16 |
| Ventilation hours 6 months after T1 | 16 | 12 | 16 | 0 | 16 | 12 | 16 |
| Ventilation hours before T2 | 14 | 12 | 12 | 0 | 12 | 8 | 16 |
| Ventilation hours 6 months after T2 | 8 | 4 | 10 | 0 | 8 | 8 | 24 |
| Number of cough assist sessions before T1 | 6 | 4 | 6 | 3 | 4 | 4 | 6 |
| Number of cough assist sessions 6 months after T1 | 6 | 3 | 4 | 2 | 3 | 3 | 4 |
| Number of cough assist sessions before T2 | 4 | 3 | 3 | 2 | 2 | 2 | 3 |
| Number of cough assist sessions 6 months after T2 | 2 | 1 | 2 | 1 | 1 | 2 | 0 |
| CHOP before T1 | 9 | 14 | 15 | 36 | 22 | 29 | 8 |
| CHOP 6 months after T1 | 25 | 43 | 35 | 46 | 34 | 33 | 29 |
| CHOP before T2 | 40 | 51 | 40 | 47 | 36 | 38 | 36 |
| CHOP 6 months after T2 | 51 | 60 | 44 | 54 | 52 | 47 | 0 |
| Patient | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Gender | M | F | M | M | F | F |
| Age at symptom onset (months) | 0.5 | 1.5 | 5 | 3 | 2 | 0.5 |
| Age at T1 (months) | 2 | 2 | 6 | 5 | 3 | 2 |
| Side effects after T1 | no side effects | no side effects | mild agitation | mild agitation | no side effects | no side effects |
| Age at first current study evaluation (months) | 14 | 14 | 18 | 16 | 12 | 10 |
| Ventilation hours at first current study evaluation | 12 | 10 | 8 | 10 | 12 | 12 |
| Ventilation hours at second current study evaluation (6 months later) | 10 | 8 | 6 | 8 | 11 | 12 |
| Number of cough assist sessions at first current study evaluation | 4 | 3 | 3 | 4 | 5 | 2 |
| Number of cough assist sessions at second current study evaluation (6 months later) | 3 | 3 | 3 | 4 | 4 | 2 |
| CHOP at first current study evaluation | 44 | 50 | 34 | 38 | 42 | 54 |
| CHOP at second current study evaluation (6 months later) | 54 | 62 | 39 | 44 | 48 | 64 |
| CHOP Evolution | CHOP Evolution in Control Group after 6 Months | CHOP Evolution in Study Group at 6 Months after T1 | CHOP Evolution in Study Group at 6 Months after T2 | |
|---|---|---|---|---|
| N | Valid | 6 | 6 | 6 |
| Missing | 0 | 0 | 0 | |
| Mean | 13.33 | 15.17 | 9.33 | |
| Median | 13.00 | 14.00 | 9.00 | |
| Minimum | 8 | 4.00 | 4.00 | |
| Maximum | 20 | 29.00 | 16.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirea, A.; Shelby, E.-S.; Axente, M.; Badina, M.; Padure, L.; Leanca, M.; Dima, V.; Sporea, C. Combination Therapy with Nusinersen and Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy Type I. J. Clin. Med. 2021, 10, 5540. https://doi.org/10.3390/jcm10235540
Mirea A, Shelby E-S, Axente M, Badina M, Padure L, Leanca M, Dima V, Sporea C. Combination Therapy with Nusinersen and Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy Type I. Journal of Clinical Medicine. 2021; 10(23):5540. https://doi.org/10.3390/jcm10235540
Chicago/Turabian StyleMirea, Andrada, Elena-Silvia Shelby, Mihaela Axente, Mihaela Badina, Liliana Padure, Madalina Leanca, Vlad Dima, and Corina Sporea. 2021. "Combination Therapy with Nusinersen and Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy Type I" Journal of Clinical Medicine 10, no. 23: 5540. https://doi.org/10.3390/jcm10235540
APA StyleMirea, A., Shelby, E.-S., Axente, M., Badina, M., Padure, L., Leanca, M., Dima, V., & Sporea, C. (2021). Combination Therapy with Nusinersen and Onasemnogene Abeparvovec-xioi in Spinal Muscular Atrophy Type I. Journal of Clinical Medicine, 10(23), 5540. https://doi.org/10.3390/jcm10235540







