Changes in Thyroid Hormone Signaling Mediate Cardiac Dysfunction in the Tg197 Mouse Model of Arthritis: Potential Therapeutic Implications
Abstract
1. Introduction
2. Methods
2.1. Mice
2.2. Experimental Protocol
- Male WT C57BL/6JxCBA mice at 11–12 weeks of age, n = 14
- Male Tg197 mice at 11–12 weeks of age, n = 13
- Female WT C57BL/6JxCBA mice at 11–12 weeks of age, n = 14
- Female Tg197 mice at 11–12 weeks of age, n = 16
2.3. Echocardiography
2.4. Measurement of Thyroid Hormones
2.5. Administration of Triiodothyronine
2.6. Protein Isolation, Sodium Dodecyl Sulfate-Protein Polyacrylamide (SDS-PAGE) Gel Electrophoresis and Immunodetection
2.7. Statistical Analysis
3. Results
3.1. Development of Left Ventricular Dysfunction in the Tg197 Arthritis Model in Both Genders
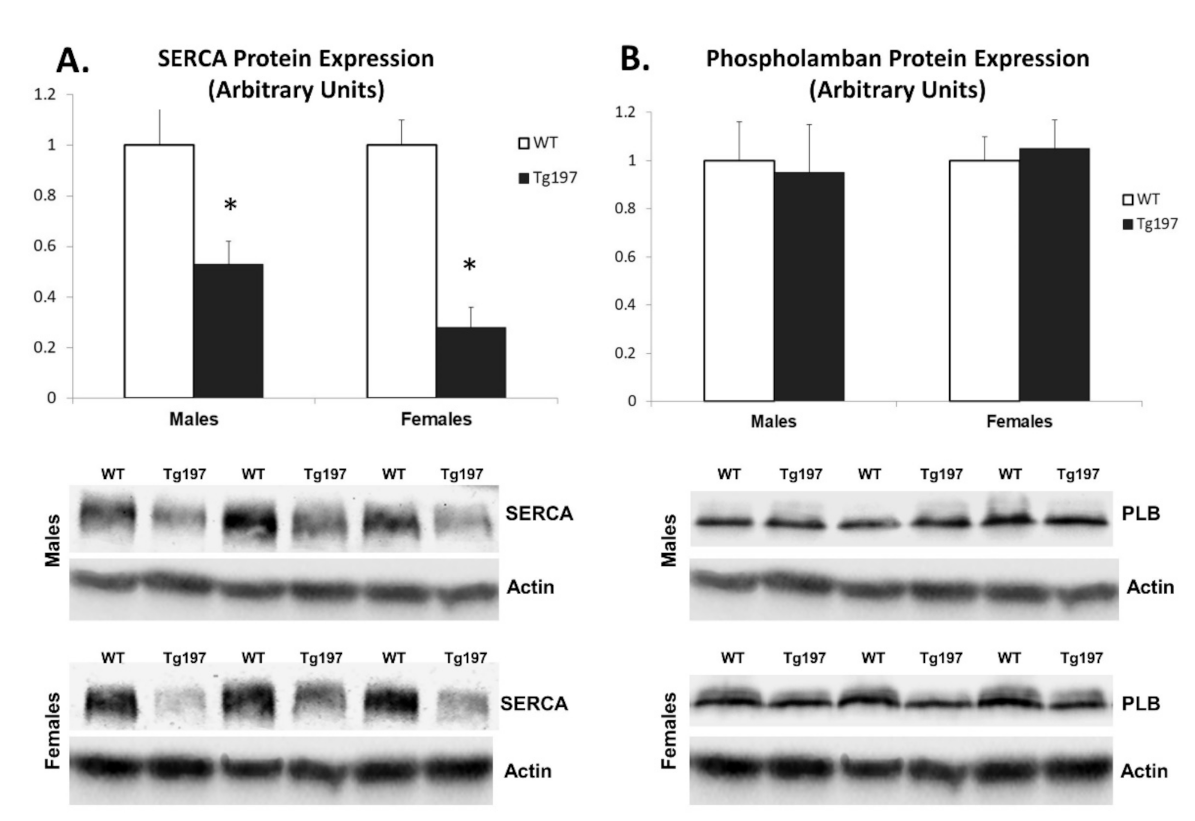
3.2. Downregulation of Calcium Cycling Protein Expression in LV of Tg197 Mice Reveals Cardiac Remodeling at the Molecular Level
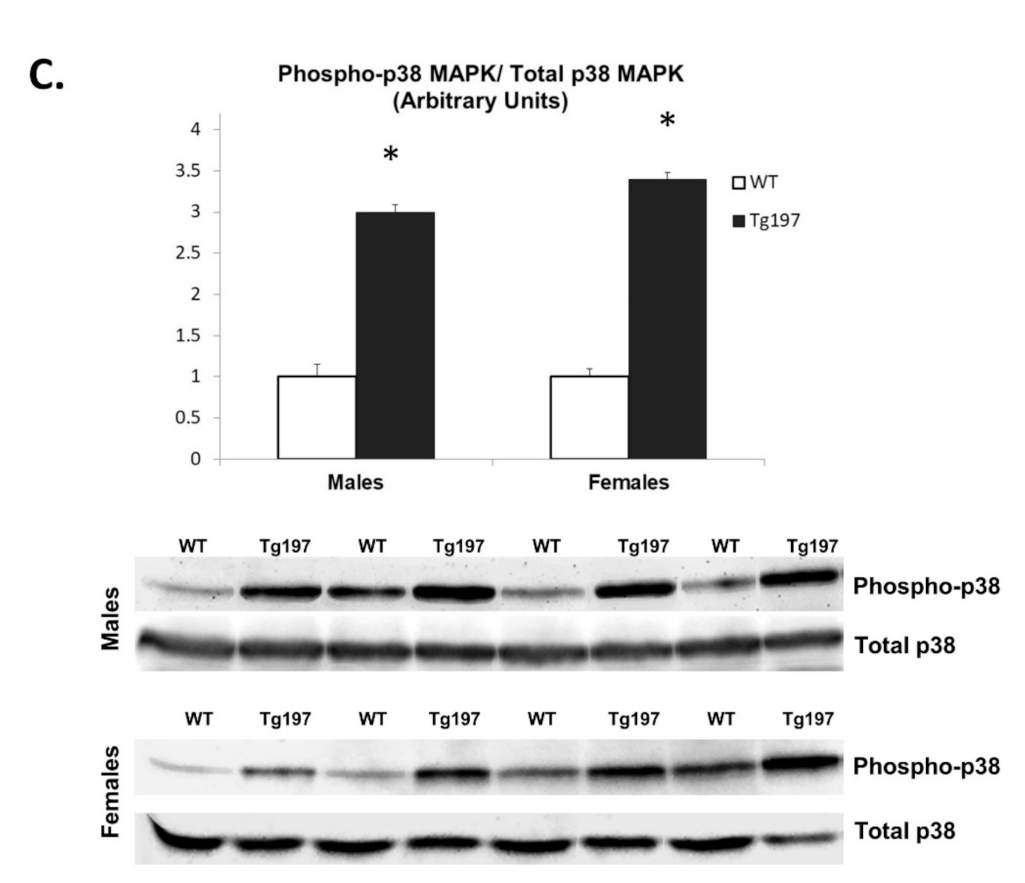
3.3. Activation of Pro-Inflammatory Kinase Signaling in Myocardium of Tg197 Animals in Both Genders
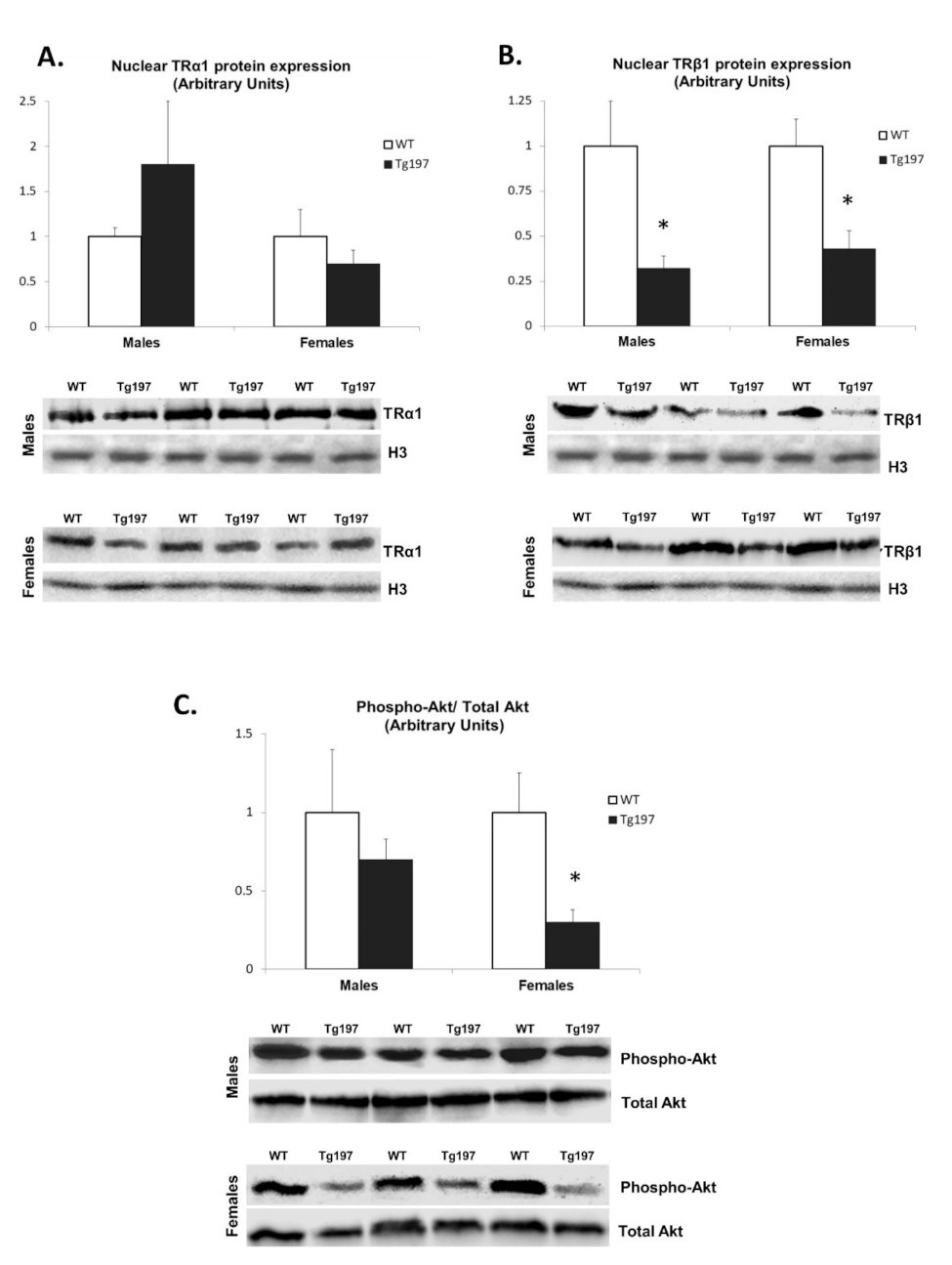
3.4. Changes in Thyroid Hormone Signaling Pathway in Myocardium of Tg197 Animals in Both Genders
3.5. Activation of TH-Related Kinase Signaling in Myocardium of Tg197 Animals in Both Genders
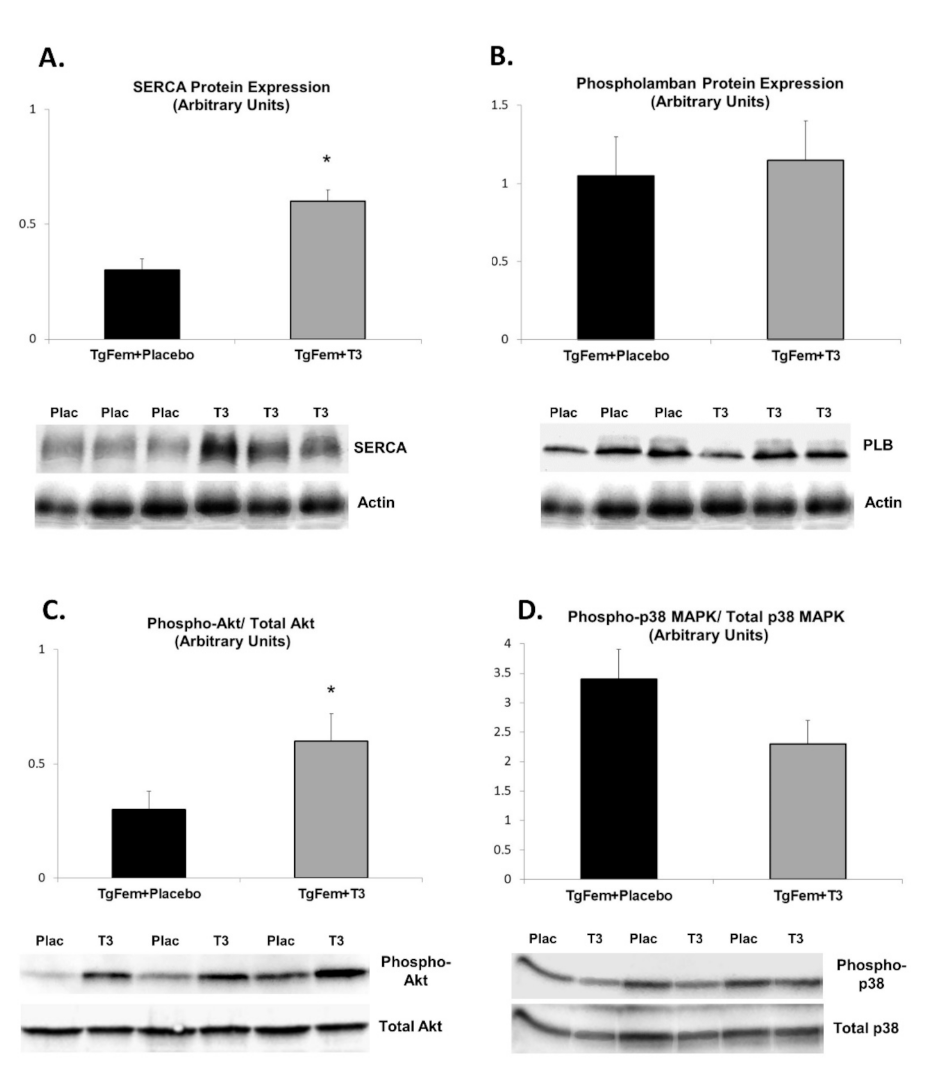
3.6. Effect of T3 Administration in Tg197 Female Mice
4. Discussion
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Innala, L.; Sjöberg, C.; Möller, B.; Ljung, L.; Smedby, T.; Södergren, A.; Magnusson, S.; Rantapää-Dahlqvist, S.; Wållberg-Jonsson, S. Co-morbidity in patients with early rheumatoid arthritis-inflammation matters. Arthritis Res. Ther. 2016, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Messina, S.; Pistone, G.; Calvo, L.; Scaglione, R.; Licata, G. Heart involvement in rheumatoid arthritis: Systematic review and meta-analysis. Int. J. Cardiol. 2013, 167, 2031–2038. [Google Scholar] [CrossRef]
- Chen, J.; Norling, L.V.; Cooper, D. Cardiac Dysfunction in Rheumatoid Arthritis: The Role of Inflammation. Cells 2021, 10, 881. [Google Scholar] [CrossRef] [PubMed]
- Løgstrup, B.B.; Ellingsen, T.; Pedersen, A.B.; Kjaersgaard, A.; Bøtker, H.E.; Maeng, M. Development of heart failure in patients with rheumatoid arthritis: A Danish population-based study. Eur. J. Clin. Investig. 2018, 48, e12915. [Google Scholar] [CrossRef]
- Nicola, P.J.; Maradit-Kremers, H.; Roger, V.L.; Jacobsen, S.J.; Crowson, C.S.; Ballman, K.V.; Gabriel, S.E. The risk of congestive heart failure in rheumatoid arthritis: A population-based study over 46 years. Arthritis Rheum. 2005, 52, 412–420. [Google Scholar] [CrossRef]
- Mantel, A.; Holmqvist, M.; Andersson, D.C.; Lund, L.H.; Askling, J. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J. Am. Coll. Cardiol. 2017, 69, 1275–1285. [Google Scholar] [CrossRef]
- Crowson, C.S.; Nicola, P.J.; Kremers, H.M.; O’Fallon, W.M.; Therneau, T.M.; Jacobsen, S.J.; Roger, V.L.; Ballman, K.V.; Gabriel, S.E. How much of the increased incidence of heart failure in rheumatoid arthritis is attributable to traditional cardiovascular risk factors and ischemic heart disease? Arthritis Rheum. 2005, 52, 3039–3044. [Google Scholar] [CrossRef]
- Mokotedi, L.; Michel, F.S.; Mogane, C.; Gomes, M.; Woodiwiss, A.J.; Norton, G.R.; Millen, A.M.E. Associations of inflammatory markers with impaired left ventricular diastolic and systolic function in collagen-induced arthritis. PLoS ONE 2020, 15, e0230657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Miao, Z.; Luo, A.; Zhu, D.; Lu, Y.; Li, P.; Feng, X.; Tan, W.; Wang, F. Identifying a marked inflammation mediated cardiac dysfunction during the development of arthritis in collagen-induced arthritis mice. Clin. Exp. Rheumatol. 2020, 38, 203–211. [Google Scholar] [PubMed]
- Keffer, J.; Probert, L.; Cazlaris, H.; Georgopoulos, S.; Kaslaris, E.; Kioussis, D.; Kollias, G. Transgenic mice expressing human tumour necrosis factor: A predictive genetic model of arthritis. Embo. J. 1991, 10, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Ntari, L.; Sakkou, M.; Chouvardas, P.; Mourouzis, I.; Prados, A.; Denis, M.C.; Karagianni, N.; Pantos, C.; Kollias, G. Comorbid TNF-mediated heart valve disease and chronic polyarthritis share common mesenchymal cell-mediated aetiopathogenesis. Ann. Rheum. Dis. 2018, 77, 926–934. [Google Scholar] [CrossRef]
- Fechtner, S.; Fox, D.A.; Ahmed, S. Transforming growth factor β activated kinase 1: A potential therapeutic target for rheumatic diseases. Rheumatology 2017, 56, 1060–1068. [Google Scholar] [CrossRef][Green Version]
- Koopman, F.A.; van Maanen, M.A.; Vervoordeldonk, M.J.; Tak, P.P. Balancing the autonomic nervous system to reduce inflammation in rheumatoid arthritis. J. Intern. Med. 2017, 282, 64–75. [Google Scholar] [CrossRef]
- Pantos, C.; Xinaris, C.; Mourouzis, I.; Kokkinos, A.D.; Cokkinos, D.V. TNF-alpha administration in neonatal cardiomyocytes is associated with differential expression of thyroid hormone receptors: A response prevented by T3. Horm. Metab. Res. 2008, 40, 731–734. [Google Scholar] [CrossRef]
- Pörings, A.S.; Lowin, T.; Dufner, B.; Grifka, J.; Straub, R.H. A thyroid hormone network exists in synovial fibroblasts of rheumatoid arthritis and osteoarthritis patients. Sci. Rep. 2019, 9, 13235. [Google Scholar] [CrossRef]
- Vale, C.; Neves, J.S.; von Hafe, M.; Borges-Canha, M.; Leite-Moreira, A. The Role of Thyroid Hormones in Heart Failure. Cardiovasc. Drugs Ther. 2019, 33, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I. Translating thyroid hormone effects into clinical practice: The relevance of thyroid hormone receptor alpha1 in cardiac repair. Heart Fail. Rev. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Isaksen, J.L.; Skov, M.W.; Graff, C.; Ellervik, C.; Kanters, J.K. Electrocardiography in euthyroid individuals: A Danish general population study. Minerva Endocrinol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.E. Cardiovascular morbidity and mortality in rheumatoid arthritis. Am. J. Med. 2008, 121, S9–S14. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Wang, M.; Tai, Y.; Tao, J.; Zhou, W.; Han, Y.; Wei, W. Triggers of Cardiovascular Diseases in Rheumatoid Arthritis. Curr. Probl. Cardiol. 2021, 100853. [Google Scholar]
- Alexopoulou, L.; Pasparakis, M.; Kollias, G. A murine transmembrane tumor necrosis factor (tnf) transgene induces arthritis by cooperative p55/p75 tnf receptor signaling. Eur. J. Immunol. 1997, 27, 2588–2592. [Google Scholar] [CrossRef] [PubMed]
- Mourouzis, I.; Mantzouratou, P.; Galanopoulos, G.; Kostakou, E.; Roukounakis, N.; Kokkinos, A.D.; Cokkinos, D.V.; Pantos, C. Dose dependent effects of thyroid hormone on post-ischaemic cardiac performance: Potential involvement of Akt and ERK signaling. Mol. Cell Biochem. 2012, 363, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Mourouzis, I.; Kostakou, E.; Galanopoulos, G.; Mantzouratou, P.; Pantos, C. Inhibition of thyroid hormone receptor alpha1 impairs post-ischemic cardiac performance after myocardial infarction in mice. Mol. Cell Biochem. 2013, 379, 97–105. [Google Scholar] [CrossRef]
- Kranias, E.G.; Hajjar, R.J. Modulation of cardiac contractility by the phospholamban/SERCA2a regulatome. Circ. Res. 2012, 110, 1646–1660. [Google Scholar] [CrossRef]
- Del Buono, M.; Abbate, A.; Toldo, S. Interplay of inflammation, oxidative stress and cardiovascular disease in rheumatoid arthritis. Heart 2018, 104, 1991–1992. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.A.; Anwer, L.A.; Poizat, C. Cardiac SERCA2A/B: Therapeutic targets for heart failure. Eur. J. Pharmacol. 2014, 724, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.; Banner, N.R.; Morley-Smith, A.; Lyon, A.R.; Harding, S.E. The Current and Future Landscape of SERCA Gene Therapy for Heart Failure: A Clinical Perspective. Hum. Gene. Ther. 2015, 26, 293–304. [Google Scholar] [CrossRef]
- Farah, H.; Young, S.P.; Mauro, C.; Jones, S.W. Metabolic dysfunction and inflammatory disease: The role of stromal fibroblasts. FEBS J. 2021, 288, 5555–5568. [Google Scholar] [CrossRef]
- Liao, P.; Georgakopoulos, D.; Kovacs, A.; Zheng, M.; Lerner, D.; Pu, H.; Saffitz, J.; Chien, K.; Xiao, R.P.; Kass, D.A.; et al. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc. Natl. Acad. Sci. USA 2001, 98, 12283–12288. [Google Scholar] [CrossRef]
- Scharf, M.; Neef, S.; Freund, R.; Geers-Knörr, C.; Franz-Wachtel, M.; Brandis, A.; Krone, D.; Schneider, H.; Groos, S.; Menon, M.B.; et al. Mitogen-activated protein kinase-activated protein kinases 2 and 3 regulate SERCA2a expression and fiber type composition to modulate skeletal muscle and cardiomyocyte function. Mol. Cell Biol. 2013, 33, 2586–2602. [Google Scholar] [CrossRef]
- Pantos, C.; Mourouzis, I.; Galanopoulos, G.; Gavra, M.; Perimenis, P.; Spanou, D.; Cokkinos, D.V. Thyroid hormone receptor alpha1 downregulation in postischemic heart failure progression: The potential role of tissue hypothyroidism. Horm. Metab. Res. 2010, 42, 718–724. [Google Scholar] [CrossRef]
- Arosio, B.; Monti, D.; Mari, D.; Passarino, G.; Ostan, R.; Ferri, E.; De Rango, F.; Franceschi, C.; Cesari, M.; Vitale, G. Thyroid hormones and frailty in persons experiencing extreme longevity. Exp. Gerontol. 2020, 138, 111000. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Monti, D.; Mari, D.; Arosio, B.; Gentilini, D.; Ferri, E.; Passarino, G.; De Rango, F.; D’Aquila, P.; Mariotti, S.; et al. Heterogeneity of Thyroid Function and Impact of Peripheral Thyroxine Deiodination in Centenarians and Semi-Supercentenarians: Association with Functional Status and Mortality. J. Gerontol. Biol. Sci. Med. Sci. 2019, 74, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Belke, D.D.; Gloss, B.; Swanson, E.A.; Dillmann, W.H. Adeno-associated virus-mediated expression of thyroid hormone receptor isoforms-alpha1 and -beta1 improves contractile function in pressure overload-induced cardiac hypertrophy. Endocrinology 2007, 148, 2870–2877. [Google Scholar] [CrossRef][Green Version]
- Emamifar, A.; Hangaard, J.; Jensen Hansen, I.M. Thyroid disorders in patients with newly diagnosed rheumatoid arthritis is associated with poor initial treatment response evaluated by disease activity score in 28 joints-C-reactive protein (DAS28-CRP): An observational cohort study. Medicine 2017, 96, e8357. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, Y.; Kim, H.H.; Ying, H.; Furuya, F.; Huang, Z.; Simoncini, T.; Noma, K.; Ueki, K.; Nguyen, N.H.; Scanlan, T.S.; et al. Rapid nongenomic actions of thyroid hormone. Proc. Natl. Acad. Sci. USA 2006, 103, 14104–14109. [Google Scholar] [CrossRef]
- Cittadini, A.; Monti, M.G.; Iaccarino, G.; Di Rella, F.; Tsichlis, P.N.; Di Gianni, A.; Strömer, H.; Sorriento, D.; Peschle, C.; Trimarco, B.; et al. Adenoviral gene transfer of Akt enhances myocardial contractility and intracellular calcium handling. Gene Ther. 2006, 13, 8–19. [Google Scholar] [CrossRef] [PubMed]
| Males | Females | |||||
|---|---|---|---|---|---|---|
| WT (n = 14) | Tg197 (n = 13) | p Value for Males | WT (n = 14) | Tg197 (n = 16) | p Value for Females | |
| Body weight, g | 28.6 ± 0.7 | 19.9 ± 1.0 | 0.00001 | 22.10 ± 0.5 | 14.2 ± 0.4 | 10−10 |
| LVEDd, mm/BW | 0.13 ± 0.002 | 0.19 ± 0.008 | 0.0001 | 0.16 ± 0.004 | 0.23 ± 0.006 | 10−9 |
| LVEDs, mm/BW | 0.08 ± 0.003 | 0.12 ± 0.006 | 0.00004 | 0.09 ± 0.003 | 0.15 ± 0.006 | 10−7 |
| LVPWd, mm/BW | 0.024 ± 0.0007 | 0.034 ± 0.0018 | 0.001 | 0.028 ± 0.0010 | 0.044 ± 0.0014 | 10−8 |
| LA, mm/BW | 0.08 ± 0.002 | 0.11 ± 0.005 | 0.00005 | 0.09 ± 0.002 | 0.15 ± 0.003 | 10−14 |
| SVPW, cm/s | 3.05 ± 0.06 | 2.46 ± 0.07 | 10−8 | 2.84 ± 0.05 | 2.02 ± 0.03 | 10−12 |
| Ejection Fraction (%) | 74.6 ± 0.8 | 67.8 ± 1.8 | 0.02 | 73.7 ± 1.0 | 64.1 ± 1.5 | 0.0001 |
| TgFem + Placebo (n = 7) | TgFem + T3 (n = 6) | p Value | |
|---|---|---|---|
| Body weight, g | 14.0 ± 0.6 | 14.2 ± 0.7 | 0.7 |
| LVEDd, mm/BW | 0.24 ± 0.009 | 0.22 ± 0.016 | 0.15 |
| LVEDs, mm/BW | 0.16 ± 0.01 | 0.11 ± 0.01 | 0.01 |
| LVPWd, mm/BW | 0.044 ± 0.0014 | 0.044 ± 0.0033 | 0.85 |
| LA, mm/BW | 0.15 ± 0.003 | 0.12 ± 0.006 | 0.001 |
| SVPW, cm/s | 2.02 ± 0.03 | 2.88 ± 0.12 | 0.0001 |
| Ejection Fraction (%) | 64.1 ± 1.5 | 80.2 ± 1.6 | 0.0005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntari, L.; Mantzouratou, P.; Katsaouni, A.; Pantos, C.; Kollias, G.; Mourouzis, I. Changes in Thyroid Hormone Signaling Mediate Cardiac Dysfunction in the Tg197 Mouse Model of Arthritis: Potential Therapeutic Implications. J. Clin. Med. 2021, 10, 5512. https://doi.org/10.3390/jcm10235512
Ntari L, Mantzouratou P, Katsaouni A, Pantos C, Kollias G, Mourouzis I. Changes in Thyroid Hormone Signaling Mediate Cardiac Dysfunction in the Tg197 Mouse Model of Arthritis: Potential Therapeutic Implications. Journal of Clinical Medicine. 2021; 10(23):5512. https://doi.org/10.3390/jcm10235512
Chicago/Turabian StyleNtari, Lydia, Polyxeni Mantzouratou, Athanasia Katsaouni, Constantinos Pantos, George Kollias, and Iordanis Mourouzis. 2021. "Changes in Thyroid Hormone Signaling Mediate Cardiac Dysfunction in the Tg197 Mouse Model of Arthritis: Potential Therapeutic Implications" Journal of Clinical Medicine 10, no. 23: 5512. https://doi.org/10.3390/jcm10235512
APA StyleNtari, L., Mantzouratou, P., Katsaouni, A., Pantos, C., Kollias, G., & Mourouzis, I. (2021). Changes in Thyroid Hormone Signaling Mediate Cardiac Dysfunction in the Tg197 Mouse Model of Arthritis: Potential Therapeutic Implications. Journal of Clinical Medicine, 10(23), 5512. https://doi.org/10.3390/jcm10235512






