Effects of Thyroid Hormone on Tissue Hypoxia: Relevance to Sepsis Therapy
Abstract
1. Introduction
2. THs and Tissue Hypoxia
| Study | Type of Treatment | Model | Outcome |
|---|---|---|---|
| Ferreyra et al., 2009 [28] | Pre-treatment with T3 | In vivo I/R in rat kidney | Reduced proteinuria |
| Erkan et al., 2003 [30] | Pre-treatment with T4 | Anoxia-reoxygenation in rabbit proximal tubule cells | Better preservation of cellular structure |
| Sutter et al., 1988 [29] | Treatment with T4 post-ischemia | In vivo I/R in rat kidney | Improved kidney function, preserved cellular morphology |
| Ferreyra et al., 2013 [31] | Pre-treatment with T3 | In vivo I/R in rat kidney | Improved clinical signs and acute tubular necrosis |
| Kim et al., 2014 [32] | Pre-treatment with T3 | In vivo I/R in rat kidney | Protection of tubular epithelial cells against apoptosis |
| Fernandez et al., 2007 [33] | Pre-treatment with T3 | In vivo I/R in rat liver | Reduced injury (serum AST and ALT levels) |
| Yang et al., 2015 [34] | Pre-treatment with T3 | In vivo I/R in rat liver | Improved liver function, reduced histological damage and apoptosis |
| Vargas and Videla 2017 [35] | Pre-treatment with T3 | In vivo I/R in rat liver | Reduced liver injury |
| Bhargava et al., 2008 [37] | Pre-treatment with T3 | Hyperoxia injury in rat lung | Increased alveolar fluid clearance |
| Hiroi et al., 2006 [44] | Treatment with T4 post-ischemia | Transient focal ischemia in mouse brain | Reduced cerebral infarct volume, and improved neurological deficit score |
| Hung et al., 2013 [39] | T4 treatment immediately after HI | Hypoxia in immature rat brain | Protected against white matter injury |
| Hung et al., 2018 [38] | T4 treatment after HI | Right carotid-artery ligation, followed by hypoxia | Protected against white matter injury |
| Li et al., 2019 [40] | T3 treatment during hypoxia | Mouse primary cortical neurons | Reduced neuronal damage |
| Keshavarz et al., 2017 [42] | Chronic T4 pre-treatment | MCAO in rats | Enhanced injury |
| Rastogi et al., 2008 [43] | Chronic T4 pre-treatment | MCAO in rats | Enhanced injury |
3. TH and Angiogenesis
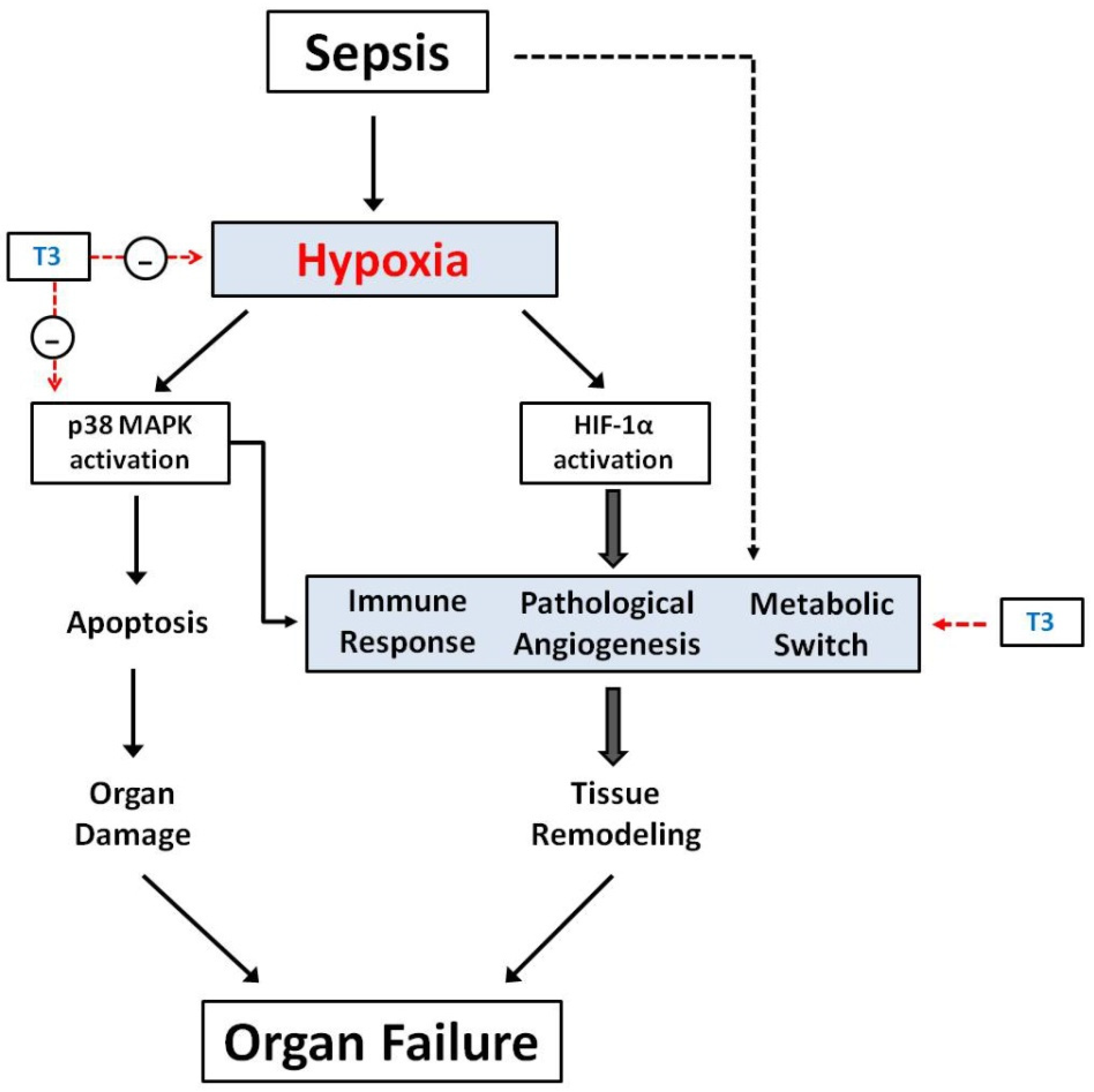
4. Sepsis and Tissue Hypoxia
4.1. Microcirculatory Dysfunction in Sepsis Leads to Tissue Hypoxia
4.2. The Molecular Basis of Tissue Hypoxia in Sepsis: HIF-1a and p38 MAPK
5. Sepsis and Neurohormonal Response
6. TH-Effects in Tissue Hypoxia Induced by Sepsis
6.1. TH and Tissue Hypoxia in Sepsis
6.2. TH and Mitochondrial Function
6.3. TH and Hemorheology
6.4. TH Effects on Relevant for Sepsis Immune System Functions
7. Clinical Safety and Feasibility of TH-Treatment in Critically Ill Patients
8. Conclusions
9. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wajner, S.M.; Maia, A.L. New Insights toward the Acute Non-Thyroidal Illness Syndrome. Front. Endocrinol. 2012, 3, 8. [Google Scholar] [CrossRef]
- Mebis, L.; van den Berghe, G. The hypothalamus-pituitary-thyroid axis in critical illness. Neth. J. Med. 2009, 67, 332–340. [Google Scholar]
- Wang, B.; Liu, S.; Li, L.; Yao, Q.; Song, R.; Shao, X.; Li, Q.; Shi, X.; Zhang, J.A. Non-thyroidal illness syndrome in patients with cardiovascular diseases: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 226, 1–10. [Google Scholar] [CrossRef]
- Alevizaki, M.; Synetou, M.; Xynos, K.; Pappa, T.; Vemmos, K.N. Low triiodothyronine: A strong predictor of outcome in acute stroke patients. Eur. J. Clin. Investig. 2007, 37, 651–657. [Google Scholar] [CrossRef]
- Caraballo, C.; Jaimes, F. Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J. Biol. Med. 2019, 92, 629–640. [Google Scholar] [PubMed]
- Ostergaard, L.; Granfeldt, A.; Secher, N.; Tietze, A.; Iversen, N.K.; Jensen, M.S.; Andersen, K.K.; Nagenthiraja, K.; Gutierrez-Lizardi, P.; Mouridsen, K.; et al. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiol. Scand. 2015, 59, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.C. The Hypoxia Response Pathways—Hats Off! N. Engl. J. Med. 2016, 375, 1687–1689. [Google Scholar] [CrossRef]
- Schonenberger, M.J.; Kovacs, W.J. Hypoxia signaling pathways: Modulators of oxygen-related organelles. Front. Cell Dev. Biol. 2015, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Burrows, N.; Babur, M.; Resch, J.; Williams, K.J.; Brabant, G. Hypoxia-inducible factor in thyroid carcinoma. J. Thyroid Res. 2011, 2011, 762905. [Google Scholar] [CrossRef]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. L-Thyroxine vs. 3,5,3’-triiodo-L-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol. Cell Physiol. 2009, 296, C980–C991. [Google Scholar] [CrossRef]
- Otto, T.; Fandrey, J. Thyroid hormone induces hypoxia-inducible factor 1alpha gene expression through thyroid hormone receptor beta/retinoid x receptor alpha-dependent activation of hepatic leukemia factor. Endocrinology 2008, 149, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Simonides, W.S.; Mulcahey, M.A.; Redout, E.M.; Muller, A.; Zuidwijk, M.J.; Visser, T.J.; Wassen, F.W.; Crescenzi, A.; da-Silva, W.S.; Harney, J.; et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J. Clin. Investig. 2008, 118, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Malliopoulou, V.; Paizis, I.; Moraitis, P.; Mourouzis, I.; Tzeis, S.; Karamanoli, E.; Cokkinos, D.D.; Carageorgiou, H.; Varonos, D.; et al. Thyroid hormone and cardioprotection: Study of p38 MAPK and JNKs during ischaemia and at reperfusion in isolated rat heart. Mol. Cell. Biochem. 2003, 242, 173–180. [Google Scholar] [CrossRef]
- Pantos, C.I.; Malliopoulou, V.A.; Mourouzis, I.S.; Karamanoli, E.P.; Paizis, I.A.; Steimberg, N.; Varonos, D.D.; Cokkinos, D.V. Long-term thyroxine administration protects the heart in a pattern similar to ischemic preconditioning. Thyroid 2002, 12, 325–329. [Google Scholar] [CrossRef]
- Pantos, C.; Malliopoulou, V.; Mourouzis, I.; Karamanoli, E.; Moraitis, P.; Tzeis, S.; Paizis, I.; Cokkinos, A.D.; Carageorgiou, H.; Varonos, D.D.; et al. Thyroxine pretreatment increases basal myocardial heat-shock protein 27 expression and accelerates translocation and phosphorylation of this protein upon ischaemia. Eur. J. Pharmacol. 2003, 478, 53–60. [Google Scholar] [CrossRef]
- Pantos, C.; Malliopoulou, V.; Mourouzis, I.; Thempeyioti, A.; Paizis, I.; Dimopoulos, A.; Saranteas, T.; Xinaris, C.; Cokkinos, D.V. Hyperthyroid hearts display a phenotype of cardioprotection against ischemic stress: A possible involvement of heat shock protein 70. Horm. Metab. Res. 2006, 38, 308–313. [Google Scholar] [CrossRef]
- Pantos, C.; Mourouzis, I.; Saranteas, T.; Brozou, V.; Galanopoulos, G.; Kostopanagiotou, G.; Cokkinos, D.V. Acute T3 treatment protects the heart against ischemia-reperfusion injury via TRalpha1 receptor. Mol. Cell. Biochem. 2011, 353, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I.; Saranteas, T.; Clave, G.; Ligeret, H.; Noack-Fraissignes, P.; Renard, P.Y.; Massonneau, M.; Perimenis, P.; Spanou, D.; et al. Thyroid hormone improves postischaemic recovery of function while limiting apoptosis: A new therapeutic approach to support hemodynamics in the setting of ischaemia-reperfusion? Basic Res. Cardiol. 2009, 104, 69–77. [Google Scholar] [CrossRef]
- Fang, L.; Xu, Z.; Lu, J.; Hong, L.; Qiao, S.; Liu, L.; An, J. Cardioprotective effects of triiodothyronine supplementation against ischemia reperfusion injury by preserving calcium cycling proteins in isolated rat hearts. Exp. Ther. Med. 2019, 18, 4935–4941. [Google Scholar] [CrossRef]
- Forini, F.; Kusmic, C.; Nicolini, G.; Mariani, L.; Zucchi, R.; Matteucci, M.; Iervasi, G.; Pitto, L. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology 2014, 155, 4581–4590. [Google Scholar] [CrossRef]
- Karakus, O.O.; Darwish, N.H.E.; Sudha, T.; Salaheldin, T.A.; Fujioka, K.; Dickinson, P.C.T.; Weil, B.; Mousa, S.A. Development of Triiodothyronine Polymeric Nanoparticles for Targeted Delivery in the Cardioprotection against Ischemic Insult. Biomedicines 2021, 9, 1713. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I. Translating thyroid hormone effects into clinical practice: The relevance of thyroid hormone receptor alpha1 in cardiac repair. Heart Fail. Rev. 2015, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kuzman, J.A.; Gerdes, A.M.; Kobayashi, S.; Liang, Q. Thyroid hormone activates Akt and prevents serum starvation-induced cell death in neonatal rat cardiomyocytes. J. Mol. Cell. Cardiol. 2005, 39, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Kobayashi, S.; Chen, J.; Redetzke, R.A.; Said, S.; Liang, Q.; Gerdes, A.M. Short term triiodo-l-thyronine treatment inhibits cardiac myocyte apoptosis in border area after myocardial infarction in rats. J. Mol. Cell. Cardiol. 2008, 44, 180–187. [Google Scholar] [CrossRef]
- Mourouzis, I.; Politi, E.; Pantos, C. Thyroid hormone and tissue repair: New tricks for an old hormone? J. Thyroid Res. 2013, 2013, 312104. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I. Thyroid hormone receptor α1 as a novel therapeutic target for tissue repair. Ann. Transl. Med. 2018, 6, 254. [Google Scholar] [CrossRef]
- Ferreyra, C.; O’Valle, F.; Osorio, J.M.; Moreno, J.M.; Rodriguez, I.; Vargas, F.; Osuna, A. Effect of preconditioning with triiodothyronine on renal ischemia/reperfusion injury and poly(ADP-ribose) polymerase expression in rats. Transplant. Proc. 2009, 41, 2073–2075. [Google Scholar] [CrossRef]
- Sutter, P.M.; Thulin, G.; Stromski, M.; Ardito, T.; Gaudio, K.M.; Kashgarian, M.; Siegel, N.J. Beneficial effect of thyroxin in the treatment of ischemic acute renal failure. Pediatr. Nephrol. 1988, 2, 1–7. [Google Scholar] [CrossRef]
- Erkan, E.; Sakarcan, A.; Haklar, G.; Yalcin, S. Thyroxine prevents reoxygenation injury in isolated proximal tubule cells. Pediatr. Nephrol. 2003, 18, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, C.; Vargas, F.; Rodriguez-Gomez, I.; Perez-Abud, R.; O’Valle, F.; Osuna, A. Preconditioning with triiodothyronine improves the clinical signs and acute tubular necrosis induced by ischemia/reperfusion in rats. PLoS ONE 2013, 8, e74960. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, S.W.; Jung, Y.J.; Min, S.I.; Min, S.K.; Kim, S.J.; Ha, J. Preconditioning with thyroid hormone (3,5,3-triiodothyronine) prevents renal ischemia-reperfusion injury in mice. Surgery 2014, 155, 554–561. [Google Scholar] [CrossRef]
- Fernandez, V.; Castillo, I.; Tapia, G.; Romanque, P.; Uribe-Echevarria, S.; Uribe, M.; Cartier-Ugarte, D.; Santander, G.; Vial, M.T.; Videla, L.A. Thyroid hormone preconditioning: Protection against ischemia-reperfusion liver injury in the rat. Hepatology 2007, 45, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Sui, M.; Liu, F.; Fu, Z.; Wang, Q.X. Tri-iodothyronine preconditioning protects against liver ischemia reperfusion injury through the regulation of autophagy by the MEK/ERK/mTORC1 axis. Biochem. Biophys. Res. Commun. 2015, 467, 704–710. [Google Scholar] [CrossRef]
- Vargas, R.; Videla, L.A. Thyroid hormone suppresses ischemia-reperfusion-induced liver NLRP3 inflammasome activation: Role of AMP-activated protein kinase. Immunol. Lett. 2017, 184, 92–97. [Google Scholar] [CrossRef]
- Ma, S.F.; Xie, L.; Pino-Yanes, M.; Sammani, S.; Wade, M.S.; Letsiou, E.; Siegler, J.; Wang, T.; Infusino, G.; Kittles, R.A.; et al. Type 2 deiodinase and host responses of sepsis and acute lung injury. Am. J. Respir. Cell Mol. Biol. 2011, 45, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, M.; Runyon, M.R.; Smirnov, D.; Lei, J.; Groppoli, T.J.; Mariash, C.N.; Wangensteen, O.D.; Ingbar, D.H. Triiodo-L-thyronine rapidly stimulates alveolar fluid clearance in normal and hyperoxia-injured lungs. Am. J. Respir. Crit. Care Med. 2008, 178, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.L.; Hsu, M.H.; Yu, H.R.; Wu, K.L.H.; Wang, F.S. Thyroxin Protects White Matter from Hypoxic-Ischemic Insult in the Immature Sprague(-)Dawley Rat Brain by Regulating Periventricular White Matter and Cortex BDNF and CREB Pathways. Int. J. Mol. Sci. 2018, 19, 2573. [Google Scholar] [CrossRef]
- Hung, P.L.; Huang, C.C.; Huang, H.M.; Tu, D.G.; Chang, Y.C. Thyroxin treatment protects against white matter injury in the immature brain via brain-derived neurotrophic factor. Stroke 2013, 44, 2275–2283. [Google Scholar] [CrossRef]
- Li, J.; Abe, K.; Milanesi, A.; Liu, Y.Y.; Brent, G.A. Thyroid Hormone Protects Primary Cortical Neurons Exposed to Hypoxia by Reducing DNA Methylation and Apoptosis. Endocrinology 2019, 160, 2243–2256. [Google Scholar] [CrossRef]
- Jo, S.; Kallo, I.; Bardoczi, Z.; Arrojo e Drigo, R.; Zeold, A.; Liposits, Z.; Oliva, A.; Lemmon, V.P.; Bixby, J.L.; Gereben, B.; et al. Neuronal hypoxia induces Hsp40-mediated nuclear import of type 3 deiodinase as an adaptive mechanism to reduce cellular metabolism. J. Neurosci. 2012, 32, 8491–8500. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, S.; Dehghani, G.A. Cerebral Ischemia/Reperfusion Injury in the Hyperthyroid Rat. Iran. J. Med. Sci. 2017, 42, 48–56. [Google Scholar]
- Rastogi, L.; Gupta, S.; Godbole, M.M. Pathophysiological basis for thyrotoxicosis as an aggravating factor in post-ischemic brain injury in rats. J. Endocrinol. 2008, 196, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, Y.; Kim, H.H.; Ying, H.; Furuya, F.; Huang, Z.; Simoncini, T.; Noma, K.; Ueki, K.; Nguyen, N.H.; Scanlan, T.S.; et al. Rapid nongenomic actions of thyroid hormone. Proc. Natl. Acad. Sci. USA 2006, 103, 14104–14109. [Google Scholar] [CrossRef]
- Colantuoni, A.; Marchiafava, P.L.; Lapi, D.; Forini, F.S.; Iervasi, G. Effects of tetraiodothyronine and triiodothyronine on hamster cheek pouch microcirculation. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1931–H1936. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Davis, F.B.; Mousa, S.A. Thyroid hormone-induced angiogenesis. Curr. Cardiol. Rev. 2009, 5, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Luidens, M.K.; Mousa, S.A.; Davis, F.B.; Lin, H.Y.; Davis, P.J. Thyroid hormone and angiogenesis. Vascul. Pharmacol. 2010, 52, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Lin, H.Y.; Hercbergs, A.A.; Keating, K.A.; Mousa, S.A. How thyroid hormone works depends upon cell type, receptor type, and hormone analogue: Implications in cancer growth. Discov. Med. 2019, 27, 111–117. [Google Scholar]
- Talhada, D.; Santos, C.R.A.; Goncalves, I.; Ruscher, K. Thyroid Hormones in the Brain and Their Impact in Recovery Mechanisms After Stroke. Front. Neurol. 2019, 10, 1103. [Google Scholar] [CrossRef]
- Mousa, S.A.; Davis, F.B.; Mohamed, S.; Davis, P.J.; Feng, X. Pro-angiogenesis action of thyroid hormone and analogs in a three-dimensional in vitro microvascular endothelial sprouting model. Int. Angiol. 2006, 25, 407–413. [Google Scholar]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef]
- Davis, F.B.; Mousa, S.A.; O’Connor, L.; Mohamed, S.; Lin, H.Y.; Cao, H.J.; Davis, P.J. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ. Res. 2004, 94, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ortmeier, S.B.; Savinova, O.V.; Nareddy, V.B.; Beyer, A.J.; Wang, D.; Gerdes, A.M. Thyroid hormone induces sprouting angiogenesis in adult heart of hypothyroid mice through the PDGF-Akt pathway. J. Cell. Mol. Med. 2012, 16, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cooper-Kuhn, C.M.; Nannmark, U.; Blomgren, K.; Kuhn, H.G. Stimulatory effects of thyroid hormone on brain angiogenesis in vivo and in vitro. J. Cereb. Blood Flow Metab. 2010, 30, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Frilling, A.; Benko, T.; Best, J.; Sheu, S.Y.; Trippler, M.; Schlaak, J.F.; Broelsch, C.E. Tri-iodothyronine as a stimulator of liver regeneration after partial and subtotal hepatectomy. Eur. Surg. Res. 2007, 39, 58–63. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, N.; Shi, Y.N.; Yuan, J.; Li, L. Thyroid hormone induced angiogenesis through the integrin alphavbeta3/protein kinase D/histone deacetylase 5 signaling pathway. J. Mol. Endocrinol. 2014, 52, 245–254. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Lin, G.L.; McGinley, J.P.; Drysdale, S.B.; Pollard, A.J. Epidemiology and Immune Pathogenesis of Viral Sepsis. Front. Immunol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Cilloniz, C.; Dominedo, C.; Magdaleno, D.; Ferrer, M.; Gabarrus, A.; Torres, A. Pure Viral Sepsis Secondary to Community-Acquired Pneumonia in Adults: Risk and Prognostic Factors. J. Infect. Dis. 2019, 220, 1166–1171. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Zhang, D.; Xu, J.; Dai, H.; Tang, N.; Su, X.; Cao, B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet 2020, 395, 1517–1520. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Markwart, R.; Saito, H.; Harder, T.; Tomczyk, S.; Cassini, A.; Fleischmann-Struzek, C.; Reichert, F.; Eckmanns, T.; Allegranzi, B. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: A systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1536–1551. [Google Scholar] [CrossRef]
- Vincent, J.L.; De Backer, D. Microvascular dysfunction as a cause of organdysfunction in severe sepsis. Crit. Care 2005, 9, S9–S12. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury. Curr. Opin. Crit. Care 2016, 22, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, H.; Toshimori, J.; Yamamoto, K. Sepsis-associated liver injury: Incidence, classification and the clinical significance. Hepatol. Res. 2013, 43, 255–266. [Google Scholar] [CrossRef]
- Gajic, O.; Dabbagh, O.; Park, P.K.; Adesanya, A.; Chang, S.Y.; Hou, P.; Anderson, H., 3rd; Hoth, J.J.; Mikkelsen, M.E.; Gentile, N.T.; et al. Early identification of patients at risk of acute lung injury: Evaluation of lung injury prediction score in a multicenter cohort study. Am. J. Respir. Crit. Care Med. 2011, 183, 462–470. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Shah, C.V.; Meyer, N.J.; Gaieski, D.F.; Lyon, S.; Miltiades, A.N.; Goyal, M.; Fuchs, B.D.; Bellamy, S.L.; Christie, J.D. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock 2013, 40, 375–381. [Google Scholar] [CrossRef]
- Zorio, V.; Venet, F.; Delwarde, B.; Floccard, B.; Marcotte, G.; Textoris, J.; Monneret, G.; Rimmele, T. Assessment of sepsis-induced immunosuppression at ICU discharge and 6 months after ICU discharge. Ann. Intensive Care 2017, 7, 80. [Google Scholar] [CrossRef]
- Samuels, J.M.; Moore, H.B.; Moore, E.E. Coagulopathy in Severe Sepsis: Interconnectivity of Coagulation and the Immune System. Surg. Infect. (Larchmt) 2018, 19, 208–215. [Google Scholar] [CrossRef]
- Sato, R.; Ariyoshi, N.; Hasegawa, D.; Crossey, E.; Hamahata, N.; Ishihara, T.; Nasu, M.; Devendra, G. Effects of Inotropes on the Mortality in Patients With Septic Shock. J. Intensive Care Med. 2021, 36, 211–219. [Google Scholar] [CrossRef]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the Surviving Sepsis Campaign database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Jagger, J.E.; Ellis, C.G. Sepsis impairs microvascular autoregulation and delays capillary response within hypoxic capillaries. Crit. Care 2015, 19, 389. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Hellman, J.; Ince, C.; Ait-Oufella, H. Endothelial Responses in Sepsis. Am. J. Respir. Crit. Care Med. 2020, 202, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Tyml, K.; Martin, C.; Sibbald, W. Microvascular perfusion is impaired in a rat model of normotensive sepsis. J. Clin. Investig. 1994, 94, 2077–2083. [Google Scholar] [CrossRef]
- Nakajima, Y.; Baudry, N.; Duranteau, J.; Vicaut, E. Microcirculation in intestinal villi: A comparison between hemorrhagic and endotoxin shock. Am. J. Respir. Crit. Care Med. 2001, 164, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Sielenkamper, A.W.; Meyer, J.; Kloppenburg, H.; Eicker, K.; Van Aken, H. The effects of sepsis on gut mucosal blood flow in rats. Eur. J. Anaesthesiol. 2001, 18, 673–678. [Google Scholar] [CrossRef]
- Ellis, C.G.; Bateman, R.M.; Sharpe, M.D.; Sibbald, W.J.; Gill, R. Effect of a maldistribution of microvascular blood flow on capillary O(2) extraction in sepsis. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H156–H164. [Google Scholar] [CrossRef]
- Humer, M.F.; Phang, P.T.; Friesen, B.P.; Allard, M.F.; Goddard, C.M.; Walley, K.R. Heterogeneity of gut capillary transit times and impaired gut oxygen extraction in endotoxemic pigs. J. Appl. Physiol. 1996, 81, 895–904. [Google Scholar] [CrossRef]
- Ko, E.; Youn, J.M.; Park, H.S.; Song, M.; Koh, K.H.; Lim, C.H. Early red blood cell abnormalities as a clinical variable in sepsis diagnosis. Clin. Hemorheol. Microcirc. 2018, 70, 355–363. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Rivers, E.P.; Knoblich, B.P.; Jacobsen, G.; Muzzin, A.; Ressler, J.A.; Tomlanovich, M.C. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit. Care Med. 2004, 32, 1637–1642. [Google Scholar] [CrossRef]
- Nguyen, H.B.; Loomba, M.; Yang, J.J.; Jacobsen, G.; Shah, K.; Otero, R.M.; Suarez, A.; Parekh, H.; Jaehne, A.; Rivers, E.P. Early lactate clearance is associated with biomarkers of inflammation, coagulation, apoptosis, organ dysfunction and mortality in severe sepsis and septic shock. J. Inflamm. 2010, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.T.; Potz, B.A.; Young, W.A.; Ayala, A. Overview of the Molecular Pathways and Mediators of Sepsis. In Sepsis: Definitions, Pathophysiology and the Challenge of Bedside Management; Ward, N.S., Levy, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 47–69. [Google Scholar]
- Gritte, R.B.; Souza-Siqueira, T.; Curi, R.; Machado, M.C.C.; Soriano, F.G. Why Septic Patients Remain Sick After Hospital Discharge? Front. Immunol. 2020, 11, 605666. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M.; Singer, M.; Skirecki, T. Sepsis therapies: Learning from 30 years of failure of translational research to propose new leads. EMBO Mol. Med. 2020, 12, e10128. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Furuta, G.T.; Taylor, C.T. Hypoxia and Innate Immunity: Keeping Up with the HIFsters. Annu. Rev. Immunol. 2020, 38, 341–363. [Google Scholar] [CrossRef]
- Ebbesen, P.; Eckardt, K.U.; Ciampor, F.; Pettersen, E.O. Linking measured intercellular oxygen concentration to human cell functions. Acta Oncol. 2004, 43, 598–600. [Google Scholar] [CrossRef]
- Issbrucker, K.; Marti, H.H.; Hippenstiel, S.; Springmann, G.; Voswinckel, R.; Gaumann, A.; Breier, G.; Drexler, H.C.; Suttorp, N.; Clauss, M. p38 MAP kinase—A molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J. 2003, 17, 262–264. [Google Scholar] [CrossRef]
- Fang, W.; Cai, S.X.; Wang, C.L.; Sun, X.X.; Li, K.; Yan, X.W.; Sun, Y.B.; Sun, X.Z.; Gu, C.K.; Dai, M.Y.; et al. Modulation of mitogenactivated protein kinase attenuates sepsisinduced acute lung injury in acute respiratory distress syndrome rats. Mol. Med. Rep. 2017, 16, 9652–9658. [Google Scholar] [CrossRef]
- Xi, Y.; Kim, T.; Brumwell, A.N.; Driver, I.H.; Wei, Y.; Tan, V.; Jackson, J.R.; Xu, J.; Lee, D.K.; Gotts, J.E.; et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat. Cell Biol. 2017, 19, 904–914. [Google Scholar] [CrossRef]
- Jahani, M.; Dokaneheifard, S.; Mansouri, K. Hypoxia: A key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J. Inflamm. (Lond.) 2020, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaeghen, T.; Vandewalle, J.; Libert, C. Hypoxia-inducible factors in metabolic reprogramming during sepsis. FEBS J. 2020, 287, 1478–1495. [Google Scholar] [CrossRef]
- Galvan-Pena, S.; O’Neill, L.A. Metabolic reprograming in macrophage polarization. Front. Immunol. 2014, 5, 420. [Google Scholar] [CrossRef]
- Sanchez, A.; Tripathy, D.; Yin, X.; Desobry, K.; Martinez, J.; Riley, J.; Gay, D.; Luo, J.; Grammas, P. p38 MAPK: A mediator of hypoxia-induced cerebrovascular inflammation. J. Alzheimers Dis. 2012, 32, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Cuadrado, A.; Nebreda, A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010, 429, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Kumar, S.; Gao, F.; Louden, C.S.; Lopez, B.L.; Christopher, T.A.; Wang, C.; Lee, J.C.; Feuerstein, G.Z.; Yue, T.L. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 1999, 99, 1685–1691. [Google Scholar] [CrossRef]
- Meineke, R.; Rimmelzwaan, G.F.; Elbahesh, H. Influenza Virus Infections and Cellular Kinases. Viruses 2019, 11, 171. [Google Scholar] [CrossRef]
- Marchant, D.; Singhera, G.K.; Utokaparch, S.; Hackett, T.L.; Boyd, J.H.; Luo, Z.; Si, X.; Dorscheid, D.R.; McManus, B.M.; Hegele, R.G. Toll-like receptor 4-mediated activation of p38 mitogen-activated protein kinase is a determinant of respiratory virus entry and tropism. J. Virol. 2010, 84, 11359–11373. [Google Scholar] [CrossRef] [PubMed]
- Celestino, I.; Checconi, P.; Amatore, D.; De Angelis, M.; Coluccio, P.; Dattilo, R.; Alunni Fegatelli, D.; Clemente, A.M.; Matarrese, P.; Torcia, M.G.; et al. Differential Redox State Contributes to Sex Disparities in the Response to Influenza Virus Infection in Male and Female Mice. Front. Immunol. 2018, 9, 1747. [Google Scholar] [CrossRef]
- Marchant, D.; Dou, Y.; Luo, H.; Garmaroudi, F.S.; McDonough, J.E.; Si, X.; Walker, E.; Luo, Z.; Arner, A.; Hegele, R.G.; et al. Bosentan enhances viral load via endothelin-1 receptor type-A-mediated p38 mitogen-activated protein kinase activation while improving cardiac function during coxsackievirus-induced myocarditis. Circ. Res. 2009, 104, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mourouzis, I.; Saranteas, T.; Ligeret, H.; Portal, C.; Perimenis, P.; Pantos, C. Phenylephrine postconditioning increases myocardial injury: Are alpha-1 sympathomimetic agonist cardioprotective? Ann. Card. Anaesth. 2014, 17, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Chen, Q.H.; Hu, Q.; Liu, Z.; Wu, Q.; Liang, S.S.; Zhang, H.G.; Zhang, Q.; Zhang, X.K. Dexmedetomidine protects intestinal ischemia-reperfusion injury via inhibiting p38 MAPK cascades. Exp. Mol. Pathol. 2020, 115, 104444. [Google Scholar] [CrossRef]
- Xiao, Y.; Lei, S.; Huang, Y.; Zhao, B.; Wang, H.; Cao, H.; Xia, Z. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the P38-MAPK/TXNIP signaling activation in streptozotocin induced diabetic rats. Acta Cir. Bras. 2017, 32, 429–439. [Google Scholar] [CrossRef]
- Martin, E.D.; Bassi, R.; Marber, M.S. p38 MAPK in cardioprotection—are we there yet? Br. J. Pharmacol. 2015, 172, 2101–2113. [Google Scholar] [CrossRef]
- Luongo, C.; Dentice, M.; Salvatore, D. Deiodinases and their intricate role in thyroid hormone homeostasis. Nat. Rev. Endocrinol. 2019, 15, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Davis, F.B.; Lin, H.Y.; Mousa, S.A.; Zhou, M.; Luidens, M.K. Translational implications of nongenomic actions of thyroid hormone initiated at its integrin receptor. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1238–E1246. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Shin, H.; Kim, W.; Lim, T.H.; Jang, B.; Cho, Y.; Choi, K.S.; Ahn, C.; Lee, J.; Na, M.K. The Value of Decreased Thyroid Hormone for Predicting Mortality in Adult Septic Patients: A Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 14137. [Google Scholar] [CrossRef] [PubMed]
- Padhi, R.; Kabi, S.; Panda, B.N.; Jagati, S. Prognostic significance of nonthyroidal illness syndrome in critically ill adult patients with sepsis. Int. J. Crit. Illn. Inj. Sci. 2018, 8, 165–172. [Google Scholar] [PubMed]
- Zhao, Y.; Wang, W.Y.; Tian, J.; Zhang, X.; Yang, M.; Chen, J.; Mu, M.; Tang, Y.D. Impact of low T3 syndrome on adverse cardiovascular events in adult patients with acute viral myocarditis. Zhonghua Xin Xue Guan Bing Za Zhi 2019, 47, 447–451. [Google Scholar]
- Pantos, C.; Mourouzis, I.; Cokkinos, D.V. Thyroid hormone and cardiac repair/regeneration: From Prometheus myth to reality? Can. J. Physiol. Pharmacol. 2012, 90, 977–987. [Google Scholar] [CrossRef]
- Warner, M.H.; Beckett, G.J. Mechanisms behind the non-thyroidal illness syndrome: An update. J. Endocrinol. 2010, 205, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fekete, C.; Lechan, R.M. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef]
- Peeters, R.P.; Wouters, P.J.; Kaptein, E.; van Toor, H.; Visser, T.J.; Van den Berghe, G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J. Clin. Endocrinol. Metab. 2003, 88, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Castro, I.; Quisenberry, L.; Calvo, R.M.; Obregon, M.J.; Lado-Abeal, J. Septic shock non-thyroidal illness syndrome causes hypothyroidism and conditions for reduced sensitivity to thyroid hormone. J. Mol. Endocrinol. 2013, 50, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Fontes, K.N.; Cabanelas, A.; Bloise, F.F.; de Andrade, C.B.V.; Souza, L.L.; Wilieman, M.; Trevenzoli, I.H.; Agra, L.C.; Silva, J.D.; Bandeira-Melo, C.; et al. Differential Regulation of Thyroid Hormone Metabolism Target Genes during Non-thyroidal [corrected] Illness Syndrome Triggered by Fasting or Sepsis in Adult Mice. Front. Physiol. 2017, 8, 828. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Davis, P.J.; Lin, H.Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E.; et al. Thyroid Hormones Interaction With Immune Response, Inflammation and Non-thyroidal Illness Syndrome. Front. Cell Dev. Biol. 2020, 8, 614030. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Harbuz, M.S.; Levy, A.; Lightman, S.L. Inhibition of the hypothalamic-pituitary-thyroid axis in response to lipopolysaccharide is independent of changes in circulating corticosteroids. Neuroimmunomodulation 1997, 4, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Mebis, L.; Debaveye, Y.; Ellger, B.; Derde, S.; Ververs, E.J.; Langouche, L.; Darras, V.M.; Fliers, E.; Visser, T.J.; Van den Berghe, G. Changes in the central component of the hypothalamus-pituitary-thyroid axis in a rabbit model of prolonged critical illness. Crit. Care 2009, 13, R147. [Google Scholar] [CrossRef]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status, and Outcome in 191 Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e926–e935. [Google Scholar] [CrossRef]
- Scappaticcio, L.; Pitoia, F.; Esposito, K.; Piccardo, A.; Trimboli, P. Impact of COVID-19 on the thyroid gland: An update. Rev. Endocr. Metab. Disord. 2020. [Google Scholar] [CrossRef]
- Mourouzis, I.S.; Lourbopoulos, A.I.; Trikas, A.G.; Tseti, I.K.; Pantos, C.I. Triiodothyronine prevents tissue hypoxia in experimental sepsis: Potential therapeutic implications. Intensive Care Med. Exp. 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I.; Cokkinos, D.V. Rebuilding the post-infarcted myocardium by activating ‘physiologic’ hypertrophic signaling pathways: The thyroid hormone paradigm. Heart Fail. Rev. 2010, 15, 143–154. [Google Scholar] [CrossRef]
- Pantos, C.; Kostopanagiotou, G.; Armaganidis, A.; Trikas, A.; Tseti, I.; Mourouzis, I. Triiodothyronine for the treatment of critically ill patients with COVID-19 infection: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Van Wyngene, L.; Vandewalle, J.; Libert, C. Reprogramming of basic metabolic pathways in microbial sepsis: Therapeutic targets at last? EMBO Mol. Med. 2018, 10, e8712. [Google Scholar] [CrossRef]
- Bloise, F.F.; Santos, A.T.; de Brito, J.; de Andrade, C.B.V.; Oliveira, T.S.; de Souza, A.F.P.; Fontes, K.N.; Silva, J.D.; Blanco, N.; Silva, P.L.; et al. Sepsis Impairs Thyroid Hormone Signaling and Mitochondrial Function in the Mouse Diaphragm. Thyroid 2020, 30, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef]
- Cho, Y.I.; Cho, D.J. Hemorheology and microvascular disorders. Korean Circ. J. 2011, 41, 287–295. [Google Scholar] [CrossRef]
- Pantos, C.; Apostolaki, V.; Kokkinos, L.; Trikas, A.; Mourouzis, I. Acute triiodothyronine treatment and red blood cell sedimentation rate (ESR) in critically ill COVID-19 patients: A novel association? Clin. Hemorheol. Microcirc. 2021. [Google Scholar] [CrossRef]
- De Vito, P.; Balducci, V.; Leone, S.; Percario, Z.; Mangino, G.; Davis, P.J.; Davis, F.B.; Affabris, E.; Luly, P.; Pedersen, J.Z.; et al. Nongenomic effects of thyroid hormones on the immune system cells: New targets, old players. Steroids 2012, 77, 988–995. [Google Scholar] [CrossRef]
- Montesinos, M.D.M.; Pellizas, C.G. Thyroid Hormone Action on Innate Immunity. Front. Endocrinol. 2019, 10, 350. [Google Scholar] [CrossRef]
- Hodkinson, C.F.; Simpson, E.E.; Beattie, J.H.; O’Connor, J.M.; Campbell, D.J.; Strain, J.J.; Wallace, J.M. Preliminary evidence of immune function modulation by thyroid hormones in healthy men and women aged 55–70 years. J. Endocrinol. 2009, 202, 55–63. [Google Scholar] [CrossRef]
- Varedi, M.; Shiri, H.; Moattari, A.; Omrani, G.H.; Amirghofran, Z. Hyperthyroid state or in vitro thyroxine treatment modulates TH1/TH2 responses during exposure to HSV-1 antigens. J. Immunotoxicol. 2014, 11, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Furuya, F.; Ishii, T.; Tamura, S.; Takahashi, K.; Kobayashi, H.; Ichijo, M.; Takizawa, S.; Kaneshige, M.; Suzuki-Inoue, K.; Kitamura, K. The ligand-bound thyroid hormone receptor in macrophages ameliorates kidney injury via inhibition of nuclear factor-kappaB activities. Sci. Rep. 2017, 7, 43960. [Google Scholar] [CrossRef]
- Lourbopoulos, A.; Mourouzis, I.; Karapanayiotides, T.; Nousiopoulou, E.; Chatzigeorgiou, S.; Mavridis, T.; Kokkinakis, I.; Touloumi, O.; Irinopoulou, T.; Chouliaras, K.; et al. Changes in thyroid hormone receptors after permanent cerebral ischemia in male rats. J. Mol. Neurosci. 2014, 54, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Lourbopoulos, A.; Erturk, A.; Hellal, F. Microglia in action: How aging and injury can change the brain’s guardians. Front. Cell. Neurosci. 2015, 9, 54. [Google Scholar] [CrossRef]
- Kwakkel, J.; Surovtseva, O.V.; de Vries, E.M.; Stap, J.; Fliers, E.; Boelen, A. A novel role for the thyroid hormone-activating enzyme type 2 deiodinase in the inflammatory response of macrophages. Endocrinology 2014, 155, 2725–2734. [Google Scholar] [CrossRef]
- Mooij, P.; Simons, P.J.; de Haan-Meulman, M.; de Wit, H.J.; Drexhage, H.A. Effect of thyroid hormones and other iodinated compounds on the transition of monocytes into veiled/dendritic cells: Role of granulocyte-macrophage colony-stimulating factor, tumour-necrosis factor-alpha and interleukin-6. J. Endocrinol. 1994, 140, 503–512. [Google Scholar] [CrossRef]
- Alamino, V.A.; Montesinos, M.M.; Rabinovich, G.A.; Pellizas, C.G. The thyroid hormone triiodothyronine reinvigorates dendritic cells and potentiates anti-tumor immunity. Oncoimmunology 2016, 5, e1064579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mascanfroni, I.; Montesinos Mdel, M.; Susperreguy, S.; Cervi, L.; Ilarregui, J.M.; Ramseyer, V.D.; Masini-Repiso, A.M.; Targovnik, H.M.; Rabinovich, G.A.; Pellizas, C.G. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 2008, 22, 1032–1042. [Google Scholar] [CrossRef]
- Mascanfroni, I.D.; Del Mar Montesinos, M.; Alamino, V.A.; Susperreguy, S.; Nicola, J.P.; Ilarregui, J.M.; Masini-Repiso, A.M.; Rabinovich, G.A.; Pellizas, C.G. Nuclear factor (NF)-kappaB-dependent thyroid hormone receptor beta1 expression controls dendritic cell function via Akt signaling. J. Biol. Chem. 2010, 285, 9569–9582. [Google Scholar] [CrossRef] [PubMed]
- Fliers, E.; Bianco, A.C.; Langouche, L.; Boelen, A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. 2015, 3, 816–825. [Google Scholar] [CrossRef]
- Carrel, T.; Eckstein, F.; Englberger, L.; Mury, R.; Mohacsi, P. Thyronin treatment in adult and pediatric heart surgery: Clinical experience and review of the literature. Eur. J. Heart Fail. 2002, 4, 577–582. [Google Scholar] [CrossRef]
- Ririe, D.G.; Butterworth, J.F.; Hines, M.; Hammon, J.W., Jr.; Zaloga, G.P. Effects of cardiopulmonary bypass and deep hypothermic circulatory arrest on the thyroid axis during and after repair of congenital heart defects: Preservation by deep hypothermia? Anesth. Analg. 1998, 87, 543–548. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Yang, Q.Y.; Xue, F.S.; Zhang, W.; Yang, G.Z.; Liao, X.; Meng, F.M. Preoperative oral thyroid hormones to prevent euthyroid sick syndrome and attenuate myocardial ischemia-reperfusion injury after cardiac surgery with cardiopulmonary bypass in children: A randomized, double-blind, placebo-controlled trial. Medicine 2018, 97, e12100. [Google Scholar] [CrossRef]
- van der Jagt, M.; Knoops, S.; de Jong, M.F.; de Jong, M.J.; Peeters, R.P.; Groeneveld, A.B. Increased Thyroxin During Therapeutic Hypothermia Predicts Death in Comatose Patients After Cardiac Arrest. Neurocrit. Care 2015, 23, 198–204. [Google Scholar] [CrossRef]
- Kaptein, E.M.; Sanchez, A.; Beale, E.; Chan, L.S. Clinical review: Thyroid hormone therapy for postoperative nonthyroidal illnesses: A systematic review and synthesis. J. Clin. Endocrinol. Metab. 2010, 95, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, A.M.; Quinn, D.W.; Pagano, D.; Edwards, N.; Faroqui, M.; Graham, T.R.; Keogh, B.E.; Mascaro, J.; Riddington, D.W.; Rooney, S.J.; et al. Glucose-insulin-potassium and tri-iodothyronine individually improve hemodynamic performance and are associated with reduced troponin I release after on-pump coronary artery bypass grafting. Circulation 2006, 114, I245–I250. [Google Scholar] [CrossRef][Green Version]
- Klemperer, J.D.; Klein, I.L.; Ojamaa, K.; Helm, R.E.; Gomez, M.; Isom, O.W.; Krieger, K.H. Triiodothyronine therapy lowers the incidence of atrial fibrillation after cardiac operations. Ann. Thorac. Surg. 1996, 61, 1323–1327, discussion 1328–1329. [Google Scholar] [CrossRef]
- Jeevanandam, V. Triiodothyronine: Spectrum of use in heart transplantation. Thyroid 1997, 7, 139–145. [Google Scholar] [CrossRef]
- Novitzky, D.; Cooper, D.K. Thyroid hormones and the stunned myocardium. J. Endocrinol. 2014, 223, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Novitzky, D.; Mi, Z.; Sun, Q.; Collins, J.; Cooper, D.K. Thyroid hormone therapy in the management of 63,593 brain-dead organ donors: A retrospective review. Transplantation 2014, 98, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Kanji, S.; Neilipovitz, J.; Neilipovitz, B.; Kim, J.; Haddara, W.M.R.; Pittman, M.; Meggison, H.; Patel, R. Triiodothyronine replacement in critically ill adults with non-thyroidal illness syndrome. Can. J. Anaesth. 2018, 65, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Hesch, R.D.; Hüsch, M.; Ködding, R.; Höffken, B.; Meyer, T. Treatment of dopamine-dependent shock with triiodothyronine. Endocr. Res. Commun. 1981, 8, 229–237. [Google Scholar] [CrossRef] [PubMed]
| Study | Type of Treatment | Model | Outcome |
|---|---|---|---|
| Pantos et al., 2002 [15] | Pre-treatment with T4 | Isolated rat heart | Increased recovery of function |
| Kuzman et al., 2005 [24] | Pre-treatment with T3 | Neonatal rat cardiomyocytes | Increased cell viability, reduced apoptosis |
| Pantos et al., 2011 [18] | Treatment with T3 post-ischemia | Isolated rat heart | Increased recovery of function, reduced injury and apoptosis |
| Pantos et al., 2009 [19] | Treatment with T3 post-ischemia | Isolated rat heart | Increased recovery of function, reduced injury |
| Chen et al., 2008 [25] | Treatment with T3 after infarction | In vivo CAL rat heart | Improved LV function, reduced apoptosis |
| Forini et al., 2014 [21] | Treatment with T3 after infarction | In vivo CAL rat heart | Reduced infarct size and mitochondrial impairment |
| Fang et al., 2019 [20] | Pre-treatment with T3 | Isolated rat heart | Improved LV function |
| Karakus et al., 2021 [22] | Pre-treatment with T3 polymeric nanoparticles | Neonatal rat cardiomyocytes | Improved hypoxic cell damage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourbopoulos, A.I.; Mourouzis, I.S.; Trikas, A.G.; Tseti, I.K.; Pantos, C.I. Effects of Thyroid Hormone on Tissue Hypoxia: Relevance to Sepsis Therapy. J. Clin. Med. 2021, 10, 5855. https://doi.org/10.3390/jcm10245855
Lourbopoulos AI, Mourouzis IS, Trikas AG, Tseti IK, Pantos CI. Effects of Thyroid Hormone on Tissue Hypoxia: Relevance to Sepsis Therapy. Journal of Clinical Medicine. 2021; 10(24):5855. https://doi.org/10.3390/jcm10245855
Chicago/Turabian StyleLourbopoulos, Athanasios I., Iordanis S. Mourouzis, Athanasios G. Trikas, Ioulia K. Tseti, and Constantinos I. Pantos. 2021. "Effects of Thyroid Hormone on Tissue Hypoxia: Relevance to Sepsis Therapy" Journal of Clinical Medicine 10, no. 24: 5855. https://doi.org/10.3390/jcm10245855
APA StyleLourbopoulos, A. I., Mourouzis, I. S., Trikas, A. G., Tseti, I. K., & Pantos, C. I. (2021). Effects of Thyroid Hormone on Tissue Hypoxia: Relevance to Sepsis Therapy. Journal of Clinical Medicine, 10(24), 5855. https://doi.org/10.3390/jcm10245855





