A Historical Cohort in Kidney Transplantation: 55-Year Follow-Up of 72 HLA-Identical, Donor-Recipient Pairs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort Construction
2.2. Primary Outcome
2.3. Analysis
3. Results
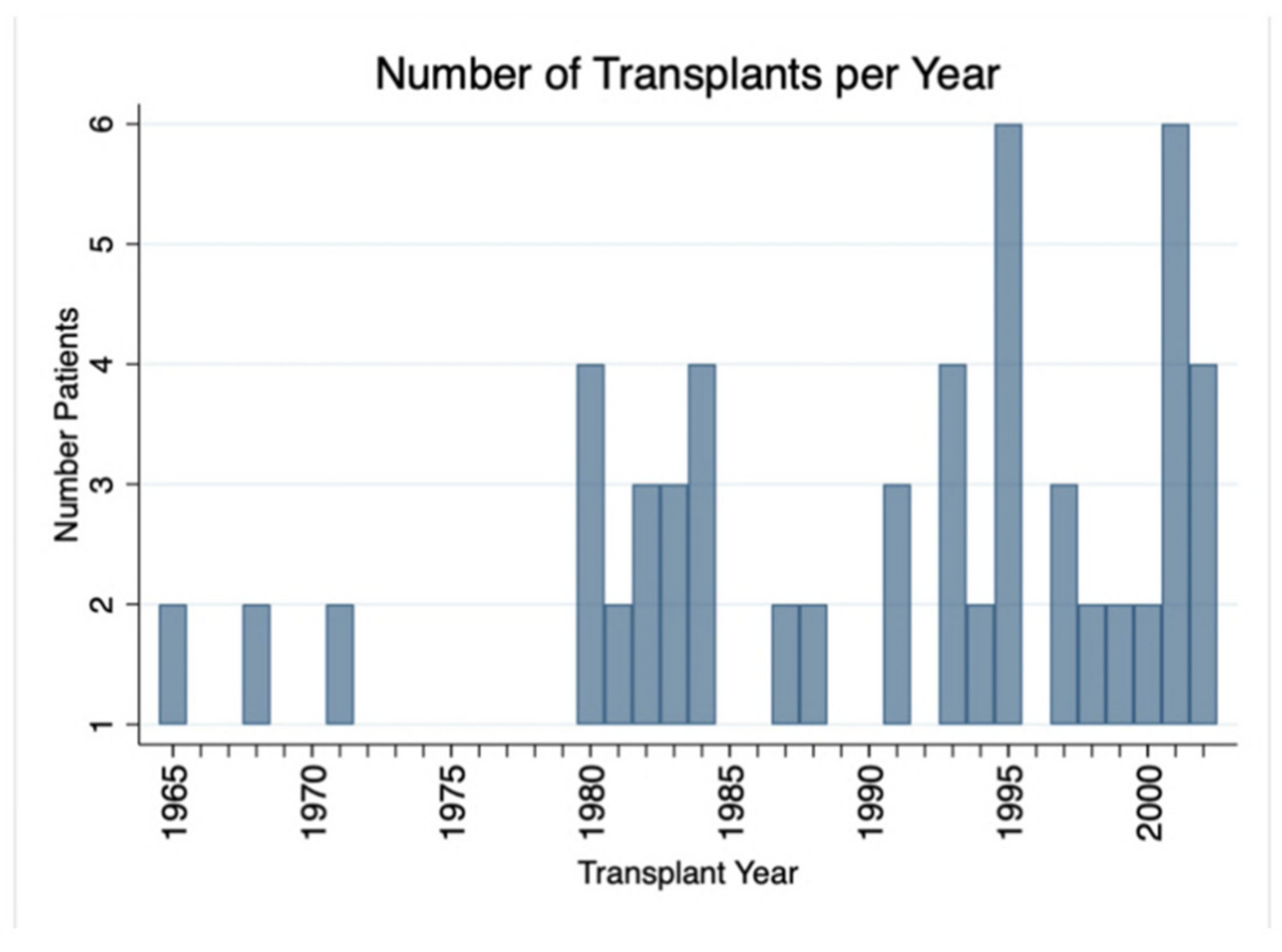
3.1. Cohort Description
3.2. Patient Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thorsby, E. A short history of HLA. Tissue Antigens 2009, 74, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Dausset, J. Iso-leuco-anticorps. Acta Haematol. 1958, 20, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.P.; Murray, J.E.; Harrison, J.H.; Guild, W.R. Successful homotransplantation of the human kidney between identical twins. J. Am. Med. Assoc. 1956, 160, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.M.; Merrill, J.P.; Miller, B.F.; Thorn, G.W. Experiences with renal homotransplantation in the human: Report of nine cases. J. Clin. Investig. 1955, 34, 327–382. [Google Scholar] [CrossRef] [Green Version]
- Merrill, J.P.; Murray, J.E.; Harrison, J.H.; Friedman, E.A.; Dealy, J.B., Jr.; Dammin, G.J. Successful homotransplantation of the kidney between nonidentical twins. N. Engl. J. Med. 1960, 262, 1251–1260. [Google Scholar] [CrossRef]
- Seigler, H.F.; Gunnells, J.C., Jr.; Robinson, R.R.; Ward, F.E.; Amos, D.B.; Rowlands, D.T.; Burkholder, P.M.; Klein, W.J.; Stickel, D.L. Renal transplantation between HL-A identical donor-recipient pairs: Functional and morphological evaluation. J. Clin. Investig. 1972, 51, 3200–3215. [Google Scholar] [CrossRef] [PubMed]
- Seigler, H.F.; Ward, F.E.; McCoy, R.C.; Gunnells, J.C.; Gutman, R.A.; Tisher, C.C.; Weinerth, J.L.; Stickel, D.L. Renal transplantation between HL-A haploidentical donor-recipient pairs: Functional and morphological evaluation. Surgery 1976, 79, 241–247. [Google Scholar]
- Seigler, H.F.; Ward, F.E.; McCoy, R.E.; Weinerth, J.L.; Gunnells, J.C.; Stickel, D.L. Long-term results with forty-five living related renal allograft recipients genotypically identical for HLA. Surgery 1977, 81, 274–283. [Google Scholar]
- Hart, A.; Smith, J.; Skeans, M.; Gustafson, S.; Wilk, A.; Castro, S.; Foutz, J.; Wainright, J.L.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2018 annual data report: Kidney. Am. J. Transplant. 2020, 20, 20–130. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Mcbride, M.A.; Cherikh, W.S.; Tolleris, C.B.; Bresnahan, B.A.; Johnson, C.P. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002, 62, 311–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matas, A.J.; Payne, W.D.; Sutherland, D.E.; Humar, A.; Gruessner, R.W.; Kandaswamy, R.; Dunn, D.L.; Gillingham, K.J.; Najarian, J.S. 2500 living donor kidney transplants: A single-center experience. Ann. Surg. 2001, 234, 149. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.C.; Opelz, G.; McGarvey, C.J.; Weil, E.J.; Chakkera, H.A. The risk of transplant failure with HLA mismatch in first adult kidney allografts from deceased donors. Transplantation 2016, 100, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Döhler, B. Effect of human leukocyte antigen compatibility on kidney graft survival: Comparative analysis of two decades. Transplantation 2007, 84, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Goubella, A.; Broeders, N.; Racapé, J.; Hamade, A.; Massart, A.; Hougardy, J.-M.; Hoang, A.D.; Mikhalski, D.; Baudoux, T.; Gankam, F.; et al. Patient and graft outcome in current era of immunosuppression: A single centre pilot study. Acta Clin. Belg. 2015, 70, 23–29. [Google Scholar] [CrossRef]
- Organ Procurement and Transplantation Network. OPTN Policies 2021. Available online: https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf (accessed on 13 October 2020).
- Su, X.; Zenios, S.A.; Chakkera, H.; Milford, E.L.; Chertow, G.M. Diminishing significance of HLA matching in kidney transplantation. Am. J. Transplant. 2004, 4, 1501–1508. [Google Scholar] [CrossRef]
- Israni, A.K.; Salkowski, N.; Gustafson, S.; Snyder, J.J.; Friedewald, J.J.; Formica, R.N.; Wang, X.; Shteyn, E.; Cherikh, W.; Stewart, D.; et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J. Am. Soc. Nephrol. 2014, 25, 1842–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, M.J.; Wen, X.; Rehman, S.; Santos, A.H.; Andreoni, K.A. Rethinking the advantage of zero-HLA mismatches in unrelated living donor kidney transplantation: Implications on kidney paired donation. Transpl. Int. 2015, 28, 401–409. [Google Scholar] [CrossRef]
- Williams, R.C.; Opelz, G.; Weil, E.J.; McGarvey, C.J.; Chakkera, H.A. The risk of transplant failure with HLA mismatch in first adult kidney allografts 2: Living donors, summary, guide. Transplant. Direct 2017, 3, e152. [Google Scholar] [CrossRef]
- Wiebe, C.; Kosmoliaptsis, V.; Pochinco, D.; Gibson, I.W.; Ho, J.; Birk, P.E.; Goldberg, A.; Karpinski, M.; Shaw, J.; Rush, D.N.; et al. HLA-DR/DQ molecular mismatch: A prognostic biomarker for primary alloimmunity. Am. J. Transpl. 2019, 19, 1708–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNOS. OPTN National Data 2021. Available online: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/ (accessed on 14 October 2020).
- Chang, P.; Gill, J.; Dong, J.; Rose, C.; Yan, H.; Landsberg, D.; Cole, E.H.; Gill, J.S. Living donor age and kidney allograft half-life: Implications for living donor paired exchange programs. Clin. J. Am. Soc. Nephrol. 2012, 7, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, D.R.; Wu, C.M.; Hariharan, S. Epidemiology of end-stage renal failure among twins and diagnosis, management, and current outcomes of kidney transplantation between identical twins. Am. J. Transpl. 2020, 20, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Institute, N.R. HLA Nomenclature 2020. Available online: http://hla.alleles.org/nomenclature/index.html (accessed on 14 October 2020).
- Ossman, R.; Jamme, M.; Moulin, B.; Legendre, C.; Morelon, E.; Frimat, L.; Hourmant, M.; Durrbach, A.; Malvezzi, P.; Rostaing, L.; et al. Immunosuppression and Graft Rejection in Living-related HLA-identical Renal Transplantation: The RADOVFULL Study. Transplantation 2020, 104, 1256–1262. [Google Scholar] [CrossRef]
- Clayton, P.A.; McDonald, S.P.; Russ, G.R.; Chadban, S.J. Long-Term Outcomes after Acute Rejection in Kidney Transplant Recipients: An ANZDATA Analysis. J. Am. Soc. Nephrol. 2019, 30, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Vernerey, D.; Tinel, C.; Aubert, O.; Duong van Huyen, J.P.; Rabant, M.; Verine, J.; Nochy, D.; Empana, J.-P.; Martinez, F.; et al. Subclinical Rejection Phenotypes at 1 Year Post-Transplant and Outcome of Kidney Allografts. J. Am. Soc. Nephrol. 2015, 26, 1721–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreso, F.; Ibernon, M.; Gomà, M.; Carrera, M.; Fulladosa, X.; Hueso, M.; Gil-Vernet, S.; Cruzado, J.M.; Torras, J.; Grinyó, J.M.; et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am. J. Transpl. 2006, 6, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, F.; Charpentier, B.; Vanrenterghem, Y.; Rostaing, L.; Bresnahan, B.; Darji, P.; Massari, P.; Mondragon-Ramirez, G.A.; Agarwal, M.; Di Russo, G.; et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am. J. Transplant. 2010, 10, 535–546. [Google Scholar] [CrossRef]
- Vincenti, F.; Rostaing, L.; Grinyo, J.; Rice, K.; Steinberg, S.; Gaite, L.; Moal, M.-C.; Mondragon-Ramirez, G.A.; Kothari, J.; Polinsky, M.S.; et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N. Engl. J. Med. 2016, 374, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Kher, V.; Jha, P.K. Paired kidney exchange transplantation—pushing the boundaries. Transpl. Int. 2020, 33, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Cantwell, L.; Ta, J.; Woodroffe, C.; D’Orsogna, L.; Holdsworth, R. Providing Better-Matched Donors for HLA Mismatched Compatible Pairs Through Kidney Paired Donation. Transplantation 2017, 101, 642–648. [Google Scholar] [CrossRef]

| Included | |
|---|---|
| N = 72 | |
| Gender (Female)-n (%) | 31 (43%) |
| Race | |
| Black | 17 (24%) |
| Other | 2 (3%) |
| White | 53 (74%) |
| Age at Transplant-Med (IQR) | 40 (30–52) |
| Diagnosis-n (%) | |
| Chronic Glomerulonephritis | 27 (39%) |
| Hypertension | 8 (11%) |
| Diabetes | 8 (11%) |
| Obstructive Uropathy | 4 (6%) |
| Lupus | 5 (7%) |
| Polycystic Kidney Disease | 4 (6%) |
| Post-streptococcal Glomerulonephritis | 5 (7%) |
| Other | 9 (13%) |
| Dialysis Type-n (%) | |
| Peritoneal Dialysis | 11 (15%) |
| Hemodialysis | 30 (42%) |
| Both | 8 (11%) |
| No Dialysis | 8 (11%) |
| Unknown | 15 (21%) |
| Duration of Dialysis (Months)-Med (IQR) | 6 (2–14) |
| Immunosuppression (Ever Used)-n (%) | |
| Azathioprine | 47 (65%) |
| Prednisone | 69 (96%) |
| Cyclophosphamide | 4 (6%) |
| Mycophenolate Mofetil | 24 (33%) |
| Calcineurin Inhibitor | 29 (40%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, B.I.; Villani, V.; Kesseli, S.J.; Nobuhara, C.; Samoylova, M.L.; Moris, D.; Collins, B.H.; McElroy, L.M.; Poh, M.; Knechtle, S.J.; et al. A Historical Cohort in Kidney Transplantation: 55-Year Follow-Up of 72 HLA-Identical, Donor-Recipient Pairs. J. Clin. Med. 2021, 10, 5505. https://doi.org/10.3390/jcm10235505
Shaw BI, Villani V, Kesseli SJ, Nobuhara C, Samoylova ML, Moris D, Collins BH, McElroy LM, Poh M, Knechtle SJ, et al. A Historical Cohort in Kidney Transplantation: 55-Year Follow-Up of 72 HLA-Identical, Donor-Recipient Pairs. Journal of Clinical Medicine. 2021; 10(23):5505. https://doi.org/10.3390/jcm10235505
Chicago/Turabian StyleShaw, Brian I., Vincenzo Villani, Samuel J. Kesseli, Chloe Nobuhara, Mariya L. Samoylova, Dimitrios Moris, Bradley H. Collins, Lisa M. McElroy, Melissa Poh, Stuart J. Knechtle, and et al. 2021. "A Historical Cohort in Kidney Transplantation: 55-Year Follow-Up of 72 HLA-Identical, Donor-Recipient Pairs" Journal of Clinical Medicine 10, no. 23: 5505. https://doi.org/10.3390/jcm10235505
APA StyleShaw, B. I., Villani, V., Kesseli, S. J., Nobuhara, C., Samoylova, M. L., Moris, D., Collins, B. H., McElroy, L. M., Poh, M., Knechtle, S. J., Barbas, A. S., & Seigler, H. F. (2021). A Historical Cohort in Kidney Transplantation: 55-Year Follow-Up of 72 HLA-Identical, Donor-Recipient Pairs. Journal of Clinical Medicine, 10(23), 5505. https://doi.org/10.3390/jcm10235505







