Clinical Impact of Hormone Replacement Therapy on Atrial Fibrillation in Postmenopausal Women: A Nationwide Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Ethical Considerations
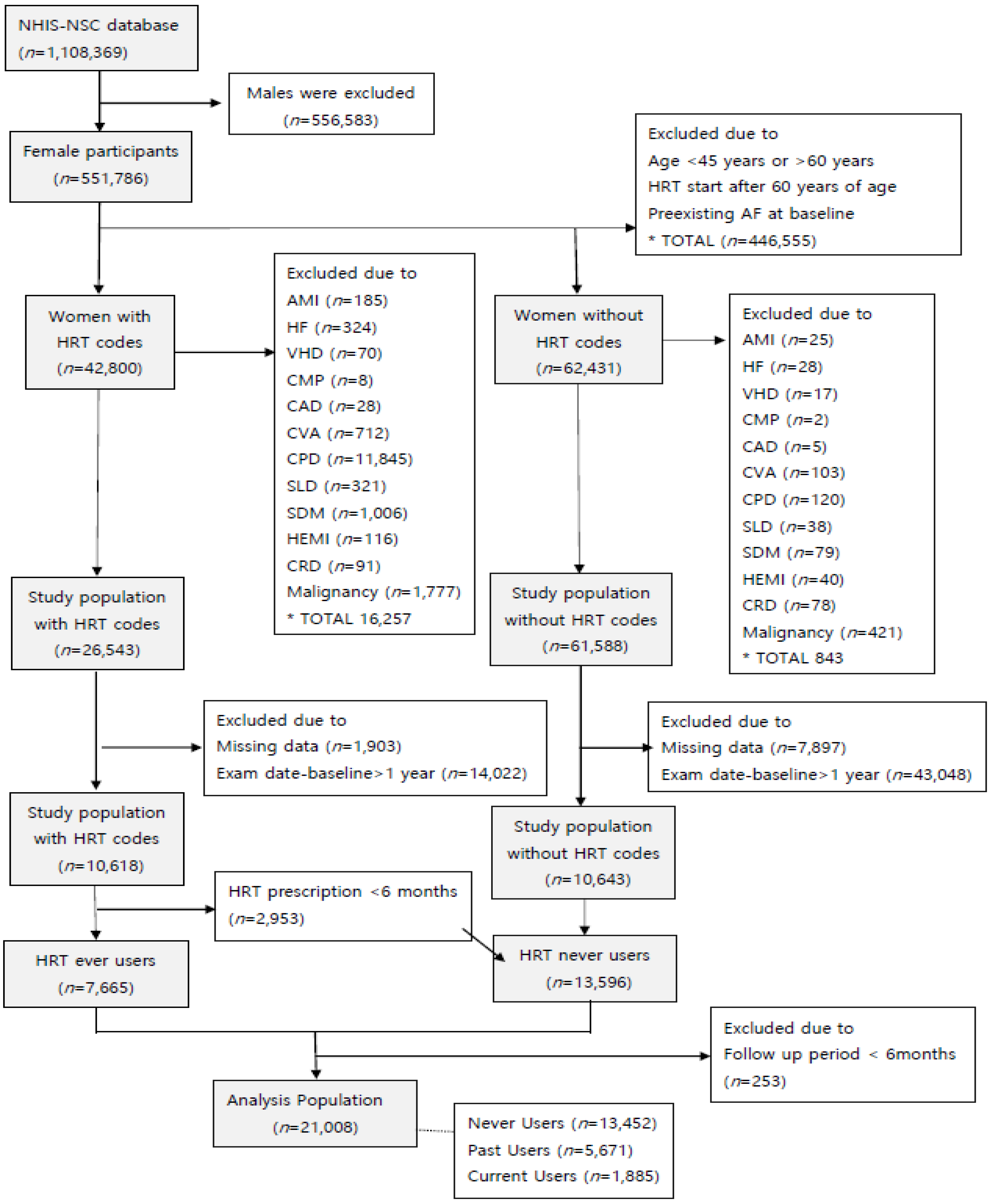
2.2. Study Population
2.3. Exposure to HRT
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. AF and HRT Use
3.3. AF in HRT Current Users
3.4. AF in HRT Past Users
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuster, V.; Rydén, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; Le Heuzey, J.Y.; Kay, G.N.; Lowe, J.E.; et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006, 114, e257–e354. [Google Scholar]
- Bushnell, C.; McCullough, L.D.; Awad, I.A.; Chireau, M.V.; Fedder, W.N.; Furie, K.L.; Howard, V.J.; Lichtman, J.H.; Lisabeth, L.D.; Piña, I.L.; et al. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 1545–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, D.; Rahman, F.; Schnabel, R.B.; Yin, X.; Benjamin, E.; Christophersen, I.E. Atrial fibrillation in women: Epidemiology, pathophysiology, presentation, and prognosis. Nat. Rev. Cardiol. 2016, 13, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Baber, R.J.; Panay, N.; Fenton, A.I. Fenton the IMS Writing Group 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef] [PubMed]
- North American Menopause Society. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017, 24, 728–753. [Google Scholar] [CrossRef] [PubMed]
- Viscoli, C.M.; Brass, L.M.; Kernan, W.N.; Sarrel, P.M.; Suissa, S.; Horwitz, R.I. A Clinical Trial of Estrogen-Replacement Therapy after Ischemic Stroke. N. Engl. J. Med. 2001, 345, 1243–1249. [Google Scholar] [CrossRef]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. For the Heart and Estrogen/progestin Replacement Study (HERS) Research Group Randomized Trial of Estrogen Plus Progestin for Secondary Prevention of Coronary Heart Disease in Postmenopausal Women. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Hulley, S.; Furberg, C.; Barrett-Connor, E.; Cauley, J.; Grady, D.; Haskell, W.; Knopp, R.; Lowery, M.; Satterfield, S.; Schrott, H.; et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 2002, 288, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Hodis, H.N.; Mack, W.J.; Lobo, R.A.; Shoupe, D.; Sevanian, A.; Mahrer, P.R.; Selzer, R.H.; Liu, C.-R.; Liu, C.-H.; Azen, S.P.; et al. Estrogen in the Prevention of Atherosclerosis. Ann. Intern. Med. 2001, 135, 939–953. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [PubMed]
- Nudy, M.; Chinchilli, V.M.; Foy, A.J. A systematic review and meta-regression analysis to examine the ‘timing hypothesis’ of hormone replacement therapy on mortality, coronary heart disease, and stroke. IJC Heart Vasc. 2019, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Boardman, H.M.; Hartley, L.; Eisinga, A.; Main, C.; i Figuls, M.R.; Cosp, X.B.; Sanchez, R.G.; Knight, B. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database Syst. Rev. 2015, Cd002229. [Google Scholar] [CrossRef]
- Perez, M.V.; Wang, P.J.; Larson, J.C.; Virnig, B.A.; Cochrane, B.; Curb, J.D.; Klein, L.; Manson, J.E.; Martin, L.W.; Robinson, J.; et al. Effects of postmenopausal hormone therapy on incident atrial fibrillation: The Women’s Health Initiative randomized controlled trials. Circ. Arrhythm. Electrophysiol. 2012, 5, 1108–1116. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.A.; Rexrode, K.M.; Sandhu, R.K.; Moorthy, M.V.; Conen, D.; Albert, C.M. Menopausal age, postmenopausal hormone therapy and incident atrial fibrillation. Heart 2017, 103, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Haung, Y.B.; Kuo, H.F.; Tang, W.H.; Hsu, P.C.; Su, H.M.; Lin, T.H.; Chu, C.S.; Jhuo, S.J.; Lee, K.T.; et al. Hormone replacement therapy and risk of atrial fibrillation in Taiwanese menopause women: A nationwide cohort study. Sci. Rep. 2016, 6, 24132. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Haung, Y.B.; Kuo, H.F.; Tang, W.H.; Hsu, P.C.; Su, H.M.; Lin, T.H.; Chu, C.S.; Jhuo, S.J.; Lee, K.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar]
- Svennberg, E.; Engdahl, J.; Al-Khalili, F.; Friberg, L.; Frykman, V.; Rosenqvist, M. Mass screening for untreated atrial fibrillation: The STROKESTOP study. Circulation 2015, 131, 2176–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, K.B.; Ng, T.P.; Ong, H.Y. Low prevalence of atrial fibrillation in community-dwelling Chinese aged 55 years or older in Singapore: A population-based study. J. Electrocardiol. 2008, 41, 94–98. [Google Scholar] [CrossRef]
- Yang, S.; Kwak, S.; Kwon, S.; Lee, H.-J.; Lee, H.; Park, J.-B.; Lee, S.-P.; Kim, H.; Han, K.; Kim, Y.-J.; et al. Association of Total Reproductive Years with Incident Atrial Fibrillation, and Subsequent Ischemic Stroke in Women with Natural Menopause. Circ. Arrhythmia Electrophysiol. 2019, 12, e007428. [Google Scholar] [CrossRef]
- Friberg, J.; Scharling, H.; Gadsbøll, N.; Truelsen, T.; Jensen, G.B. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am. J. Cardiol. 2004, 94, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Conen, D.; Chae, C.U.; Glynn, R.J.; Tedrow, U.B.; Everett, B.M.; Buring, J.E.; Albert, C.M. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA 2011, 305, 2080–2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.J.; Massaro, J.M.; Levy, D.; Vasan, R.S.; Wolf, P.A.; D’Agostino, R.B.; Larson, M.G.; Kannel, W.B.; Benjamin, E.J. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: The Framingham Heart Study. JAMA 2003, 290, 1049–1056. [Google Scholar] [CrossRef]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- Song, S.O.; Jung, C.H.; Song, Y.D.; Park, C.Y.; Kwon, H.S.; Cha, B.S.; Park, J.Y.; Lee, K.U.; Ko, K.S.; Lee, B.W. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 2014, 38, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Beral, V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003, 362, 419–427. [Google Scholar] [CrossRef]
- Nelson, H.D.; Humphrey, L.L.; Nygren, P.; Teutsch, S.M.; Allan, J.D. Postmenopausal hormone replacement therapy: Scientific review. JAMA 2002, 288, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Hernández-Díaz, S.; Logan, R.; Rossouw, J.E.; Hernán, M.A. Coronary heart disease in postmenopausal recipients of estrogen plus progestin therapy: Does the increased risk ever disappear? A randomized trial. Ann. Intern. Med. 2010, 152, 211–217. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Walsh, J.M.; Greyber, E.; Salpeter, E.E. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J. Gen. Intern. Med. 2006, 21, 363–366. [Google Scholar] [CrossRef] [Green Version]
- Salpeter, S.R.; Cheng, J.; Thabane, L.; Buckley, N.S.; Salpeter, E.E. Bayesian meta-analysis of hormone therapy and mortality in younger postmenopausal women. Am. J. Med. 2009, 122, 1016–1022.e1. [Google Scholar] [CrossRef] [PubMed]
- Shifren, J.L.; Rifai, N.; Desindes, S.; McIlwain, M.; Doros, G.; Mazer, N.A. A comparison of the short-term effects of oral conjugated equine estrogens versus transdermal estradiol on C-reactive protein, other serum markers of inflammation, and other hepatic proteins in naturally menopausal women. J. Clin. Endocrinol. Metab. 2008, 93, 1702–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, B.; Potpara, T.S.; Lip, G.Y. Stroke prevention in atrial fibrillation. Lancet 2016, 388, 806–817. [Google Scholar] [CrossRef]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [Green Version]
- Oliver-Williams, C.; Glisic, M.; Shahzad, S.; Brown, E.; Pellegrino Baena, C.; Chadni, M.; Chowdhury, R.; Franco, O.H.; Muka, T. The route of administration, timing, duration and dose of postmenopausal hormone therapy and cardiovascular outcomes in women: A systematic review. Hum. Reprod. Update 2019, 25, 257–271. [Google Scholar] [CrossRef]
- Grodstein, F.; Manson, J.E.; Stampfer, M.J.; Rexrode, K. Postmenopausal hormone therapy and stroke: Role of time since menopause and age at initiation of hormone therapy. Arch. Intern. Med. 2008, 168, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Estrogen regulation of protein expression and signaling pathways in the heart. Biol. Sex. Differ. 2014, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Carnethon, M.R.; Anthony, M.S.; Cascio, W.E.; Folsom, A.R.; Rautaharju, P.M.; Liao, D.; Evans, G.W.; Heiss, G. A prospective evaluation of the risk of QT prolongation with hormone replacement therapy: The atherosclerosis risk in communities study. Ann. Epidemiol. 2003, 13, 530–536. [Google Scholar] [CrossRef]
- Sedlak, T.; Shufelt, C.; Iribarren, C.; Merz, C.N. Sex hormones and the QT interval: A review. J. Womens Health 2012, 21, 933–941. [Google Scholar] [CrossRef]
- Tse, H.F.; Oral, H.; Pelosi, F.; Knight, B.P.; Strickberger, S.A.; Morady, F. Effect of gender on atrial electrophysiologic changes induced by rapid atrial pacing and elevation of atrial pressure. J. Cardiovasc. Electrophysiol. 2001, 12, 986–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadish, A.H.; Greenland, P.; Limacher, M.C.; Frishman, W.H.; Daugherty, S.A.; Schwartz, J.B. Estrogen and progestin use and the QT interval in postmenopausal women. Ann. Noninvasive Electrocardiol. 2004, 9, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yang, P.S.; Kim, D.; Yu, H.T.; Uhm, J.S.; Kim, J.Y.; Pak, H.N.; Lee, M.H.; Joung, B.; Lip, G.Y. CHA(2)DS(2)-VASc Score for Identifying Truly Low-Risk Atrial Fibrillation for Stroke: A Korean Nationwide Cohort Study. Stroke 2017, 48, 2984–2990. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 21,008) | Never Users (n = 13,452) | Past Users (n = 5671) | Current Users (n = 1885) | p Value | |
|---|---|---|---|---|---|
| Mean follow-up period, days | 3747.59 (1478.30) | 4168.95 (1295.09) | 3263.79 (1401.50) | 2196.15 (1449.33) | <0.001 |
| Age, years | 50.99 (3.93) | 51.10 (4.08) | 51 (3.64) | 50.18 (3.51) | <0.001 |
| BMI, kg/m2 | 23.74 (3) | 23.98 (3.08) | 23.51 (2.82) | 22.76 (2.64) | <0.001 |
| Height, cm | 155.69 (5.18) | 155.46 (5.22) | 155.93 (5.1) | 156.57 (4.96) | <0.001 |
| Weight, kg | 57.56 (7.75) | 57.96 (7.97) | 57.18 (7.37) | 55.80 (6.93) | <0.001 |
| SBP | 122 (17.08) | 123.02 (17.57) | 120.79 (16.46) | 118.39 (14.50) | <0.001 |
| DBP | 76.35 (11.25) | 76.89 (11.48) | 75.73 (10.95) | 74.38 (10.07) | <0.001 |
| Total cholesterol | 202.48 (42.22) | 202.86 (45.05) | 202.54 (37.01) | 199.62 (35.35) | 0.008 |
| HTN, n (%) | 7193 (34.24) | 4881 (36.28) | 1868 (32.94) | 444 (23.55) | <0.001 |
| Diabetes, n (%) | 2073 (9.87) | 1497 (11.13) | 474 (8.36) | 102 (5.41) | <0.001 |
| HCL, n (%) | 2999 (14.28) | 1966 (14.61) | 814 (14.35) | 219 (11.62) | 0.004 |
| HRT, n (%) | <0.001 | ||||
| E only | 1222 (5.82) | 0 (0) | 984 (17.35) | 238 (12.63) | |
| E + P | 3279 (15.61) | 0 (0) | 2440 (43.03) | 841 (44.62) | |
| Tibolone | 3049 (14.51) | 0 (0) | 2247 (39.62) | 806 (42.76) | |
| Never | 13,458 (64.06) | 13,458 (100) | 0 (0) | 0 (0) | |
| Mean duration of HRT use, days | 447.85 (653.68) | - | 327.37 (475.79) | 930.56 (871.19) | <0.001 |
| Smoking, n (%) | <0.001 | ||||
| Current | 583 (2.78) | 312 (2.32) | 184 (3.24) | 87 (4.62) | |
| Former | 227 (1.08) | 107 (0.80) | 81 (1.43) | 39 (2.07) | |
| Never | 20,198 (96.14) | 13,033 (96.89) | 5406 (95.33) | 1759 (93.32) | |
| Alcohol consumption, n (%) | <0.001 | ||||
| Rarely/never | 18,413 (87.65) | 12,178 (90.53) | 4811 (75.52) | 1424 (75.54) | |
| 1–2 drinks/week | 2016 (9.6) | 974 (7.24) | 679 (19.32) | 363 (19.26) | |
| 3–4 drinks/week | 383 (1.82) | 185 (1.38) | 122 (4.04) | 76 (4.03) | |
| >5 drinks/week | 196 (0.93) | 115 (0.85) | 59 (1.12) | 22 (1.17) | |
| Exercise, n (%) | <0.001 | ||||
| Rarely/never | 11,347 (54.01) | 8027 (59.67) | 2679 (47.24) | 641 (34.01) | |
| 1–2 times/week | 5268 (25.08) | 2974 (22.11) | 1621 (28.58) | 673 (35.70) | |
| 3–4 times/week | 2687 (12.79) | 1379 (10.25) | 906 (15.98) | 402 (21.33) | |
| 5–6 times/week | 640 (3.05) | 340 (2.53) | 194 (3.42) | 106 (5.62) | |
| 7 times/week | 1066 (5.07) | 732 (5.44) | 271 (4.78) | 63 (3.34) | |
| HRT Use | ||||
|---|---|---|---|---|
| Total | Never | Past | Current | |
| Number of AF cases | 381 | 279 | 68 | 34 |
| Person-years | 215,697.10 | 153,645.81 | 50,709.50 | 11,341.79 |
| AF incidence rate * (95% CI) | 1.77 (1.60–1.95) | 1.82 (1.61–2.04) | 1.34 (1.06–1.70) | 3.00 (2.14–4.19) |
| Age-adjusted AF incidence rate * (95% CI) | 1.77 (1.76–1.78) | 1.82 (1.81–1.83) | 1.34 (1.32–1.35) | 3.00 (2.52–2.62) |
| Age-adjusted HR (95% CI) | Reference | 0.78 (0.60–1.01) | 2.01 (1.40–2.88) | |
| p = 0.063 | p < 0.001 | |||
| Model 1 † | Reference | 0.78 (0.59–1.01) | 2.17 (1.50–3.13) | |
| p = 0.063 | p < 0.001 | |||
| Model 2 ‡ | Reference | 0.78 (0.60–1.02) | 2.24 (1.55–3.23) | |
| p = 0.069 | p < 0.001 | |||
| HRT Type (Current Users) | |||||
|---|---|---|---|---|---|
| Total (n = 15,337) | None (n = 13,452) | E Only (n = 238) | E + P (n = 841) | Tibolone (n = 806) | |
| Number of AF cases | 313 | 279 | 5 | 13 | 16 |
| Person-years | 164,987.60 | 153,645.81 | 1570.46 | 4673.92 | 5097.41 |
| AF incidence rate * (95% CI) | 1.90 (1.70–2.12) | 1.82 (1.61–2.04) | 3.18 (1.33–7.64) | 2.78 (1.62–4.79) | 3.14 (1.92–5.12) |
| Age-adjusted AF incidence rate * (95% CI) | 1.90 (1.89–1.91) | 1.82 (1.81–1.83) | 3.20 (3.05–3.34) | 2.81 (2.73–2.89) | 3.12 (3.04–3.20) |
| Age-adjusted HR (95% CI) | Reference | 2.05 (0.85–4.98) | 1.96 (1.12–3.45) | 2.02 (1.21–3.35) | |
| p = 0.11 | p = 0.02 | p = 0.007 | |||
| Model 1 † | Reference | 1.95 (0.80–4.74) | 2.25 (1.28–3.97) | 2.15 (1.29–3.59) | |
| p = 0.141 | p = 0.005 | p = 0.004 | |||
| Model 2 ‡ | Reference | 2.04 (0.84–4.97) | 2.30 (1.30–4.06) | 2.23 (1.34–3.73) | |
| p = 0.115 | p = 0.004 | p = 0.002 | |||
| HRT Regimen (Current Users) | |||||||
|---|---|---|---|---|---|---|---|
| Total (n = 15,337) | None (n = 13,452) | CEE (n = 48) | E2 (n = 190) | E2 + P (n = 841) | CEE + P (n = 0) | Tibolone (n = 806) | |
| Number of AF cases | 313 | 279 | 2 | 3 | 13 | 0 | 16 |
| Person-years | 164,987.60 | 153,645.81 | 293.34 | 1277.12 | 4673.92 | - | 5097.41 |
| AF incidence rate * (95% CI) | 1.90 (1.70–2.12) | 1.82 (1.61–2.04) | 6.82 (1.71–27.13) | 2.35 (0.76–7.27) | 2.78 (1.62–4.79) | 3.14 (1.92–5.12) | |
| Age-adjusted AF incidence rate * (95% CI) | 1.90 (1.89–1.91) | 1.82 (1.81–1.83) | 7.91 (7.33–8.48) | 2.35 (2.21–2.49) | 2.81 (2.73–2.89) | 3.12 (3.04–3.20) | |
| Age-adjusted HR (95% CI) | Reference | 4.48 (1.11–18.04) | 1.51 (0.48–4.71) | 1.96 (1.12–3.45) | - | 2.02 (1.21–3.35) | |
| p = 0.035 | p = 0.479 | p = 0.019 | p = 0.007 | ||||
| Model 1 † | Reference | 4.95 (1.22–20.05) | 1.39 (0.44–4.34) | 2.25 (1.28–4.00) | 2.15 (1.29–3.60) | ||
| p = 0.025 | p = 0.573 | p = 0.005 | p = 0.003 | ||||
| Model 2 ‡ | Reference | 5.35 (1.32–21.66) | 1.45 (0.46–4.53) | 2.30 (1.30–4.07) | 2.23 (1.34–3.73) | ||
| p = 0.019 | p = 0.524 | p = 0.004 | p = 0.002 | ||||
| HRT Type (Past Users) | |||||
|---|---|---|---|---|---|
| Total (n = 19,123) | None (n = 13,452) | E Only (n = 984) | E + P (n = 2440) | Tibolone (n = 2247) | |
| Number of AF cases | 347 | 279 | 15 | 20 | 33 |
| Person-years | 204,355.31 | 153,645.81 | 9819.02 | 20,901.44 | 19,989.04 |
| AF incidence rate * (95% CI) | 1.90 (1.53–1.89) | 1.82 (1.61–2.04) | 1.53 (0.92–2.53) | 0.28 (0.62–1.48) | 1.65 (1.17–2.32) |
| Age-adjusted AF incidence rate * (95% CI) | 1.70 (1.89–1.91) | 1.82 (1.81–1.83) | 1.51 (1.47–1.55) | 0.96 (0.93–0.98) | 1.65 (1.62–1.68) |
| Age-adjusted HR (95% CI) | Reference | 0.85 (0.51–1.43) | 0.59 (0.37–0.93) | 0.93 (0.65–1.34) | |
| p = 0.543 | p = 0.022 | p = 0.693 | |||
| Model 1 † | Reference | 0.80 (0.48–1.35) | 0.60 (0.38–0.94) | 0.93 (0.65–1.34) | |
| p = 0.411 | p = 0.026 | p = 0.705 | |||
| Model 2 ‡ | Reference | 0.81 (0.48–1.37) | 0.60 (0.38–0.94) | 0.94 (0.65–1.35) | |
| 0.85 (0.51–1.43) | 0.59 (0.37–0.93) | 0.93 (0.65–1.34) | |||
| HRT Regimen (Past Users) | |||||||
|---|---|---|---|---|---|---|---|
| Total (n = 19,123) | None (n = 13,452) | CEE (n = 541) | E2 (n = 443) | E2 + P (n = 2286) | CEE + P (n = 154) | Tibolone (n = 2247) | |
| Number of AF cases | 347 | 279 | 12 | 3 | 17 | 3 | 33 |
| Person-years | 204,355.31 | 153,645.81 | 6273.93 | 3545.09 | 18,883.21 | 2018.24 | 19,989.04 |
| AF incidence rate * (95% CI) | 1.70 (1.53–1.89) | 1.82 (1.61–2.04) | 1.91 (1.09–3.37) | 0.85 (0.27–2.62) | 0.90 (0.56–1.45) | 1.49 (0.48–4.60) | 1.65 (1.17–2.32) |
| Age-adjusted AF incidence rate * (95% CI) | 1.70 (1.89–1.91) | 1.82 (1.81–1.83) | 1.89 (1.84–1.95) | 0.85 (0.80–0.90) | 0.90 (0.88–0.92) | 1.48 (1.40–1.57) | 1.65 (1.62–1.68) |
| Age-adjusted HR (95% CI) | Reference | 1.00 (0.56–1.78) | 0.54 (0.17–1.67) | 0.57 (0.35–0.92) | 0.76 (0.24–2.36) | 0.93 (0.65–1.33) | |
| p = 0.994 | p = 0.282 | p = 0.023 | p = 0.632 | p = 0.691 | |||
| Model 1 † | Reference | 0.91 (0.51–1.62) | 0.55 (0.18–1.71) | 0.58 (0.35–0.94) | 0.74 (0.24–2.30) | 0.93 (0.65–1.34) | |
| p = 0.747 | p = 0.301 | p = 0.028 | p = 0.599 | p = 0.702 | |||
| Model 2 ‡ | Reference | 0.92 (0.51–1.64) | 0.55 (0.12–1.74) | 0.57 (0.35–0.94) | 0.75 (0.24–2.35) | 0.94 (0.65–1.35) | |
| p = 0.768 | p = 0.311 | p = 0.027 | p = 0.626 | p = 0.730 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, Y.; Park, H.; Kim, C.; Cho, S.; Kim, J. Clinical Impact of Hormone Replacement Therapy on Atrial Fibrillation in Postmenopausal Women: A Nationwide Cohort Study. J. Clin. Med. 2021, 10, 5497. https://doi.org/10.3390/jcm10235497
Lee J, Kim Y, Park H, Kim C, Cho S, Kim J. Clinical Impact of Hormone Replacement Therapy on Atrial Fibrillation in Postmenopausal Women: A Nationwide Cohort Study. Journal of Clinical Medicine. 2021; 10(23):5497. https://doi.org/10.3390/jcm10235497
Chicago/Turabian StyleLee, Jaehoon, Yuntae Kim, Hyunji Park, Changsoo Kim, Sihyun Cho, and Jongyoun Kim. 2021. "Clinical Impact of Hormone Replacement Therapy on Atrial Fibrillation in Postmenopausal Women: A Nationwide Cohort Study" Journal of Clinical Medicine 10, no. 23: 5497. https://doi.org/10.3390/jcm10235497
APA StyleLee, J., Kim, Y., Park, H., Kim, C., Cho, S., & Kim, J. (2021). Clinical Impact of Hormone Replacement Therapy on Atrial Fibrillation in Postmenopausal Women: A Nationwide Cohort Study. Journal of Clinical Medicine, 10(23), 5497. https://doi.org/10.3390/jcm10235497






