Lymphocytopenia and Anti-CD38 Directed Treatment Impact the Serological SARS-CoV-2 Response after Prime Boost Vaccination in Patients with Multiple Myeloma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
- (a)
- Aged 18 years and older;
- (b)
- Had a confirmed diagnosis of MM, smoldering MM (sMM), monoclonal gammopathy of clinical significance (MGCS) and systematic light chain amyloidosis (AL) according to the 2014 updated diagnostic criteria of the International Myeloma Working Group (IMWG) [24];
- (c)
- (d)
- Provided written informed consent.
2.2. Detection of Anti-SARS-CoV-2 Antibodies
2.3. Flow Cytometry Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Eng. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.L.; Lutrick, K.; et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Eng. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Eng. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Addeo, A.; Shah, P.K.; Bordry, N.; Hudson, R.D.; Albracht, B.; Di Marco, M.; Kaklamani, V.; Dietrich, P.-Y.; Taylor, B.S.; Simand, P.F.; et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021, 39, 1081–1090.e2. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Diao, L.; Yang, Y.; Yi, X.; Rodriguez, B.L.; Li, Y.; Villalobos, P.A.; Cascone, T.; Liu, X.; Tan, L.; et al. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov. 2018, 8, 1156–1175. [Google Scholar] [CrossRef] [Green Version]
- Joshua, D.; Suen, H.; Brown, R.; Bryant, C.; Ho, P.J.; Hart, D.; Gibson, J. The T Cell in Myeloma. Clin. Lymphoma Myeloma Leuk. 2016, 16, 537–542. [Google Scholar] [CrossRef]
- Pratt, G.; Goodyear, O.; Moss, P. Immunodeficiency and immunotherapy in multiple myeloma. Br. J. Haematol. 2007, 138, 563–579. [Google Scholar] [CrossRef]
- Rawstron, A.; Davies, F.; Owen, R.G.; English, A.; Pratt, G.; Child, J.A.; Jack, A.S.; Morgan, G. B-lymphocyte suppression in multiple myeloma is a reversible phenomenon specific to normal B-cell progenitors and plasma cell precursors. Br. J. Haematol. 1998, 100, 176–183. [Google Scholar] [CrossRef]
- Blimark, C.; Holmberg, E.; Mellqvist, U.-H.; Landgren, O.; Björkholm, M.; Hultcrantz, M.; Kjellander, C.; Turesson, I.; Kristinsson, S.Y. Multiple myeloma and infections: A population-based study on 9253 multiple myeloma patients. Haematologica 2014, 100, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Chari, A.; Samur, M.K.; Martinez-Lopez, J.; Cook, G.; Biran, N.; Yong, K.; Hungria, V.; Engelhardt, M.; Gay, F.; Feria, A.G.; et al. Clinical features associated with COVID-19 outcome in multiple myeloma: First results from the International Myeloma Society data set. Blood 2020, 136, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Branagan, A.; Lei, M.; Yee, A.J.; O’Donnell, E.; Castillo, J.J.; Raje, N.; Treon, S.T.; Flynn, C.; Burke, J.; Harrington, C.; et al. COVID-19 Vaccine Responsiveness in Patients with Multiple Myeloma and Waldenström Macroglobulinemia. Clin. Lymphoma Myeloma Leuk. 2021, 21, S29. Available online: https://events.jspargo.com/IMW21/CUSTOM/IMWAbstractBook.pdf (accessed on 18 October 2021). [CrossRef]
- Stampfer, S.D.; Goldwater, M.-S.; Jew, S.; Bujarski, S.; Regidor, B.; Daniely, D.; Chen, H.; Xu, N.; Li, M.; Green, T.; et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pimpinelli, F.; Marchesi, F.; Piaggio, G.; Giannarelli, D.; Papa, E.; Falcucci, P.; Pontone, M.; Di Martino, S.; Laquintana, V.; Malfa, A.L.; et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: Preliminary data from a single institution. J. Hematol. Oncol. 2021, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Briasoulis, A.; Gumeni, S.; Malandrakis, P.; Fotiou, D.; Papanagnou, E.-D.; Migkou, M.; Theodorakakou, F.; et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Van Oekelen, O.; Gleason, C.R.; Agte, S.; Srivastava, K.; Beach, K.F.; Aleman, A.; Kappes, K.; PVI/Seronet Team; Mouhieddine, T.K.; Wang, B.; et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell 2021, 39, 1028–1030. [Google Scholar] [CrossRef]
- Terpos, E.; Trougakos, I.P.; Gavriatopoulou, M.; Papassotiriou, I.; Sklirou, A.D.; Ntanasis-Stathopoulos, I.; Papanagnou, E.-D.; Fotiou, D.; Kastritis, E.; Dimopoulos, M.A.; et al. Low Neutralizing Antibody Responses Against SARS-CoV-2 in Elderly Myeloma Patients After the First BNT162b2 Vaccine Dose. Blood 2021, 137, 3674–3676. [Google Scholar] [CrossRef]
- Bird, S.; Panopoulou, A.; Shea, R.L.; Tsui, M.; Saso, R.; Sud, A.; West, S.; Smith, K.; Barwood, J.; Kaczmarek, E.; et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021, 8, 389–392. [Google Scholar] [CrossRef]
- Moreno, L.; Perez, C.; Zabaleta, A.; Manrique, I.; Alignani, D.; Ajona, D.; Blanco, L.; Lasa, M.; Maiso, P.; Rodriguez, I.; et al. The Mechanism of Action of the Anti-CD38 Monoclonal Antibody Isatuximab in Multiple Myeloma. Clin. Cancer Res. 2019, 25, 3176–3187. [Google Scholar] [CrossRef] [Green Version]
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Trans. Med. 2013, 5, 173ra23. [Google Scholar] [CrossRef] [PubMed]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; Van De Donk, N.W.C.J.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghandili, S.; Schönlein, M.; Lütgehetmann, M.; Schulze zur Wiesch, J.; Becher, H.; Bokemeyer, C.; Sinn, M.; Weisel, K.C.; Leypoldt, L.B. Post-Vaccination Anti-SARS-CoV-2-Antibody Response in Patients with Multiple Myeloma Correlates with Low CD19+ B-Lymphocyte Count and Anti-CD38 Treatment. Cancers 2021, 13, 3800. [Google Scholar] [CrossRef] [PubMed]
- International-Myeloma-Society. Recommendations for anti-Covid-19 Vaccination in Patients with Multiple Myeloma (MM) and Related Conditions, AL Amyloidosis and Other Monoclonal Gammopathies of Clinical Significance. Available online: https://myelomasociety.org/wp-content/uploads/2021/03/PM-COVID-vaccination-in-MM-guidelines-The-Final.pdf (accessed on 18 October 2021).
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Brehm, T.T.; Schwinge, D.; Lampalzer, S.; Schlicker, V.; Küchen, J.; Thompson, M.; Ullrich, F.; Huber, S.; Schmiedel, S.; Addo, M.M.; et al. Seroprevalence of SARS-CoV-2 antibodies among hospital workers in a German tertiary care center: A sequential follow-up study. Int. J. Hyg. Environ. Health 2021, 232, 113671. [Google Scholar] [CrossRef]
- Resman Rus, K.; Korva, M.; Knap, N.; Avsic Zupanc, T.; Poljak, M. Performance of the rapid high-throughput automated electrochemiluminescence immunoassay targeting total antibodies to the SARS-CoV-2 spike protein receptor binding domain in comparison to the neutralization assay. J. Clin. Virol. 2021, 139, 104820. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef]
- Greenberger, L.M.; Saltzman, L.A.; Senefeld, J.W.; Johnson, P.W.; DeGennaro, L.J.; Nichols, G.L. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021, 39, 1031–1033. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P.; et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- U.S.FDA. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA: U.S.FDA; 2021 Updated Thu, 08/12/2021—23:07. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised (accessed on 18 October 2021).
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Eng. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef]
- Ehmsen, S.; Asmussen, A.; Jeppesen, S.S.; Nilsson, A.C.; Østerlev, S.; Vestergaard, H.; Justesen, U.S.; Johansen, I.S.; Frederiksen, H.; Ditzel, H.J. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell 2021, 39, 1034–1036. [Google Scholar] [CrossRef] [PubMed]


| Variable, n (%) If Not Other Identified | Total |
|---|---|
| Age, median age in years (range) | 68 (35–85) |
| Male sex | 49 (60) |
| Type of plasma-cell-related neoplasia | |
| MM | 74 (90.2) |
| sMM | 2 (2.4) |
| MGCS | 2 (2.4) |
| AL | 4 (4.9) |
| Newly diagnosed | 48 (58.5) |
| Refractory or relapsed | 34 (41.4) |
| Therapy lines, median number in total (range) | 1 (0–8) |
| Anti-myeloma therapy | |
| Anti-CD38 directed therapy | 37 (45.1) |
| Daratumumab-based | 27 |
| Isatuximab-based | 10 |
| Elotuzumab-based | 2 (2.4) |
| IMiD-based therapies in total | 52 (63.4) |
| Thalidomide-based | 1 |
| Lenalidomide-based | 48 |
| Pomalidomide-based | 4 |
| Proteasome inhibitor-based in total | 17 (20.7) |
| Bortezomib-based | 4 |
| Carfilzomib-based | 13 |
| No current therapy | 13 (15.9) |
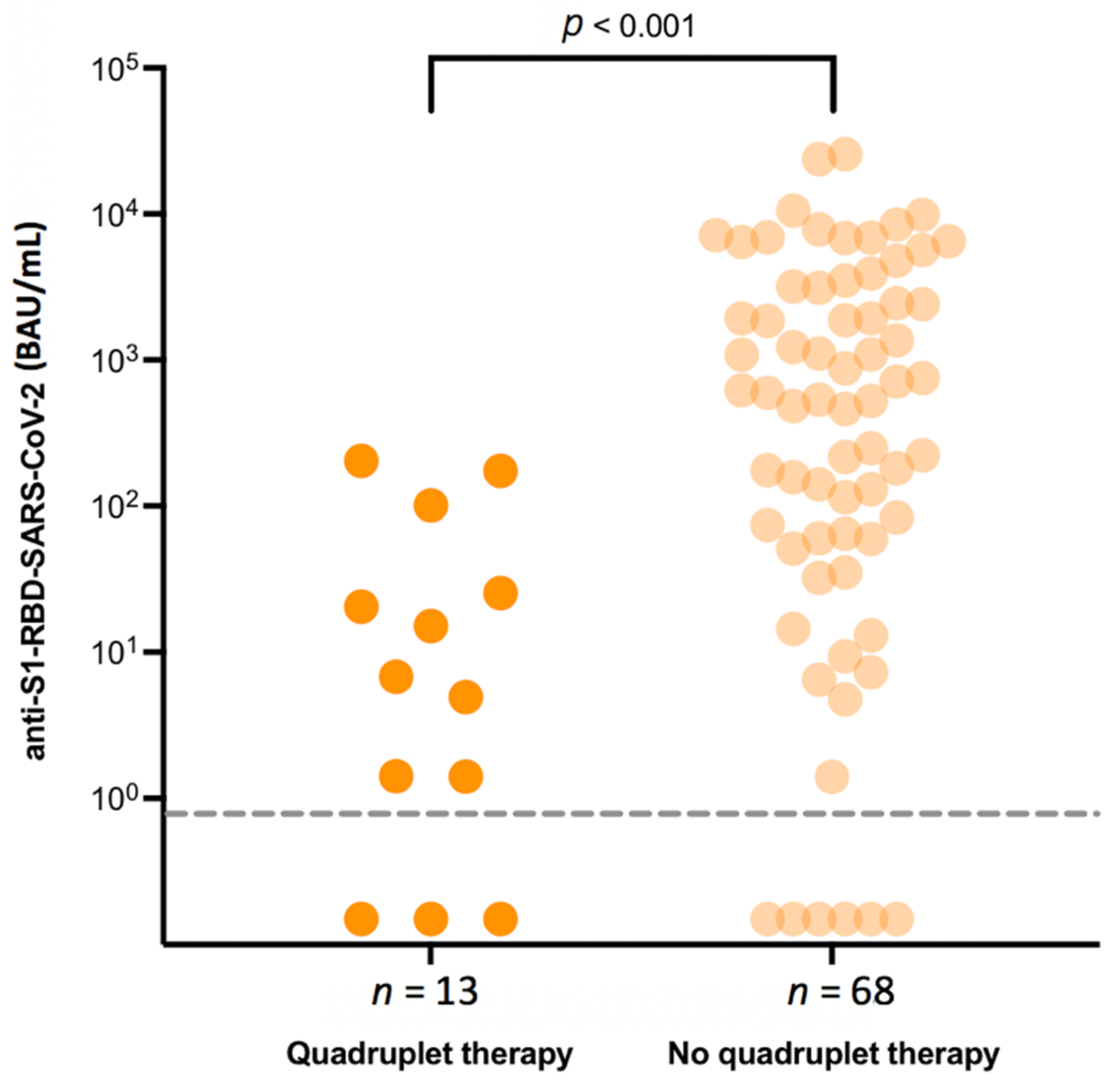
| Quadruplet treatment | 13 (15.9) |
| Remission status: deep remission ≥ VGPR | 62 (75.6) |
| Vaccination type | |
| mRNA-based | 67 |
| Vector-based | 8 |
| Heterologous | 4 |
| Parameter | Coefficient | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Constant | 6.83 | [3.54; 10.10] | <0.001 |
| CD 19 + B cells (logarithmized) | 1.01 | [0.71; 1.30] | <0.001 |
| Age | −0.10 | [−0.14; −0.06] | <0.001 |
| Vaccine (reference: mRNA) Vector Heterologous | 0.30 2.73 | [−1.18; 1.78] [0.11; 5.36] | 0.69 0.042 |
| Days post second vaccination | 0.03 | [−0.02; 0.08] | 0.19 |
| Quadruplet therapy (reference: other therapy) | −1.44 | [−2.75; −0.13] | 0.032 |
| Deep remission ≥ VGPR (reference: ≤ PR) | 0.87 | [−0.37; 2.12] | 0.17 |
| Age (Years) | Sex | CD19+ Cells/µL | Vaccine Type | Disease Type | ND/RR | Current Treatment | Line of Therapy | Remission | |
|---|---|---|---|---|---|---|---|---|---|
| I | 69 | female | 2 | mRNA | MM | RR | Elo-PCd | 5 | SD |
| II | 61 | male | 1 | vector | MM | ND | IsaKRd | 1 | sCR |
| III | 68 | female | 9 | mRNA | MM | RR | Rd | 2 | VGPR |
| IV | 78 | female | 11 | mRNA | MM | RR | PCd | 8 | PD |
| V | 66 | female | 1 | mRNA | MM/AL | RR | DRd | 2 | VGPR |
| VI | 81 | male | 9 | mRNA | MM | ND | DRd | 1 | VGPR |
| VII | 63 | female | 1 | mRNA | MM | ND | IsaKRd | 1 | sCR |
| VIII | 78 | male | 1 | mRNA | MM | RR | PCd | 3 | PR |
| IX | 76 | male | 19 | mRNA | MGCS (renal) | ND | Vd | 1 | n/a |
| Variable, n (%) If Not Other Identified | Myeloma-Patient Group (n = 49) | Control Group (n = 78) | p-Value |
|---|---|---|---|
| Mean age in years (SD) | 59.6 (8.4) | 51.3 (7.5) | <0.001 |
| Male sex | 28 (57%) | 45 (58%) | 0.95 |
| Days post second vaccination, mean (SD) | 20.4 (9.0) | 88.0 (37.2) | <0.001 |
| Vaccination type | 0.78 | ||
| mRNA-based | 39 (81%) | 67 (86%) | |
| Vector-based | 6 (13%) | 7 (9%) | |
| Heterologous | 3 (6%) | 4 (5%) | |
| TOTAL | n = 49 | n = 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghandili, S.; Schönlein, M.; Wiessner, C.; Becher, H.; Lütgehetmann, M.; Brehm, T.T.; Schulze zur Wiesch, J.; Bokemeyer, C.; Sinn, M.; Weisel, K.C.; et al. Lymphocytopenia and Anti-CD38 Directed Treatment Impact the Serological SARS-CoV-2 Response after Prime Boost Vaccination in Patients with Multiple Myeloma. J. Clin. Med. 2021, 10, 5499. https://doi.org/10.3390/jcm10235499
Ghandili S, Schönlein M, Wiessner C, Becher H, Lütgehetmann M, Brehm TT, Schulze zur Wiesch J, Bokemeyer C, Sinn M, Weisel KC, et al. Lymphocytopenia and Anti-CD38 Directed Treatment Impact the Serological SARS-CoV-2 Response after Prime Boost Vaccination in Patients with Multiple Myeloma. Journal of Clinical Medicine. 2021; 10(23):5499. https://doi.org/10.3390/jcm10235499
Chicago/Turabian StyleGhandili, Susanne, Martin Schönlein, Christian Wiessner, Heiko Becher, Marc Lütgehetmann, Thomas Theo Brehm, Julian Schulze zur Wiesch, Carsten Bokemeyer, Marianne Sinn, Katja C. Weisel, and et al. 2021. "Lymphocytopenia and Anti-CD38 Directed Treatment Impact the Serological SARS-CoV-2 Response after Prime Boost Vaccination in Patients with Multiple Myeloma" Journal of Clinical Medicine 10, no. 23: 5499. https://doi.org/10.3390/jcm10235499
APA StyleGhandili, S., Schönlein, M., Wiessner, C., Becher, H., Lütgehetmann, M., Brehm, T. T., Schulze zur Wiesch, J., Bokemeyer, C., Sinn, M., Weisel, K. C., & Leypoldt, L. B. (2021). Lymphocytopenia and Anti-CD38 Directed Treatment Impact the Serological SARS-CoV-2 Response after Prime Boost Vaccination in Patients with Multiple Myeloma. Journal of Clinical Medicine, 10(23), 5499. https://doi.org/10.3390/jcm10235499







