Algorithms for Automated Calibration of Transcutaneous Spinal Cord Stimulation to Facilitate Clinical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimulation and Recording Setup
2.3. Automatic Determination of Electrodes and Parameters
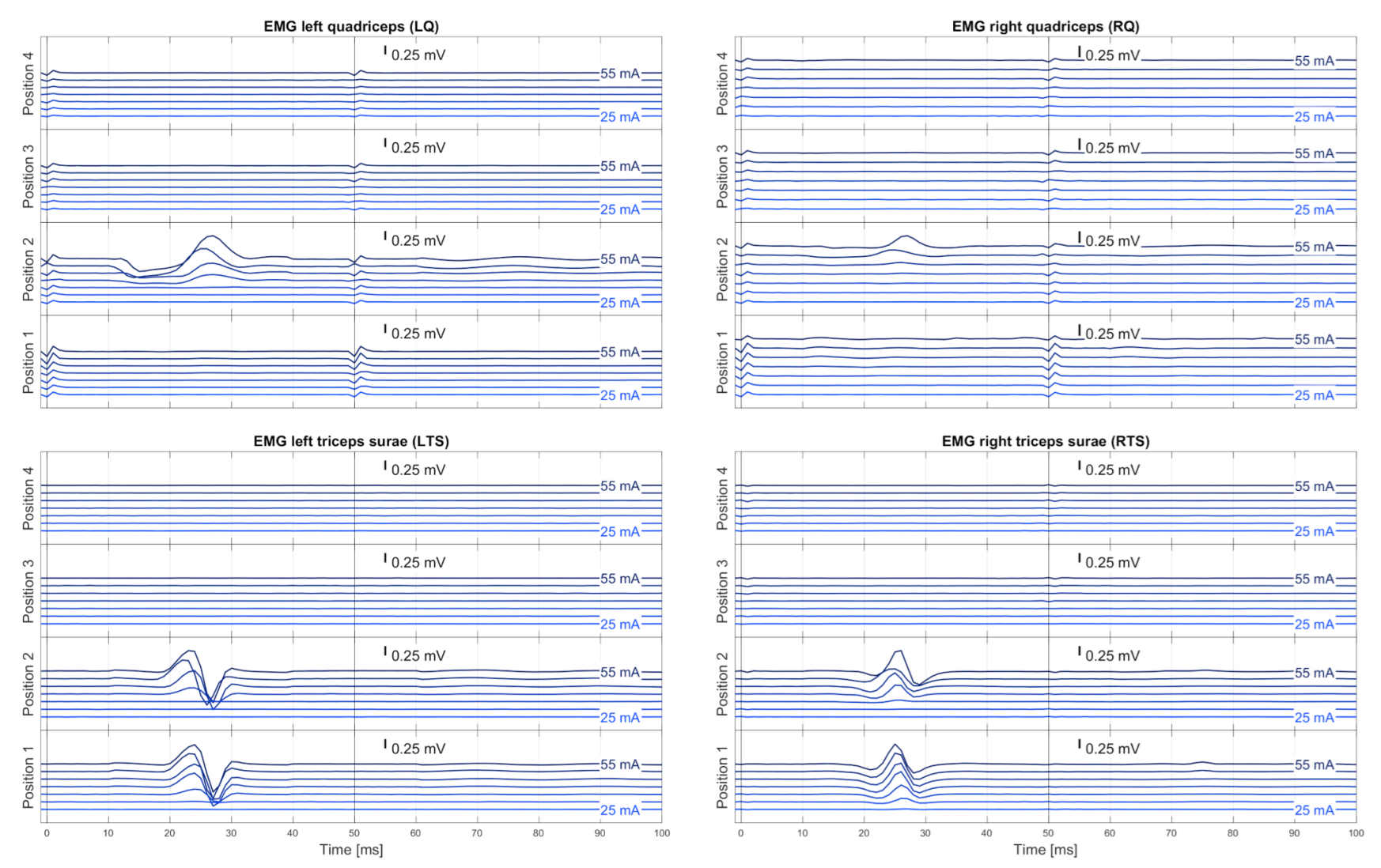
2.3.1. Automated Stimulation Protocol
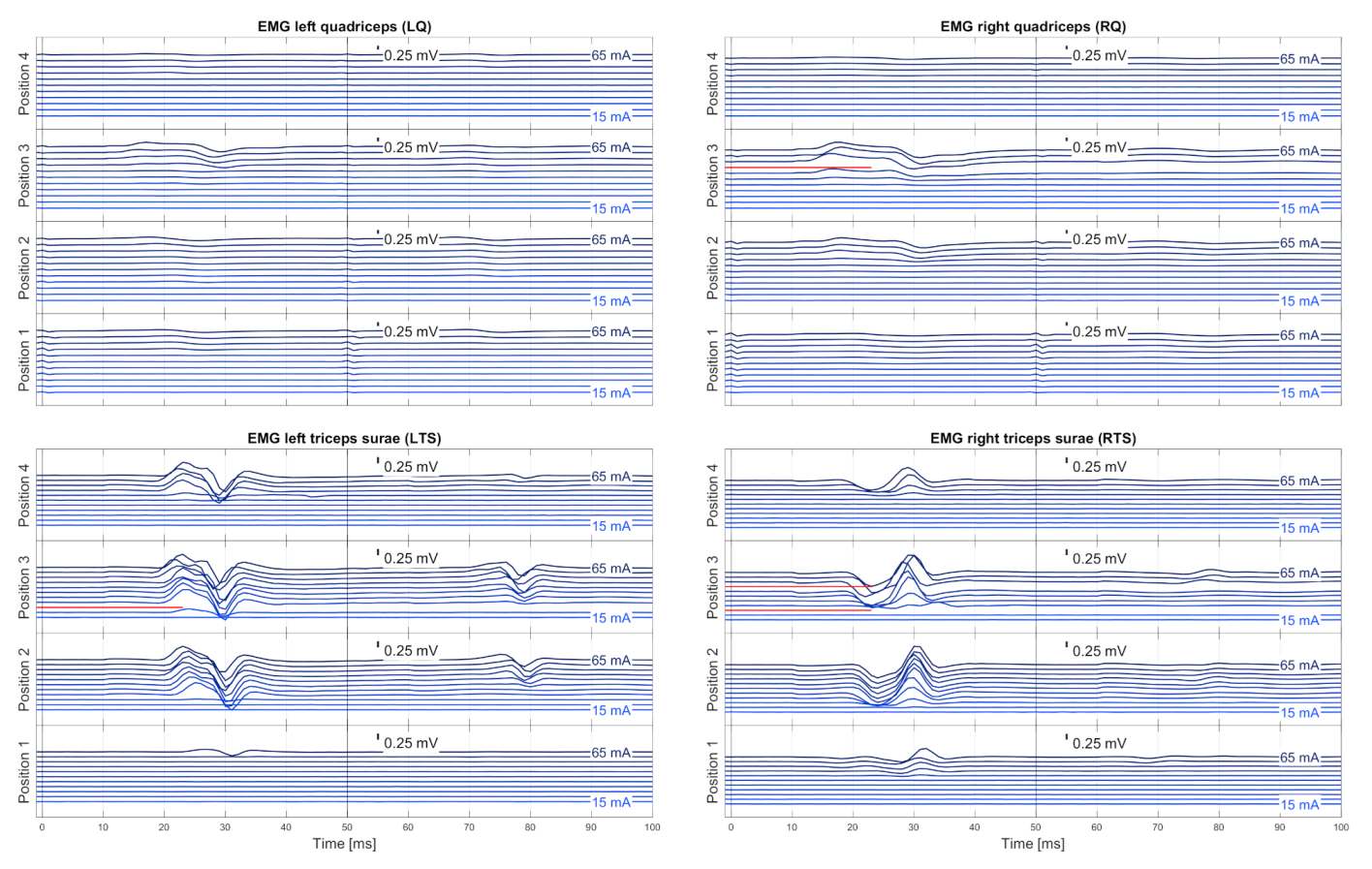
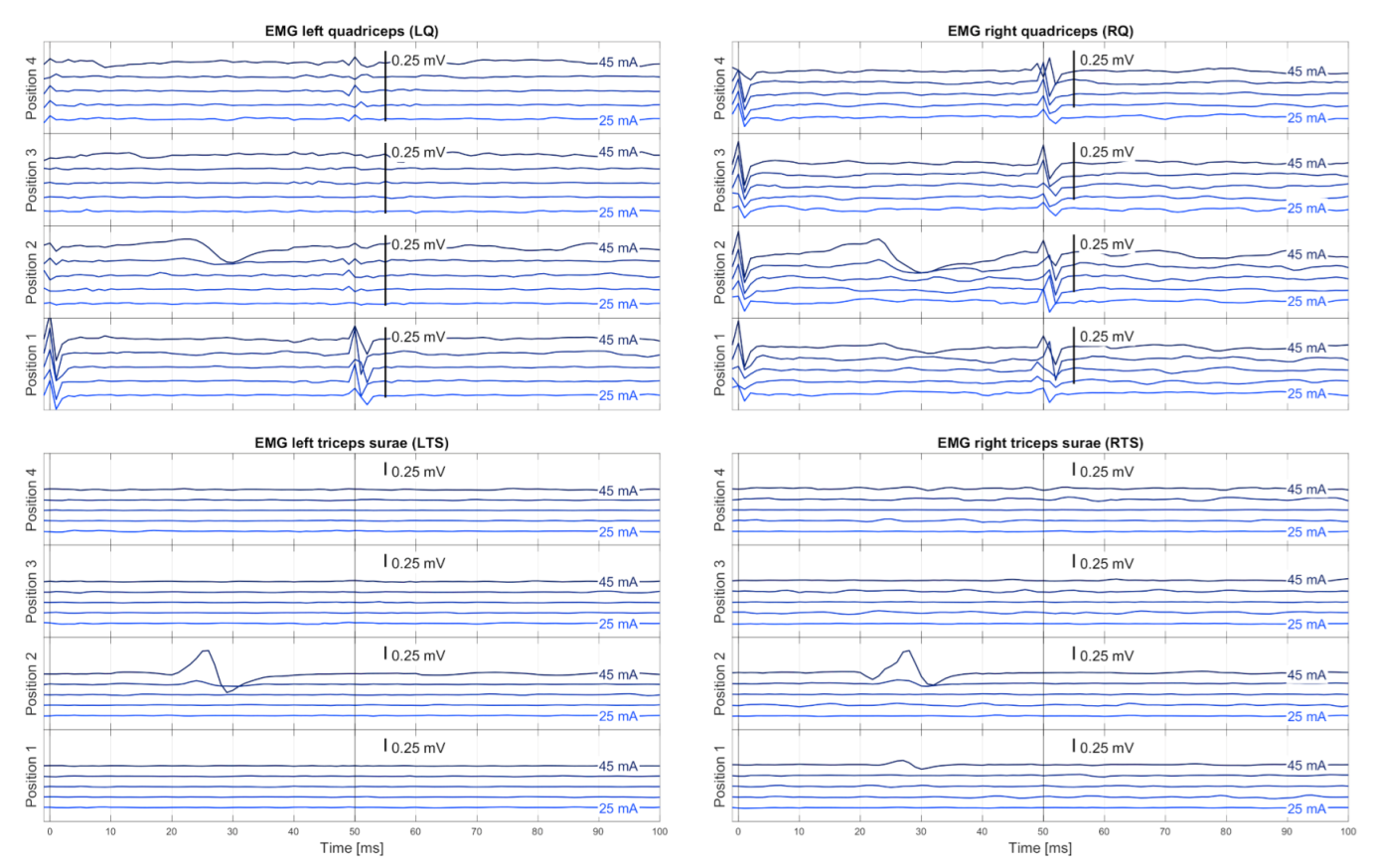
2.3.2. EMG Pre-Processing
EMG Synchronization
EMG Pre-Filtering
Muscle-Specific Base Noise Level
Similarity Check and Averaging
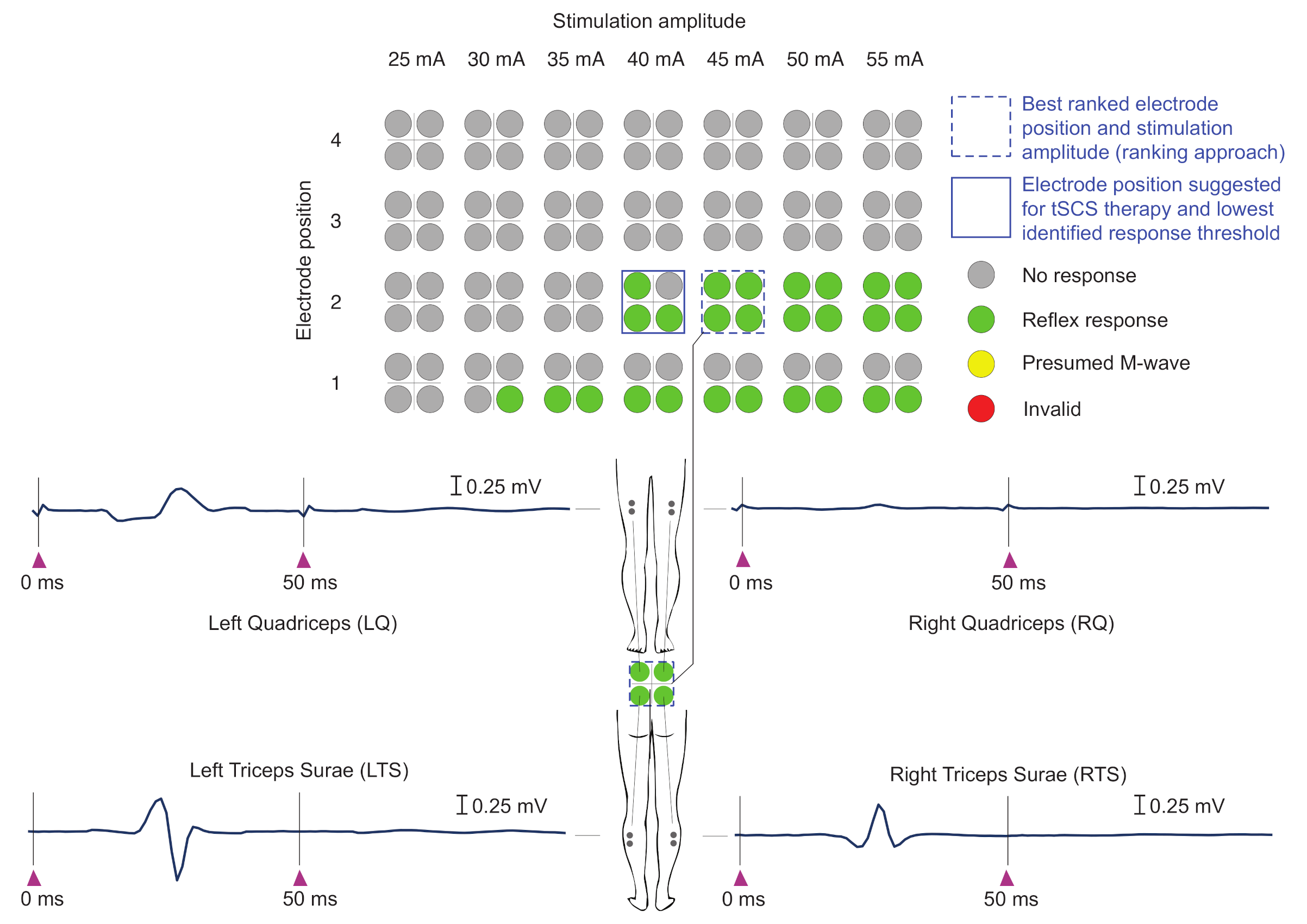
2.3.3. Automatic Evaluation of EMG Responses
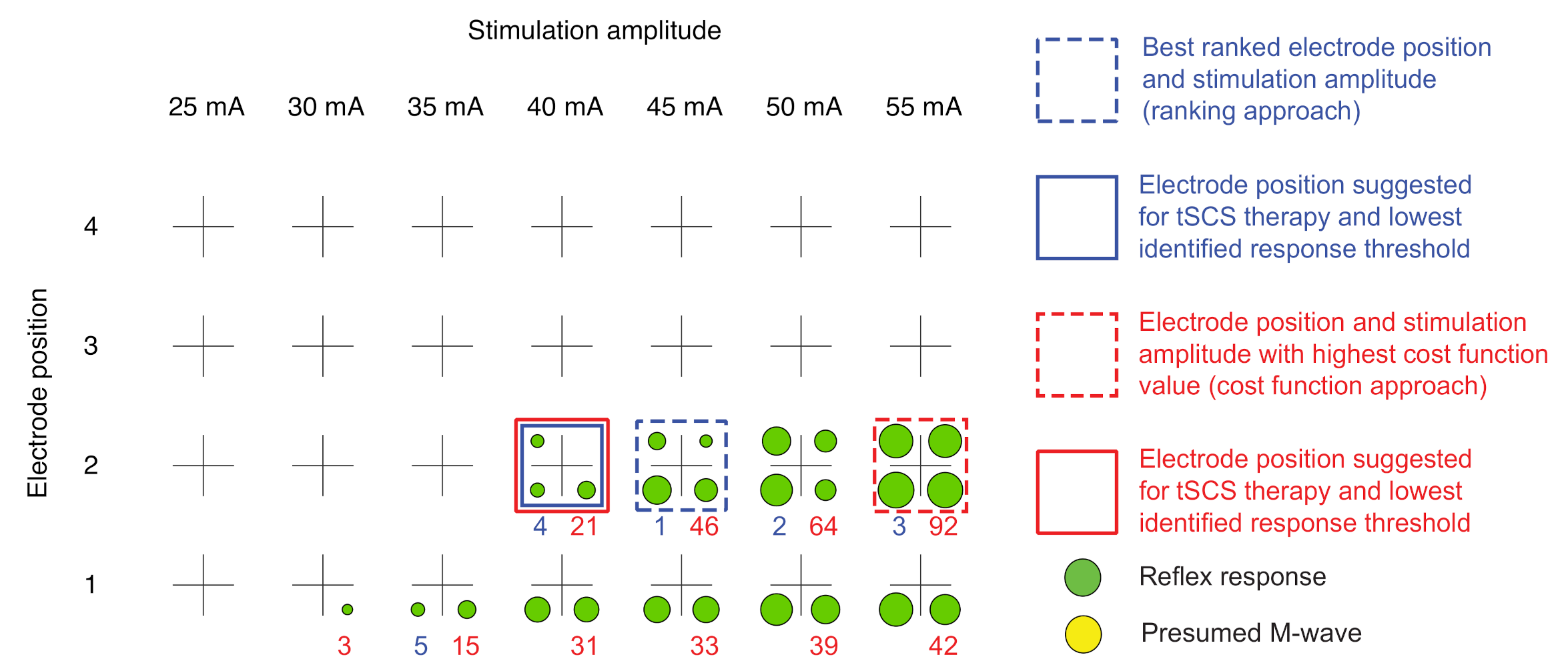
2.3.4. Automatic Delineation of Stimulation Position and Amplitude for tSCS Therapy
Ranking Approach
- 1.
- At least two green labels present (i.e., reflex responses elicited in at least two of the four muscle groups studied; minimal requirement);
- 2.
- Largest number of green labels (i.e., reflex responses);
- 3.
- Smallest current level difference between stimulation amplitude I and , at which the first green label was obtained (amplitude threshold);
- 4.
- Lowest stimulation amplitude I.
Cost Function Approach
Graphical Visualization
2.4. Validation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIS | American Spinal Cord Injury Association Impairment Scale; |
| EES | Epidural electrical stimulation; |
| EMG | Electromyography; |
| FD | Years since first diagnosis of PD; |
| H and Y | Hoehn and Yahr scale; |
| LQ | Left quadriceps; |
| LTS | Left tricpes surae; |
| PD | Parkinson’s disease; |
| PRM reflex | Posterior root-muscle reflex; |
| RQ | Right quadriceps; |
| RTS | Right tricpes surae; |
| SCI | Spinal cord injury; |
| tSCS | Transcutaneous spinal cord stimulation. |
Appendix A

References
- Wagner, F.B.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Minassian, K.; Jilge, B.; Rattay, F.; Pinter, M.; Binder, H.; Gerstenbrand, F.; Dimitrijevic, M.R. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: Electromyographic study of compound muscle action potentials. Spinal Cord. 2004, 42, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Ladenbauer, J.; Minassian, K.; Hofstoetter, U.; Dimitrijevic, M.R.; Rattay, F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: A computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 637–645. [Google Scholar] [CrossRef]
- Pinter, M.; Gerstenbrand, F.; Dimitrijevic, M. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord. 2000, 38, 524–531. [Google Scholar] [CrossRef] [Green Version]
- Angeli, C.; Edgerton, V.; Gerasimenko, Y.; Harkema, S. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef] [Green Version]
- Minassian, K.; McKay, W.; Binder, H.; Hofstoetter, U. Targeting Lumbar Spinal Neural Circuitry by Epidural Stimulation to Restore Motor Function After Spinal Cord Injury. Neurotherapeutics 2016, 13, 284–294. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, E.M.; Ghilardi, M.G.; Cury, R.G.; Barbosa, E.R.; Fuentes, R.; Teixeira, M.J.; Fonoff, E.T. Spinal cord stimulation for Parkinson’s disease: A systematic review. Neurosurg. Rev. 2015, 39, 27–35. [Google Scholar] [CrossRef]
- Pinto de Souza, C.; Hamani, C.; Oliveira Souza, C.; Lopez Contreras, W.O.; dos Santos Ghilardi, M.G.; Cury, R.G.; Reis Barbosa, E.; Jacobsen Teixeira, M.; Talamoni Fonoff, E. Spinal cord stimulation improves gait in patients with Parkinson’s disease previously treated with deep brain stimulation. Mov. Disord. 2017, 32, 278–282. [Google Scholar] [CrossRef]
- de Lima-Pardini, A.; Coelho, D.; Pinto de Souza, C.; Souza, C.O.; dos Santos Ghilardi, M.G.; Garcia, T.; Voos, M.; Milosevic, M.; Hamani, C.; Teixeira, L.A.; et al. Effects of spinal cord stimulation on postural control in Parkinson’s disease patients with freezing of gait. Elife 2018, 7, e37727. [Google Scholar] [CrossRef]
- Fonoff, E.; de Lima-Pardini, A.; Coelho, D.; Monaco, B.; Machado, B.; Pinto de Souza, C.; dos Santos Ghilardi, M.; Hamani, C. Spinal cord stimulation for freezing of gait: From bench to bedside. Front. Neurol. 2019, 10, 905. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Aguirre-Padilla, D.; Poon, Y.; Kalsi-Ryan, S.; Lozano, A.M.; Fasano, A. Spinal Cord Stimulation for Very Advanced Parkinson’s Disease: A 1-Year Prospective Trial. Mov. Disord. 2020, 35, 1082–1083. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Spinal cord stimulation as a neuromodulatory intervention for altered motor control following spinal cord injury. In Advanced Technologies for the Rehabilitation of Gait and Balance Disorders; Biosystems & Biorobotics; Springer: Cham, Switzerland, 2018; Volume 19, pp. 501–521. [Google Scholar] [CrossRef]
- Courtine, G.; Harkema, S.; Dy, C.; Gerasimenko, Y.P.; Dyhre-Poulsen, P. Modulation of multisegmental monosynaptic responses in a variety of leg muscles during walking and running in humans. J. Physiol. 2007, 582, 1125–1139. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.; Frank, R. Transcutaneous lumbar posterior root stimulation for motor control studies and modification of motor activity after spinal cord injury. In Restorative Neurology of Spinal Cord Injury; Dimitrijevic, M., Kakulas, B., McKay, W., Vrbová, G., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 226–255. [Google Scholar]
- Hofstoetter, U.; Freundl, B.; Binder, H.; Minassian, K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef] [Green Version]
- Hofstoetter, U.; McKay, W.; Tansey, K.; Mayr, W.; Kern, H.; Minassian, K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord. Med. 2014, 37, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Hofstoetter, U.; Krenn, M.; Danner, S.; Hofer, C.; Kern, H.; McKay, W.B.; Mayr, W.; Minassian, K. Augmentation of Voluntary Locomotor Activity by Transcutaneous Spinal Cord Stimulation in Motor-Incomplete Spinal Cord-Injured Individuals. Artif. Organs 2015, 39, E176–E186. [Google Scholar] [CrossRef]
- Estes, S.P.; Iddings, J.A.; Field-Fote, E.C. Priming neural circuits to modulate spinal reflex excitability. Front. Neurol. 2017, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.; Edgerton, V. Weight Bearing Over-ground Stepping in an Exoskeleton with Non-invasive Spinal Cord Neuromodulation after Motor Complete Paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Hofstoetter, U.S.; Hubli, M.; Hassani, R.H.; Rinaldo, C.; Curt, A.; Bolliger, M. Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury. J. Clin. Med. 2020, 9, 3541. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.; Freundl, B.; Lackner, P.; Binder, H. Transcutaneous Spinal Cord Stimulation Enhances Walking Performance and Reduces Spasticity in Individuals with Multiple Sclerosis. Brain Sci. 2021, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.; Atkinson, D.; Manson, G.; Markley, R.; Kaldis, T.; Britz, G.; Horner, P.; Vette, A.; Sayenko, D. Transcutaneous spinal cord stimulation improves postural stability in individuals with multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 52, 103009. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Perret, I.; Bayart, A.; Lackner, P.; Binder, H.; Freundl, B.; Minassian, K. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience 2021, 24, 101930. [Google Scholar] [CrossRef]
- Murg, M.; Binder, H.; Dimitrijevic, M. Epidural electric stimulation of posterior structures of the human lumbar spinal cord: 1. muscle twitches—A functional method to define the site of stimulation. Spinal Cord. 2000, 38, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M.R.; Hofer, C.; Kern, H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerv. 2007, 35, 327–336. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Minassian, K.; Hofer, C.; Mayr, W.; Rattay, F.; Dimitrijevic, M.R. Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif. Organs 2008, 32, 644–648. [Google Scholar] [CrossRef]
- Calvert, J.S.; Grahn, P.J.; Strommen, J.A.; Lavrov, I.A.; Beck, L.A.; Gill, M.L.; Linde, M.B.; Brown, D.A.; Van Straaten, M.G.; Veith, D.D.; et al. Electrophysiological Guidance of Epidural Electrode Array Implantation over the Human Lumbosacral Spinal Cord to Enable Motor Function after Chronic Paralysis. J. Neurotrauma 2019, 36, 1451–1460. [Google Scholar] [CrossRef] [Green Version]
- Rattay, F.; Minassian, K.; Dimitrijevic, M. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord. 2000, 38, 473–489. [Google Scholar] [CrossRef] [Green Version]
- Capogrosso, M.; Wenger, N.; Raspopovic, S.; Musienko, P.; Beauparlant, J.; Luciani, L.B.; Courtine, G.; Micera, S. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J. Neurosci. 2013, 33, 19326–19340. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [Green Version]
- Salchow-Hömmen, C.; Dikau, C.; Mueller, P.; Hofstoetter, U.; Kühn, A.; Schauer, T.; Wenger, N. Characterization of optimal electrode configurations for transcutaneous spinal cord stimulation. In Proceedings of the IFESS Conference at Rehab Week, Toronto, ON, Canada, 24–28 June 2019. [Google Scholar]
- Sayenko, D.; Atkinson, D.; Dy, C.; Gurley, K.; Smith, V.; Angeli, C.; Harkema, S.; Edgerton, V.; Gerasimenko, Y. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J. Appl. Physiol. 2015, 118, 1364–1374. [Google Scholar] [CrossRef] [Green Version]
- Sojka, M.; Píša, P. Usable Simulink Embedded Coder Target for Linux. In Proceedings of the 16th Real Time Linux Workshop, Düsseldorf, Germany, 12–13 October 2014. [Google Scholar]
- Estes, S.; Zarkou, A.; Hope, J.M.; Suri, C.; Field-Fote, E.C. Combined Transcutaneous Spinal Stimulation and Locomotor Training to Improve Walking Function and Reduce Spasticity in Subacute Spinal Cord Injury: A Randomized Study of Clinical Feasibility and Efficacy. J. Clin. Med. 2021, 10, 1167. [Google Scholar] [CrossRef]
- Pierantozzi, M.; Palmieri, M.; Galati, S.; Stanzione, P.; Peppe, A.; Tropepi, D.; Brusa, L.; Pisani, A.; Moschella, V.; Marciani, M.G.; et al. Pedunculopontine nucleus deep brain stimulation changes spinal cord excitability in Parkinson’s disease patients. J. Neural. Transm. 2008, 115, 731–735. [Google Scholar] [CrossRef]
- Baudry, S.; Penzer, F.; Duchateau, J. Input-output characteristics of soleus homonymous Ia afferents and corticospinal pathways during upright standing differ between young and elderly adults. Acta Physiol. 2014, 210, 667–677. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.S.; Danner, S.M.; Mayr, W.; Bruce, J.A.; McKay, W.B.; Tansey, K.E. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord–injured individuals. Neurorehabil. Neural. Repair. 2016, 30, 233–243. [Google Scholar] [CrossRef]
- McHugh, L.; Miller, A.; Leech, K.; Salorio, C.; Martin, R. Feasibility and utility of transcutaneous spinal cord stimulation combined with walking-based therapy for people with motor incomplete spinal cord injury. Spinal Cord. Ser. Cases 2020, 6, 104. [Google Scholar] [CrossRef]
- Danner, S.; Krenn, M.; Hofstoetter, U.; Toth, A.; Mayr, W.; Minassian, K. Body position influences which neural structures are recruited by lumbar transcutaneous spinal cord stimulation. PLoS ONE 2016, 11, e0147479. [Google Scholar] [CrossRef] [Green Version]


| Group | Subject | Sex | Age | Height | Weight | SCI | PD | ||
|---|---|---|---|---|---|---|---|---|---|
| Years | cm | kg | Level | AIS | H and Y | FD | |||
| Control | C1 | m | 36 | 184 | 82 | - | - | - | - |
| (n = 5) | C2 | f | 30 | 177 | 65 | - | - | - | - |
| C3 | m | 35 | 190 | 78 | - | - | - | - | |
| C4 | m | 33 | 181 | 70 | - | - | - | - | |
| C5 | f | 30 | 168 | 56 | - | - | - | - | |
| SCI | S1 | m | 40 | 182 | 80 | T5-6 | A | - | - |
| (n = 5) | S2 | m | 57 | 180 | 74 | T5-6 | A | - | - |
| S3 | f | 49 | 179 | 76 | T5-6 | B | - | - | |
| S4 | m | 58 | 185 | 75 | C7 | B | - | - | |
| S5 | f | 31 | 165 | 49 | T1-3 | B | - | - | |
| PD | P1 | m | 60 | 180 | 70 | - | - | 2 | 12 |
| (n = 5) | P2 | f | 77 | 175 | 68 | - | - | 2 | 9 |
| P3 | f | 74 | 172 | 80 | - | - | 2 | 5 | |
| P4 | m | 70 | 176 | 92 | - | - | 2 | 14 | |
| P5 | m | 70 | 178 | 72 | - | - | 2 | 2 | |
| Color Code | Description | Condition | |
|---|---|---|---|
| • | gray | No response | |
| • | green | Reflex response | |
| • | yellow | Presumed M-wave | |
| • | red | Invalid | No similar EMG signals obtained for stimulation from a given electrode position and with given stimulation amplitude |
| Subject | Expert’s Selection | Automatic Selection | ||||||
|---|---|---|---|---|---|---|---|---|
| Expert #1 | Expert #2 | Cost Function | Ranking | |||||
| C1 | 2 | 40 mA | 2 | 35 mA | 2 | 40 mA | 2 | 40 mA |
| C2 | 2 | 25 mA | 2 | 25 mA | 2 | 25 mA | 2 | 25 mA |
| C3 | 3 | 25 mA | 3 | 25 mA | 3 | 25 mA | 3 | 25 mA |
| C4 | 2 | 20 mA | 2 | 20 mA | 2 | 20 mA | 2 | 20 mA |
| C5 | 3 | 35 mA | 3 | 35 mA | 3 | 35 mA | 3 | 35 mA |
| S1 | 2 | 25 mA | 1 | 35 mA | 2 | 25 mA | 1 | 35 mA |
| S2 | 3 | 30 mA | 3 | 30 mA | 3 | 20 mA | 3 | 20 mA |
| S3 | 2 | 45 mA | 2 | 40 mA | 2 | 35 mA | 2 | 35 mA |
| S4 | 2 | 25 mA | 3 | 25 mA | 3 | 25 mA | 3 | 25 mA |
| S5 | 2 | 25 mA | 2 | 20 mA | 2 | 20 mA | 2 | 20 mA |
| P1 | 2 | 45 mA | 2 | 40 mA | 2 | 40 mA | 2 | 40 mA |
| P2 | 1 | 45 mA | 1 | 45 mA | 1 | 45 mA | 1 | 45 mA |
| P3 | - | - | - | - | - | - | - | - |
| P4 | - | - | - | - | - | - | - | - |
| P5 | 1 | 35 mA | 1 | 35 mA | 1 | 40 mA | 1 | 40 mA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salchow-Hömmen, C.; Schauer, T.; Müller, P.; Kühn, A.A.; Hofstoetter, U.S.; Wenger, N. Algorithms for Automated Calibration of Transcutaneous Spinal Cord Stimulation to Facilitate Clinical Applications. J. Clin. Med. 2021, 10, 5464. https://doi.org/10.3390/jcm10225464
Salchow-Hömmen C, Schauer T, Müller P, Kühn AA, Hofstoetter US, Wenger N. Algorithms for Automated Calibration of Transcutaneous Spinal Cord Stimulation to Facilitate Clinical Applications. Journal of Clinical Medicine. 2021; 10(22):5464. https://doi.org/10.3390/jcm10225464
Chicago/Turabian StyleSalchow-Hömmen, Christina, Thomas Schauer, Philipp Müller, Andrea A. Kühn, Ursula S. Hofstoetter, and Nikolaus Wenger. 2021. "Algorithms for Automated Calibration of Transcutaneous Spinal Cord Stimulation to Facilitate Clinical Applications" Journal of Clinical Medicine 10, no. 22: 5464. https://doi.org/10.3390/jcm10225464
APA StyleSalchow-Hömmen, C., Schauer, T., Müller, P., Kühn, A. A., Hofstoetter, U. S., & Wenger, N. (2021). Algorithms for Automated Calibration of Transcutaneous Spinal Cord Stimulation to Facilitate Clinical Applications. Journal of Clinical Medicine, 10(22), 5464. https://doi.org/10.3390/jcm10225464







