Challenging Visualization of Sentinel Lymph Nodes in Upper Urinary Tract Urothelial Carcinoma
Abstract
1. Introduction
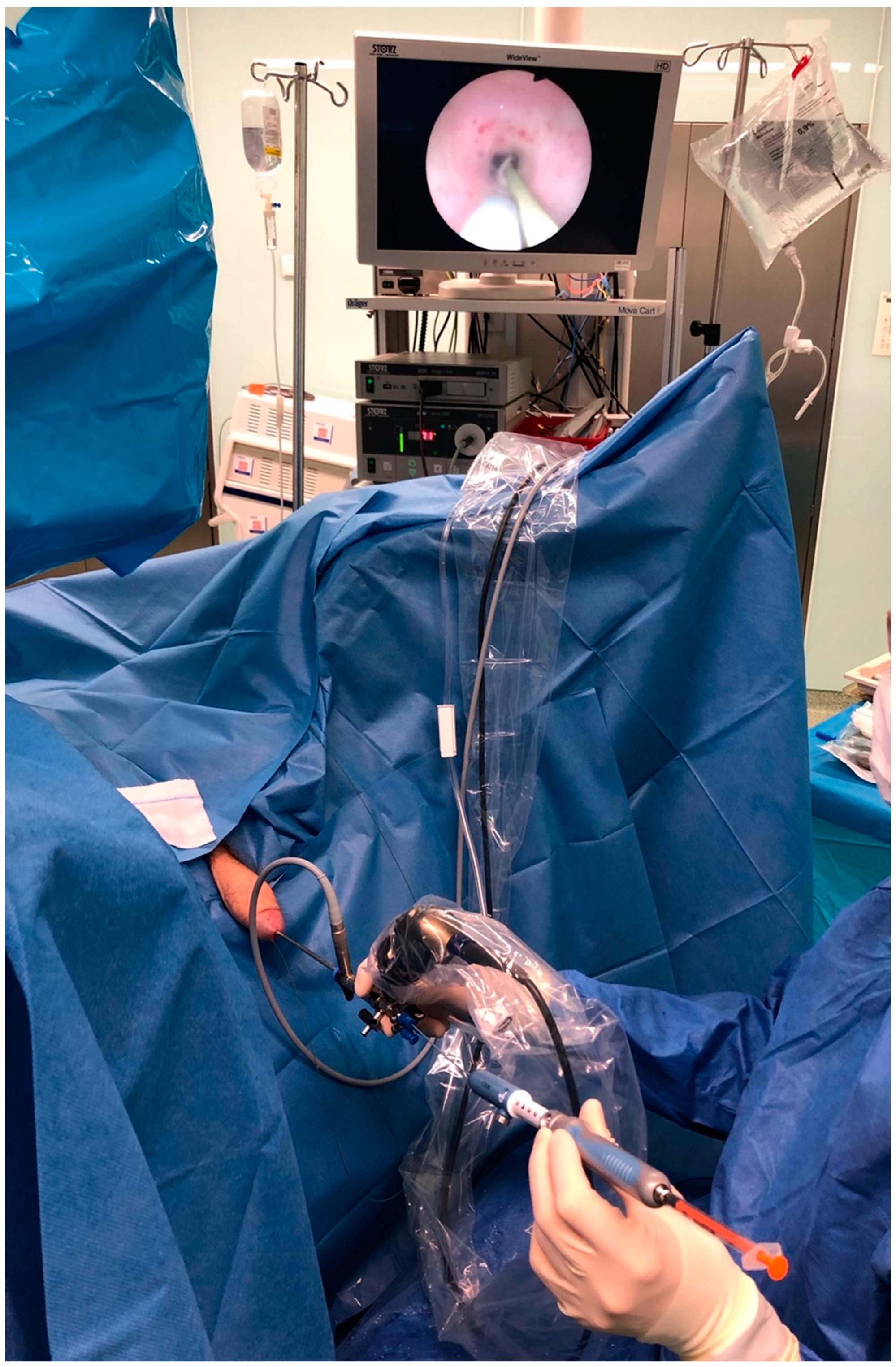
2. Materials and Methods
3. Results
3.1. Patients’ Characteristics
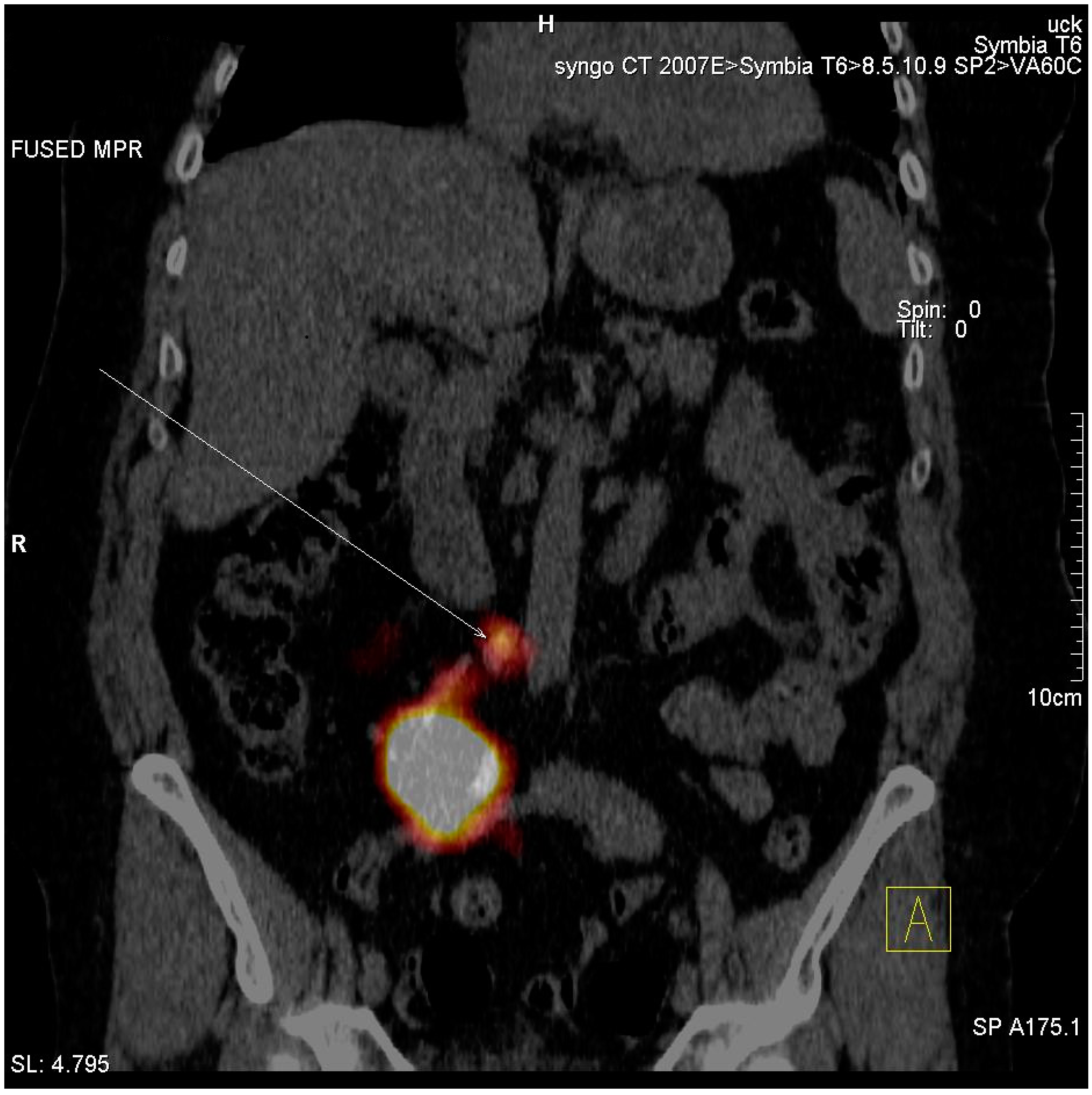
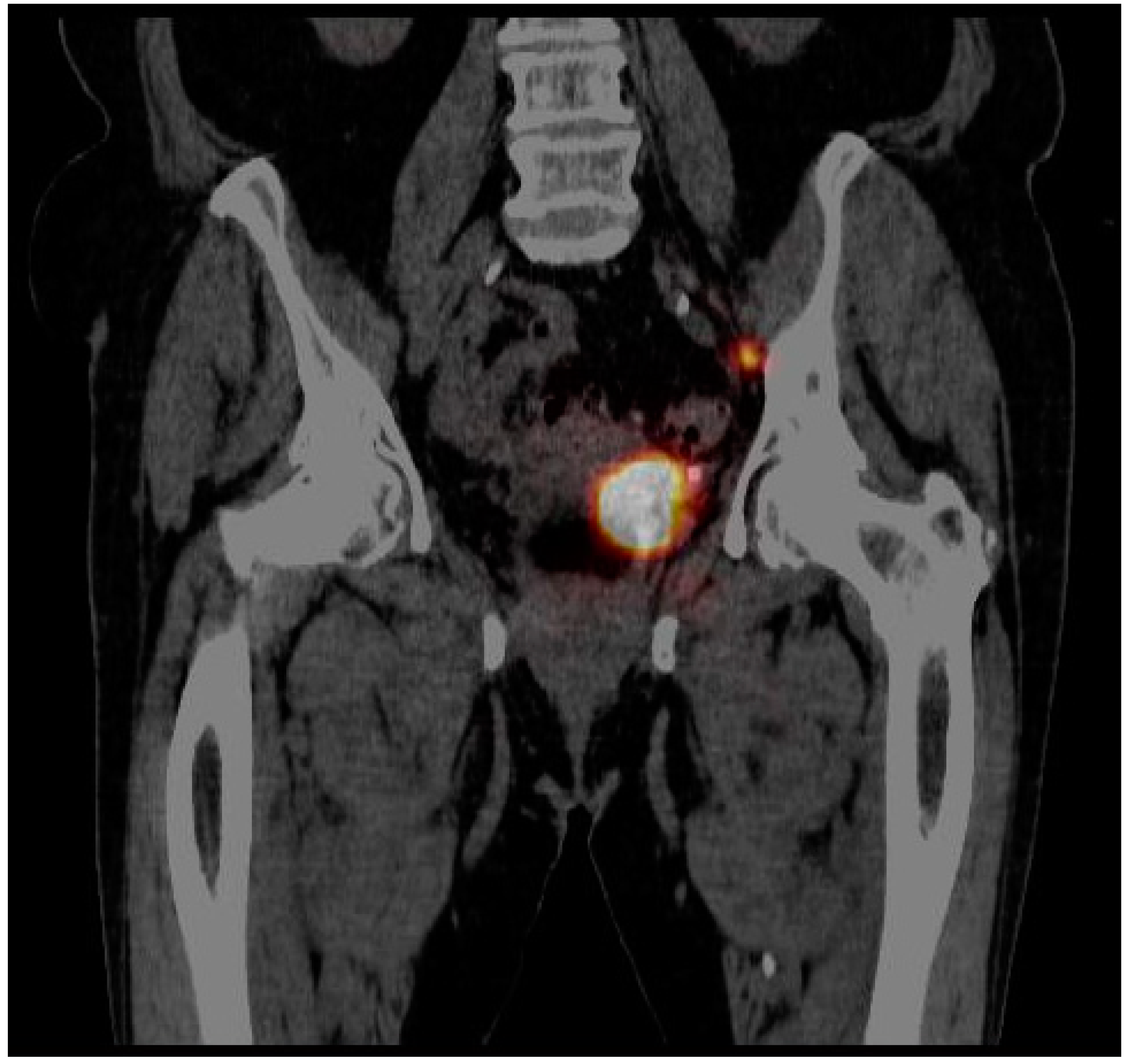
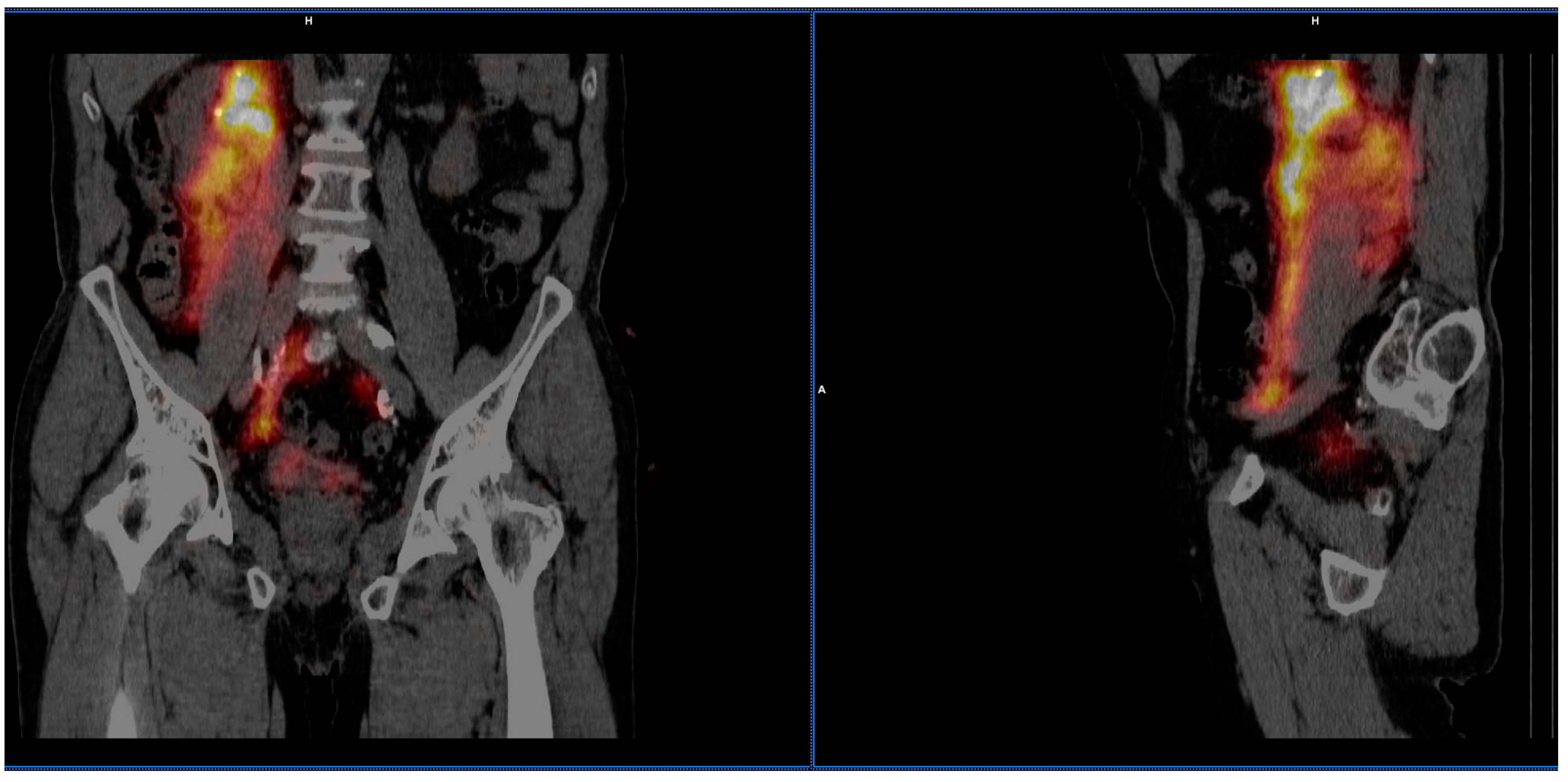
3.2. Imaging of Lymphatic Outflow
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Munoz, J.J.; Ellison, L.M. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J. Urol. 2000, 164, 1523–1525. [Google Scholar] [CrossRef]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Mostafid, A.H.; et al. EAU Guidelines on Upper Urinary Tract Urothelial Carcinoma 2021. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Soria, F.; Shariat, S.F.; Lerner, S.P.; Fritsche, H.M.; Rink, M.; Kassouf, W.; Spiess, P.E.; Lotan, Y.; Ye, D.; Fernández, M.I.; et al. Epidemiology, diagnosis, preoperative evaluation, and prognostic assessment of upper-tract urothelial carcinoma (UTUC). World J. Urol. 2017, 35, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Burger, M.; Margulis, V.; Matin, S.F.; Novara, G.; Roupret, M.; Shariat, S.F.; Wood, C.G.; Zigeuner, R. Prognostic factors in upper urinary tract urothelial carcinomas: A comprehensive review of the current literature. Eur. Urol. 2012, 62, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Yafi, F.A.; Novara, G.; Shariat, S.F.; Gupta, A.; Matsumoto, K.; Walton, T.J.; Fritsche, H.M.; El-Hakim, A.; Trischler, S.; Martínez-Salamanca, J.I.; et al. Impact of tumour location versus multifocality in patients with upper tract urothelial carcinoma treated with nephroureterectomy and bladder cuff excision: A homogeneous series without perioperative chemotherapy. BJU Int. 2012, 110, E7–E13. [Google Scholar] [CrossRef]
- Cosentino, M.; Palou, J.; Gaya, J.M.; Breda, A.; Rodriguez-Faba, O.; Villavicencio-Mavrich, H. Upper urinary tract urothelial cell carcinoma: Location as a predictive factor for concomitant bladder carcinoma. World J. Urol. 2013, 31, 141–145. [Google Scholar] [CrossRef]
- Xylinas, E.; Rink, M.; Margulis, V.; Karakiewicz, P.; Novara, G.; Shariat, S.F. Multifocal carcinoma in situ of the upper tract is associated with high risk of bladder cancer recurrence. Eur. Urol. 2012, 61, 1069–1070. [Google Scholar] [CrossRef]
- Li, W.M.; Shen, J.T.; Li, C.C.; Ke, H.L.; Wei, Y.C.; Wu, W.J.; Chou, Y.H.; Huang, C.H. Oncologic outcomes following three different approaches to the distal ureter and bladder cuff in nephroureterectomy for primary upper urinary tract urothelial carcinoma. Eur. Urol. 2010, 57, 963–969. [Google Scholar] [CrossRef]
- Margulis, V.; Shariat, S.F.; Matin, S.F.; Kamat, A.M.; Zigeuner, R.; Kikuchi, E.; Lotan, Y.; Weizer, A.; Raman, J.D.; Wood, C.G.; et al. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009, 115, 1224–1233. [Google Scholar] [CrossRef]
- Shariat, S.F.; Favaretto, R.L.; Gupta, A.; Fritsche, H.M.; Matsumoto, K.; Kassouf, W.; Walton, T.J.; Tritschler, S.; Baba, S.; Matsushita, K.; et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J. Urol. 2011, 29, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Yates, D.R.; Comperat, E.; Cussenot, O. Upper Urinary Tract Urothelial Cell Carcinomas and Other Urological Malignancies Involved in the Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) Tumor Spectrum. Eur. Urol. 2008, 54, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Colin, P.; Koenig, P.; Ouzzane, A.; Berthon, N.; Villers, A.; Biserte, J.; Rouprêt, M. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int. 2009, 104, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, J.J.; Xylinas, E.; Kluth, L.A.; Rieken, M.; Rink, M.; Shariat, S.F. Effect of smoking on outcomes of urothelial carcinoma: A systematic review of the literature. Eur. Urol. 2014, 65, 742–754. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.K.; Silverman, D.T.; Hsing, A.W.; Ross, R.K.; Schoenberg, J.B.; Mimi, C.Y.; Stemhagen, A.; Lynch, C.F.; Blot, W.J.; Fraumeni, J.F. Cigarette smoking and cancers of the renal pelvis and ureter. Cancer Res. 1992, 52, 254–257. [Google Scholar] [PubMed]
- Zaitsu, M.; Kawachi, I.; Takeuchi, T.; Kobayashi, Y. Alcohol consumption and risk of upper-tract urothelial cancer. Cancer Epidemiol. 2017, 48, 36–40. [Google Scholar] [CrossRef]
- Ouzzane, A.; Ghoneim, T.P.; Udo, K.; Verhasselt-Crinquette, M.; Puech, P.; Betrouni, N.; Rouprêt, M.; Villers, A.; Leroy, X.; Colin, P. Small cell carcinoma of the upper urinary tract (UUT-SCC): Report of a rare entity and systematic review of the literature. Cancer Treat. Rev. 2011, 37, 366–372. [Google Scholar] [CrossRef]
- Sakano, S.; Matsuyama, H.; Kamiryo, Y.; Hayashida, S.; Yamamoto, N.; Kaneda, Y.; Nasu, T.; Baba, Y.; Shimabukuro, T.; Suga, A.; et al. Impact of variant histology on disease aggressiveness and outcome after nephroureterectomy in Japanese patients with upper tract urothelial carcinoma. Int. J. Clin. Oncol. 2015, 20, 362–368. [Google Scholar] [CrossRef]
- Rink, M.; Robinson, B.D.; Green, D.A.; Cha, E.K.; Hansen, J.; Comploj, E.; Margulis, V.; Raman, J.D.; Ng, C.K.; Remzi, M.; et al. Impact of histological variants on clinical outcomes of patients with upper urinary tract urothelial carcinoma. J. Urol. 2012, 188, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Masson-Lecomte, A.; Colin, P.; Bozzini, G.; Nison, L.; de La Taille, A.; Comperat, E.; Zerbib, M.; Rozet, F.; Cathelineau, X.; Valeri, A.; et al. Impact of micropapillary histological variant on survival after radical nephroureterectomy for upper tract urothelial carcinoma. World J. Urol. 2014, 32, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Perez-Montiel, D.; Wakely, P.E.; Hes, O.; Michal, M.; Suster, S. High-grade urothelial carcinoma of the renal pelvis: Clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod. Pathol. 2006, 19, 494–503. [Google Scholar] [CrossRef]
- Olgac, S.; Mazumdar, M.; Dalbagni, G.; Reuter, V.E. Urothelial carcinoma of the renal pelvis: A clinicopathologic study of 130 cases. Am. J. Surg. Pathol. 2004, 28, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Moon, K.C.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Variant histology as a significant predictor of survival after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Urol. Oncol. 2017, 35, 458.e9–458.e15. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Vermeeren, L.; de Windt, G.; Prevoo, W.; Horenblas, S.; Olmos, R.A. Feasibility of sentinel node detection in renal cell carcinoma: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hashimoto, Y.; Kobayashi, H.; Iizuka, J.; Nakazawa, H.; Ito, F.; Tanabe, K. Template-based lymphadenectomy in urothelial carcinoma of the upper urinary tract: Impact on patient survival. Int. J. Urol. 2010, 17, 848–854. [Google Scholar] [CrossRef]
- Fajkovic, H.; Cha, E.K.; Jeldres, C.; Donner, G.; Chromecki, T.F.; Margulis, V.; Novara, G.; Lotan, Y.; Raman, J.D.; Kassouf, W.; et al. Prognostic value of extranodal extension and other lymph node parameters in patients with upper tract urothelial carcinoma. J. Urol. 2012, 187, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Moschini, M.; Foerster, B.; Abufaraj, M.; Soria, F.; Seisen, T.; Roupret, M.; Colin, P.; De la Taille, A.; Peyronnet, B.; Bensalah, K.; et al. Trends of lymphadenectomy in upper tract urothelial carcinoma (UTUC) patients treated with radical nephroureterectomy. World J. Urol. 2017, 35, 1541–1547. [Google Scholar] [CrossRef]
- Lughezzani, G.; Jeldres, C.; Isbarn, H.; Shariat, S.F.; Sun, M.; Pharand, D.; Widmer, H.; Arjane, P.; Graefen, M.; Montorsi, F.; et al. A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology 2010, 75, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Escrig, J.L.; Peyronnet, B.; Seisen, T.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Böhle, A.; Burger, M.; Compérat, E.M.; Gontero, P.; et al. Potential Benefit of Lymph Node Dissection During Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the European Association of Urology Guidelines Panel on Non-muscle-invasive Bladder Cancer. Eur. Urol. Focus 2019, 5, 224–241. [Google Scholar] [CrossRef] [PubMed]
- Roscigno, M.; Brausi, M.; Heidenreich, A.; Lotan, Y.; Margulis, V.; Shariat, S.F.; Van Poppel, H.; Zigeuner, R. Lymphadenectomy at the time of nephroureterectomy for upper tract urothelial cancer. Eur. Urol. 2011, 60, 776–783. [Google Scholar] [CrossRef]
- Gould, E.; Winship, T.; Philbin, P.; Kerr, H. Observations on a “sentinel node” in cancer of the parotid. Cancer 1960, 13, 77–78. [Google Scholar] [CrossRef]
- Cabanas, R.M. An approach for the treatment of penile carcinoma. Cancer 1977, 39, 456–466. [Google Scholar] [CrossRef]
- Sherif, A.; De La Torre, M.; Malmstrom, P.U.; Thorn, M. Lymphatic mapping and detection of sentinel nodes in patients with bladder cancer. J. Urol. 2001, 166, 812–815. [Google Scholar] [CrossRef]
- Polom, W.; Markuszewski, M.; Cytawa, W.; Lass, P.; Matuszewski, M. Radio-Guided Lymph Node Mapping in Bladder Cancer Using SPECT/CT and Intraoperative Gamma-Probe Methods. Clin. Nucl. Med. 2016, 41, e362–e367. [Google Scholar] [CrossRef] [PubMed]
- Ozsahin, M.; Zouhair, A.; Villa, S.; Storme, G.; Chauvet, B.; Taussky, D.; Gouders, D.; Ries, G.; Bontemps, P.; Coucke, P.A.; et al. Prognostic factors in urothelial renal pelvis and ureter tumours: A multicentre Rare Cancer Network study. Eur. J. Cancer 1999, 35, 738–743. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. Are urothelial carcinomas of the upper urinary tract a distinct entity from urothelial carcinomas of the urinary bladder? Behavior of urothelial carcinoma after radical surgery with respect to anatomical location: A case control study. BMC Cancer 2015, 15, 149. [Google Scholar] [CrossRef]
- Park, J.; Ha, S.H.; Min, G.E.; Song, C.; Hong, B.; Hong, J.H.; Kim, C.S.; Ahn, H. The protective role of renal parenchyma as a barrier to local tumor spread of upper tract transitional cell carcinoma and its impact on patient survival. J. Urol. 2009, 182, 894–899. [Google Scholar] [CrossRef]
- Lughezzani, G.; Jeldres, C.; Isbarn, H.; Sun, M.; Shariat, S.F.; Alasker, A.; Pharand, D.; Widmer, H.; Arjane, P.; Graefen, M.; et al. Nephroureterectomy and segmental ureterectomy in the treatment of invasive upper tract urothelial carcinoma: A population-based study of 2299 patients. Eur. J. Cancer 2009, 45, 3291–3297. [Google Scholar] [CrossRef]
- Kamihira, O.; Hattori, R.; Yamaguchi, A.; Kawa, G.; Ogawa, O.; Habuchi, T.; Kawauchi, A.; Uozumi, J.; Yokoi, S.; Tsujihata, M.; et al. Laparoscopic radical nephroureterectomy: A multicenter analysis in Japan. Eur. Urol. 2009, 55, 1397–1407. [Google Scholar] [CrossRef]
- Novara, G.; De Marco, V.; Gottardo, F.; Dalpiaz, O.; Bouygues, V.; Galfano, A.; Martignoni, G.; Patard, J.J.; Artibani, W.; Ficarra, V. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: Multi-institutional dataset from 3 European centers. Cancer 2007, 110, 1715–1722. [Google Scholar] [CrossRef]
- Rabbani, F.; Perrotti, M.; Russo, P.; Herr, H.W. Upper-tract tumors after an initial diagnosis of bladder cancer: Argument for long-term surveillance. J. Clin. Oncol. 2001, 19, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Roscigno, M.; Shariat, S.F.; Margulis, V.; Karakiewicz, P.; Remzi, M.; Kikuchi, E.; Langner, C.; Lotan, Y.; Weizer, A.; Bensalah, K.; et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J. Urol. 2009, 181, 2482–2489. [Google Scholar] [CrossRef]
- Alvarez-Maestro, M.; Rivas, J.G.; Gregorio, S.A.Y.; de Castro Guerin, C.; Gomez, A.T.; Ledo, J.C. Current role of lymphadenectomy in the upper tract urothelial carcinoma. Cent. Eur. J. Urol. 2016, 69, 384–390. [Google Scholar]
- Seisen, T.; Shariat, S.F.; Cussenot, O.; Peyronnet, B.; Renard-Penna, R.; Colin, P.; Rouprêt, M. Contemporary role of lymph node dissection at the time of radical nephroureterectomy for upper tract urothelial carcinoma. World J. Urol. 2017, 35, 535–548. [Google Scholar] [CrossRef] [PubMed]

| Patients n (%) | |
|---|---|
| Gender | |
| Male | 7 (36.7%) |
| Female | 12 (63.3%) |
| Age | |
| Median ≤ 69 years | 61.6 |
| Median > 69 years | 79.4 |
| Pathological stage | |
| pT0 | 8 (42%) |
| pTa | 7 (36%) |
| pT1 | 4 (21%) |
| CIS | 0 |
| 2004/2016 WHO grade | |
| Low grade | 7 (36%) |
| High grade | 4 (21%) |
| Lymphatic outflow analysis | |
| Number of patients with identified SLNs | 2 (10.5%) |
| Number of patients with no lymphatic outflow | 17 (89.5%) |
| Number of identified SLNs | 2 (10.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polom, W.; Cytawa, W.; Polom, A.; Frankiewicz, M.; Szurowska, E.; Lass, P.; Matuszewski, M. Challenging Visualization of Sentinel Lymph Nodes in Upper Urinary Tract Urothelial Carcinoma. J. Clin. Med. 2021, 10, 5465. https://doi.org/10.3390/jcm10235465
Polom W, Cytawa W, Polom A, Frankiewicz M, Szurowska E, Lass P, Matuszewski M. Challenging Visualization of Sentinel Lymph Nodes in Upper Urinary Tract Urothelial Carcinoma. Journal of Clinical Medicine. 2021; 10(23):5465. https://doi.org/10.3390/jcm10235465
Chicago/Turabian StylePolom, Wojciech, Wojciech Cytawa, Anna Polom, Mikołaj Frankiewicz, Edyta Szurowska, Piotr Lass, and Marcin Matuszewski. 2021. "Challenging Visualization of Sentinel Lymph Nodes in Upper Urinary Tract Urothelial Carcinoma" Journal of Clinical Medicine 10, no. 23: 5465. https://doi.org/10.3390/jcm10235465
APA StylePolom, W., Cytawa, W., Polom, A., Frankiewicz, M., Szurowska, E., Lass, P., & Matuszewski, M. (2021). Challenging Visualization of Sentinel Lymph Nodes in Upper Urinary Tract Urothelial Carcinoma. Journal of Clinical Medicine, 10(23), 5465. https://doi.org/10.3390/jcm10235465







